Uterine fibroids, or leiomyomas, are the most common benign tumors in women of childbearing age (Reference Walker and Stewart42). Approximately 25 percent of women with fibroids experience symptoms such as heavy bleeding, pelvic pain, and pregnancy complications (Reference Brahma, Martel and Christman7). In addition to the clinical burden, the economic burden of fibroids is also substantial; the total direct cost of uterine fibroids in the United States was recently estimated to be $2.2 billion annually (Reference Flynn, Jamison, Datta and Myers15).
Although medications such as nonsteroidal anti-inflammatory drugs (NSAIDs) and/or hormonal therapy may be used to manage fibroid symptoms, some women require more aggressive forms of treatment. Historically, the most common treatment for fibroids has been hysterectomy; alternative options include myomectomy or uterine artery embolization (UAE). Each of these options has its advantages and disadvantages. For example, while hysterectomy eliminates symptoms permanently, it is associated with risks of surgical complications, lengthy recovery times, missed work, and potentially negative quality-of-life outcomes associated with the loss of the uterus (Reference Beinfeld, Bosch, Isaacson and Gazelle5). Hysterectomy is also not appropriate for women who desire future childbearing. Myomectomy is often the treatment of choice for women who want to preserve fertility, but it is not completely effective in eliminating symptoms (Reference Reed, Newton and Thompson29;Reference Subramanian, Clark and Isaacson37) and is associated with surgical risks (2). UAE has a lower rate of major complications than hysterectomy or myomectomy, but a higher rate of minor complications, and is associated with more urgent visits and rehospitalizations (Reference Gupta, Sinha, Lumsden and Hickey19), and is not approved for women who desire future fertility.
Magnetic Resonance Guided Focused Ultrasound (MRgFUS) is a minimally invasive procedure that uses ultrasound as the treatment modality and magnetic resonance to provide guidance and real time feedback. MRgFUS has received FDA approval for the treatment of uterine fibroids for women who no longer desired fertility, and authors of published studies report that MRgFUS is a safe and effective treatment for fibroids (14;Reference Smart, Hindley, Regan and Gedroyc30;Reference Stewart, Gostout and Rabinovici35;Reference Stewart, Rabinovici and Tempany36).
Treatment choice depends not only on the fibroids' size and location, but also on patient preference, quality of life implications, and cost. Several economic studies of uterine fibroid treatments have been published (1;Reference Beinfeld, Bosch and Gazelle4;Reference Beinfeld, Bosch, Isaacson and Gazelle5;Reference Dembek, Pelletier, Isaacson and Spies10;Reference Goldberg, Bussard, McNeil and Diamond18;Reference Mauskopf, Flynn and Thieda24;Reference Oderda, Asche and Jones26;Reference Zowall, Carins and Brewer43), but no study to date has examined the cost-effectiveness of MRgFUS relative to each of the other treatment options available in the United States. Accordingly, we conducted a cost-effectiveness analysis of MRgFUS relative to hysterectomy, myomectomy, UAE, and pharmacotherapy in the treatment of uterine fibroids among premenopausal women in the United States.
METHODS
Model Overview
We developed a Markov model to estimate the long-term outcomes and costs among a cohort of premenopausal women receiving treatment for symptomatic uterine fibroids. The model assumes that women are offered first-line treatment with hysterectomy, myomectomy, UAE, MRgFUS, or pain management with pharmacotherapy only. The model is comprised of health states defined by presence/absence of symptoms and treatment received, with transitions across health states occurring at 6-month intervals. Each health state is associated with a utility and an economic cost. Estimation of the model involves tracking patients' transitions across health states over time and tabulating their quality-adjusted life-years (QALYs) and costs until death.
With the exception of the pharmacotherapy arm, all patients entering the model undergo routine diagnostic tests before the procedure (Figure 1a). Women initiated with UAE or MRgFUS are assumed to undergo additional imaging tests to assess eligibility (Reference Cura, Cura and Bugnone9), as a proportion of women in each group will not be eligible due to the size and location of fibroids. Those who are determined to be ineligible for UAE or MRgFUS receive an alternative procedure. All women are assumed to be eligible for hysterectomy and myomectomy.

Figure 1a. Screening and eligibility for treatments for uterine fibroids.
Patients in the model are at risk for procedure-related complications or death (Figure 1b). Those who survive will either experience symptom relief or have treatment failure; those who fail are assumed a priori to receive second-line treatment with an alternative procedure. Women who are successfully treated are at risk of symptom recurrence until they reach menopause. In the model, women can receive a maximum of three rounds of treatment due to treatment failure or symptom recurrence. Third-line treatment is assumed to be hysterectomy in all cases, which completely eliminates the fibroids.
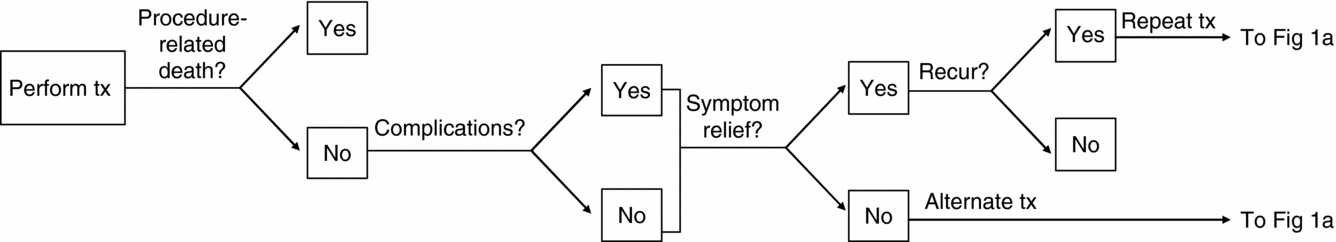
Figure 1b. Outcomes associated with treatments for uterine fibroids.
Patients initiated in the model are assumed to be premenopausal women (mean age, 40 years) with previously untreated symptomatic fibroids. In base-case analyses, we estimated total costs (in 2005 dollars), QALYs, and cost-per-QALY gained from a societal perspective, including lost productivity costs and effects on quality of life. We evaluated an alternative scenario in which productivity costs were omitted, according to the Reference Case recommendations of the U.S. Panel on Cost-Effectiveness in Health and Medicine (Reference Gold, Siegel, Russell and Weinstein17).
Treatment of Uterine Fibroids
With the exception of hysterectomy, no treatment completely eliminates uterine fibroids. Consequently, many women require follow-up treatment to achieve symptom resolution (Reference Spies, Bruno and Czeyda-Pommersheim33;Reference Subramanian, Clark and Isaacson37). Unfortunately, we are unaware of any published data on retreatment patterns (e.g., the distribution of second-line treatments received among women who fail first-line treatment). Furthermore, there are no published data on treatment patterns among women who are clinically ineligible for UAE or MRgFUS.
Given the absence of published data, we made several assumptions regarding treatment patterns among women with uterine fibroids. First, we assumed that nearly all women who are determined to be ineligible for either MRgFUS or UAE would prefer the least invasive of the remaining treatment options (excluding pharmacotherapy). Second, based on the premise that women seeking treatment desire complete elimination of symptoms, we assumed that once a patient undergoes a procedure, she continues to be treated until her symptoms have resolved. If a patient fails first-line treatment, it is assumed that she receives second-line treatment with one of the more invasive strategies (based on treatment patterns estimated from a large administrative database). Patients who experience symptom recurrence are assumed to be retreated with the first-line strategy. All patients requiring a third-line strategy—whether for second-line failure or symptom recurrence—are assumed to receive hysterectomy. Finally, we assumed that pharmacotherapy was used only in instances in which the patient did not want to undergo an invasive procedure; consequently no further treatment was modeled for this strategy.
Model Parameters and Data Sources
Model parameters and corresponding data sources are summarized in Table 1 and described below.
Table 1. Model parameters, base-case estimates, and data sources
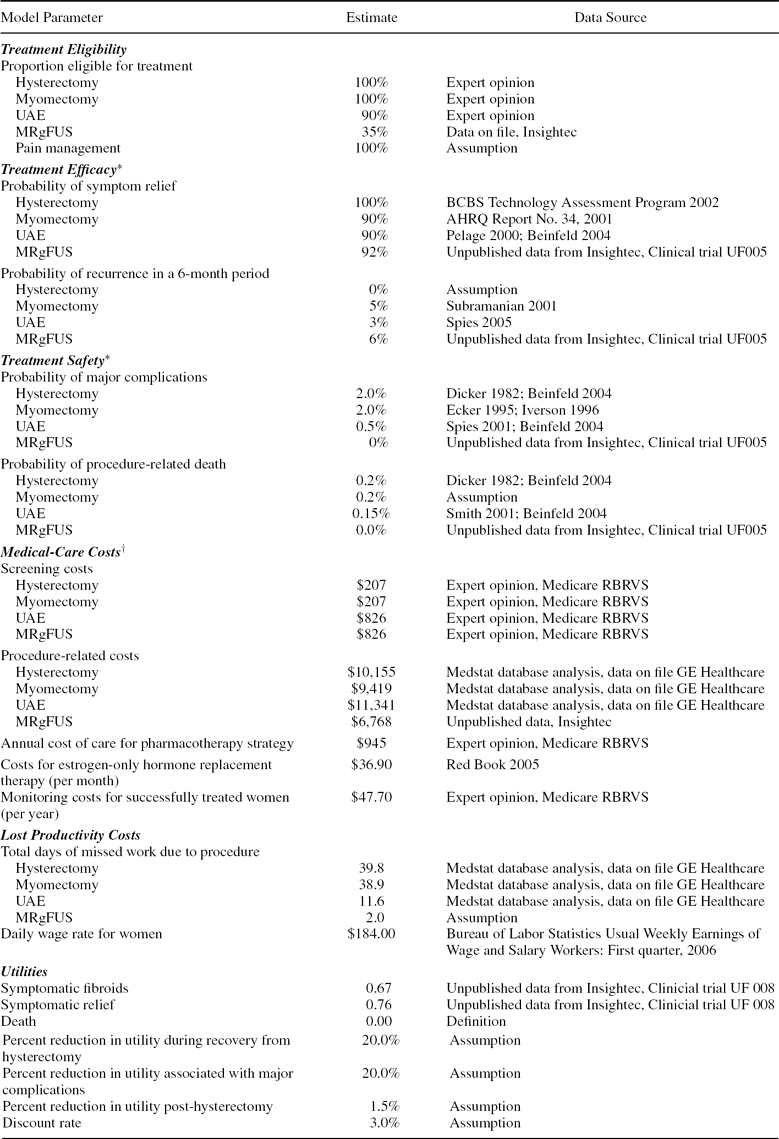
*Efficacy and safety with second- and third-line treatment are assumed to be the same as for the corresponding first-line treatment.
†Costs are in 2005 US dollars. Screening for all procedures is assumed to require 2 office visits, an ultrasound, and lab tests; screening for UAE and MRgFUS also requires an MRI. Costs for treating complications are included in procedure costs. Costs for the pharmacotherapy strategy include those for 4 office visits, 2 ultrasounds, and pain medication.
Treatment Eligibility
The proportion of women eligible for MRgFUS was estimated based on data from InSightec (Tirat Carmel, Isreal). Eligibility for UAE was estimated based on expert opinion (E.A.S.). It was assumed that all women are eligible for treatment with hysterectomy, myomectomy, or pharmacotherapy.
Treatment Efficacy
We estimated the proportions of women experiencing symptom relief and recurrence with hysterectomy (6), myomectomy (1), and UAE (6;Reference Pelage, Dref and Soyer27;Reference Spies, Ascher and Roth32) from Technology Assessment Reports and published studies. The efficacy of MRgFUS was estimated from unpublished clinical trial data (22). We used data from a subgroup of patients treated according to a set of “expanded” guidelines, as these guidelines closely resemble the commercial guidelines approved for MRgFUS in 2004.
Due to a scarcity of data on recurrence rates following treatment of uterine fibroids, we used retreatment rates to proxy for recurrence. We reviewed studies of various follow-up times and standardized all retreatment rates to 6-month risks using an exponential failure time model. In the model, we assumed a constant risk of recurrence in every cycle until menopause.
The likelihood of recurrence with hysterectomy (6), myomectomy (Reference Subramanian, Clark and Isaacson37), and UAE (Reference Spies, Bruno and Czeyda-Pommersheim33) were estimated from published studies. Recurrence with MRgFUS was based on retreatment rates from the same trial from which efficacy data were obtained (22). Probabilities of symptom relief and recurrence with each strategy were assumed to be the same regardless of whether the procedure served as a first-, second-, or third-line strategy.
Treatment Safety
Consistent with Beinfeld et al. (Reference Beinfeld, Bosch, Isaacson and Gazelle5), we obtained the rates of major complications and procedure-related death with hysterectomy from a large prospective study (Reference Dicker, Greenspan and Strauss11). Rates of major complications and procedure-related death for myomectomy were assumed to be equal to those for hysterectomy, based data from two studies (Reference Ecker, Foster and Friedman13;Reference Iverson, Chelmow and Strohbehn23). The risks of major complications (Reference Spies, Ascher and Roth32) and procedure-related death (Reference Smith31) for UAE were obtained from published studies. Rates of major complications and death for MRgFUS were obtained from trial data (Reference Fennessy, Tempany and McDannold14;Reference Hesley, Felmlee and Gebhart21).
Medical-Care Costs
Costs (in 2005 US$) associated with treatment of uterine fibroids are comprised of direct medical as well as lost productivity costs. Medical-care costs include those for preprocedure screening tests, the procedure, procedure-related complications, medications, and postprocedure monitoring.
Routine screening for all procedures was assumed to require two office visits (one each for CPT code 99212 and 99213), an ultrasound (CPT code 76856), and lab tests (i.e., complete blood count and urine pregnancy test). In addition, screening for UAE and MRgFUS was assumed to require an MRI (CPT code 72196).
Procedure costs for hysterectomy, myomectomy, and UAE were derived from a large, retrospective study of commercially insured women (Reference Carls, Lee and Ozminkowski8). Costs included payments from all inpatient, outpatient, and prescription drug claims on the day(s) spent in hospital for the procedure. Costs for inpatient procedures included those for all services rendered during the hospital stay, and, therefore, included costs for treating procedure-related complications. The cost for MRgFUS was obtained from InSightec, and includes costs of the following: the ExAblate 2000® device used to perform MRgFUS (assuming a 60-month lease and routine maintenance); MRI time; physician (radiologist and surgeon), nursing, and technician time (including hourly wage plus fringe benefits); patient sedation; and recovery room.
Women who are managed with pharmacotherapy alone are assumed to require four office visits, two ultrasounds, and two complete blood tests annually. Medication costs for analgesics and hormone therapy were obtained from a review of the economic burden of uterine fibroids (Reference Mauskopf, Flynn and Thieda24) and weighted by the distribution of women who are managed with each strategy as reported in the Thomson Medstat database (Reference Carls, Lee and Ozminkowski8).
We assumed that a proportion of women (20 percent) who receive a hysterectomy also receive estrogen-only hormone replacement therapy following surgery. Drug acquisition costs for these therapies were estimated based on average wholesale prices (12). Finally, women who are successfully treated for their fibroids were assumed to receive one physician visit and one ultrasound annually.
Lost Productivity Costs
Costs due to lost productivity were estimated by multiplying the work days missed due to the procedure and recovery by the loaded daily wage rate. Days missed due to procedure and recovery for hysterectomy, myomectomy, and UAE were obtained from a study by Carls and colleagues (Reference Carls, Lee and Ozminkowski8). Days missed for MRgFUS were estimated based on data from early trials (Reference Hesley, Felmlee and Gebhart21;Reference Smart, Hindley, Regan and Gedroyc30). We estimated the daily wage rate using data from the U.S. Bureau of Labor Statistics on the median weekly earnings for women aged 35 to 54 years in the United States (40).
Health-Related Quality of Life
Utility values for women with symptomatic fibroids and symptom relief were obtained from a clinical trial of MRgFUS (22). Consistent with Beinfeld et al. (Reference Beinfeld, Bosch, Isaacson and Gazelle5), we assumed that patients who receive a hysterectomy are subject to a 0.20 decrement in utility for the 6-month period following the procedure to account for reduced quality of life during the recovery period; women who experience hysterectomy-related complications are subject to an additional 0.20 decrement. Finally, women who receive a hysterectomy are assumed to have a 0.015 decrement in quality of life for the remainder of their lives to account for the long-term effects of hysterectomy on a woman's quality of life.
Discount Rate
All costs and QALYs were discounted to the beginning of the model period using a 3 percent annual discount rate.
Analyses
Base-Case Analyses
Cumulative costs (including those for lost productivity) and QALYs were calculated from initial screening until death, and were used to estimate the incremental cost-per-QALY gained for each of the comparators. Incremental cost-effectiveness ratios were estimated by rank-ordering the treatment regimens by increasing cost and then comparing the more costly to the next-most costly strategy by dividing the additional cost by the additional benefit. Dominated treatment strategies, including those that were more costly and less effective than an alternative (“strong dominance”) and those that had a less favorable incremental cost-effectiveness ratio than a more expensive alternative (“extended dominance”) were eliminated from the incremental cost-effectiveness analysis using conventional methods (Reference Gold, Siegel, Russell and Weinstein17). Base-case analyses were performed from a societal perspective, including costs of lost productivity and all effects on quality of life.
Sensitivity Analyses
To assess uncertainty in the base-case results, we conducted one-way sensitivity analyses by varying key model parameters one at a time through plausible ranges and examining the effects on the incremental cost-effectiveness ratios. As in the base-case analysis, productivity costs were included in total cost estimates. In each one-way sensitivity analysis we identified which of the competing strategies would be the most cost-effective at thresholds of $50,000 or $100,000 per QALY gained.
Reference Case Analysis
In addition to the base case, we performed a Reference Case analysis as defined by the U.S. Panel on Cost-Effectiveness in Health and Medicine (Reference Gold, Siegel, Russell and Weinstein17). The Panel recommends omitting the costs of lost productivity from the numerator of the cost-effectiveness ratio, under the assumption that effects on productivity are captured in the utility weights in the denominator. Therefore, in the Reference Case analysis we excluded these costs.
Alternative Analyses
Due to the paucity of data on the efficacy and safety of treatment for uterine fibroids, in addition to one-way sensitivity analyses we conducted a variety of alternative analyses in which we varied one or more parameters according to less conservative estimates than those used in the base case. As rates of complications with hysterectomy, myomectomy, and UAE vary widely in the literature, in the first alternative analysis we assumed that the rates of complications after hysterectomy, myomectomy, and UAE are equal to those used in a UK cost-effectiveness study of MRgFUS (Reference Zowall, Carins and Brewer43) (i.e, 6.2 percent for hysterectomy and myomectomy and 4 percent for UAE). In addition, in the same analysis we assumed that there exist complication costs for these procedures that are not included in our base-case procedure cost (i.e., assuming that the complications occur after discharge). As costs of complications associated with these procedures are scarce in the literature, for the purposes of this alternative analysis we assumed that the costs of complications occurring postdischarge are equal to 50 percent of the procedure cost.
In the second alternative analysis, we assumed a recurrence rate for MRgFUS of 3.6 percent. This is equal to the weighed average of recurrence rates used in the study by Zowall et al. (Reference Zowall, Carins and Brewer43). Zowall et al. used recurrence rates of 0.8 percent in the first 6 months, 6.49 percent in 6–12 months and 3.63 percent in 12–24 months based on pooled data from a selected group of patients (those with the nonperfused volume relative to total fibroids volume of 60 percent) from four clinical trials of MRgFUS.
In the third alternative analysis, we vary the eligibility rate for MRgFUS to 50 percent and 90 percent, under the assumption that the proportion of patients eligible will increase in future years as the procedure is more widely incorporated into clinical practice.
RESULTS
Base-Case Results
Lifetime discounted total costs (including lost productivity) per patient were lowest for the pharmacotherapy strategy at $9,200. Among the procedures, hysterectomy was the least costly ($19,800), followed by MRgFUS ($27,300), UAE ($28,900), and myomectomy ($35,100). The proportions of total costs attributable to screening, the procedure, lost productivity, and other costs varied by treatment strategy (Figure 2). For example, procedure costs were highest for UAE, comprising over 68 percent of the total costs for that strategy, while hysterectomy and myomectomy had the largest proportions of total costs attributable to lost productivity (37 percent and 41 percent, respectively). Productivity costs for myomectomy are higher than those for hysterectomy because it results in the most follow-up procedures. In the model, myomectomy patients who failed initial treatment were assumed to undergo a hysterectomy, thus these patients incurred productivity losses due to myomectomy as well as hysterectomy. In contrast, hysterectomy is assumed to be 100 percent effective, so none of the hysterectomy patients required subsequent treatment.
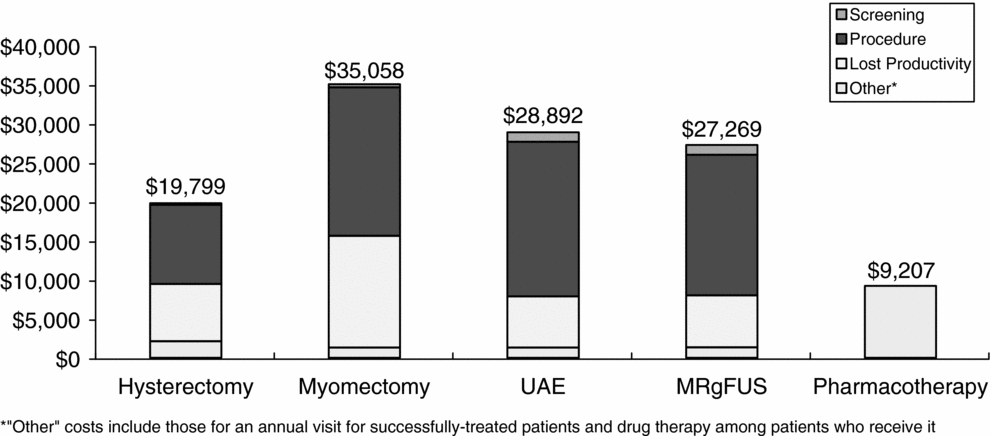
Figure 2. Total costs by component per patient receiving alternative uterine fibroid treatments.
Under base-case assumptions, treatment with UAE was associated with the most discounted QALYs (17.39), followed by MRgFUS (17.36), myomectomy (17.31), hysterectomy (17.18), and pharmacotherapy (16.70). The incremental cost-effectiveness ratios, or costs-per-QALY gained, were $21,800 for hysterectomy (relative to pharmacotherapy), $41,400 for MRgFUS (relative to hysterectomy), and $54,200 for UAE (relative to MRgFUS) (Table 2). Myomectomy was strongly dominated, meaning that it was more costly than at least one more effective strategy.
Table 2. Cost-effectiveness of treatments for uterine fibroids.
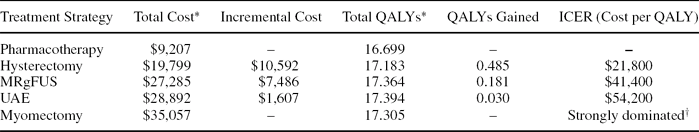
*per patient, discounted at 3.0%
†‘Strongly dominated’ meaning the strategy is both more costly and less effective than a more effective strategy
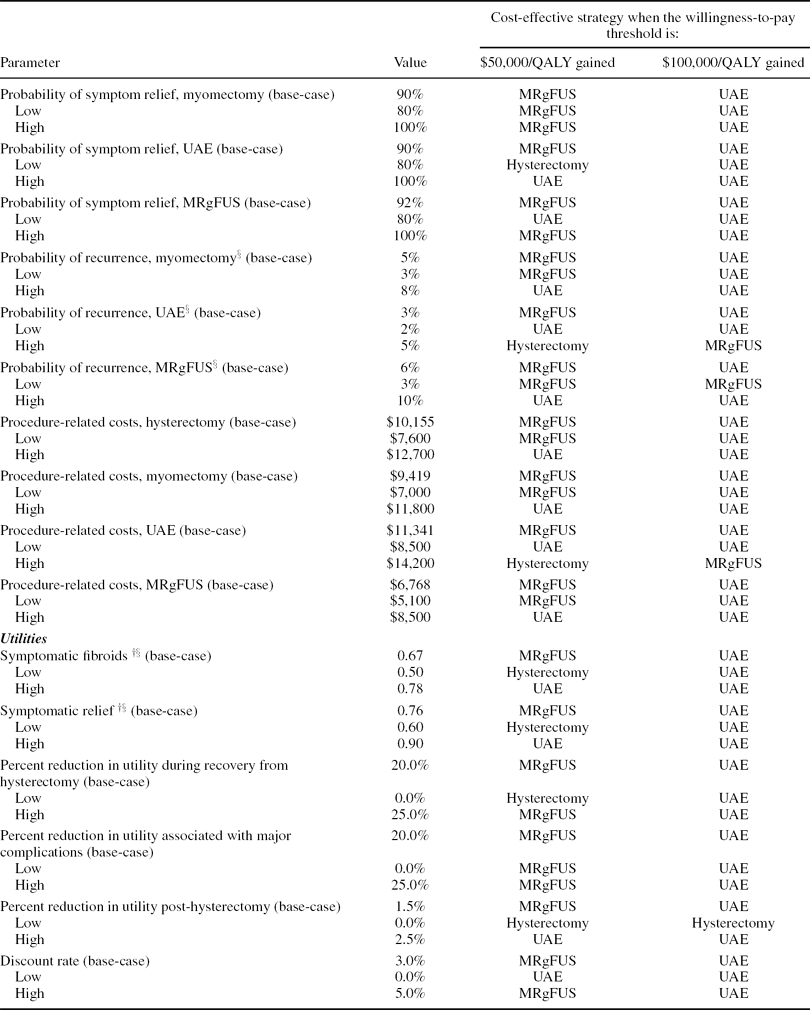
One-Way Sensitivity Analyses
Results of the one-way sensitivity analysis are shown in Table 3. Model parameters were varied across plausible ranges and the strategies that would be cost-effective at willingness-to-pay thresholds of $50,000 and $100,000 per QALY gained, alternatively, were identified. Note that the strategy that is most cost-effective at a specified threshold is not necessarily the one with the lowest ratio, but rather the one with a ratio that comes closest to the threshold without exceeding it. For example, in the base case, if a payer's willingness to pay is $50,000 per QALY gained, the cost-effective strategy would not be hysterectomy ($21,800 per QALY gained), but MRgFUS ($41,400 per QALY gained), as this is the alternative that would provide the greatest benefit at an incremental cost-effectiveness ratio not exceeding the specified criterion of $50,000 per QALY gained. As UAE ($54,200 per QALY gained) has an incremental cost-effectiveness ratio relative to MRgFUS that comes closest to a $100,000 threshold without going over it, it would be the treatment of choice for a payer with a threshold of $100,000 per QALY gained.
Table 3. The impact of variation in model parameters on the incremental cost-effectiveness of MRgFUS.
Ranges estimated based on reasonable estimates of model values unless otherwise noted
§Range for parameter based on literature
†High and low ranges for utilities for symptomatic fibroids and symptomatic relief moved together
The cost-effectiveness ratios and the most cost-effective treatment choices at the specified thresholds were most sensitive to the probability of symptom relief, the probability of symptom recurrence, and procedure costs for UAE and MRgFUS. For example, when the probability of recurrence with UAE is varied from its low (2 percent) to its high (5 percent) value (base case = 3 percent), its incremental cost-effectiveness ratio ranges from $26,100 to $317,300 per QALY gained relative to MRgFUS. Therefore, at a cost-per-QALY threshold of $50,000 per QALY, UAE would be cost-effective if its recurrence rate was at the low end of the range, whereas MRgFUS was cost-effective based on this threshold in the base case. At the high end of the range of UAE recurrence rates, hysterectomy becomes cost-effective; because UAE is the second-line treatment for MRgFUS, factors that make UAE less attractive also adversely affect the MRgFUS strategy. If MRgFUS had a recurrence rate as low as 3 percent (base case = 6 percent), the incremental cost-effectiveness ratios for MRgFUS (vs. hysterectomy) and UAE (vs. MRgFUS) would be $28,700 and $603,000 per QALY gained respectively. Alternatively, at a threshold of 7 percent recurrence, MRgFUS becomes dominated by UAE. The lifetime utility decrement associated with hysterectomy also has a substantial impact on the cost-effectiveness ratios. When the decrement is varied from 0.0 to 0.025 (base case = 0.015), MRgFUS ranges from being dominated by hysterectomy to having an incremental cost-per-QALY gained of $30,100. Results were fairly insensitive to variations in model parameters not contained in Table 3.
Reference Case Analysis
In the Reference Case analysis, productivity losses are assumed to be reflected in the utility estimates (and, therefore, omitted from the cost estimates). In this case, myomectomy remains strongly dominated, and the conclusions from the cost-effectiveness analysis are similar to base case. The incremental cost-effectiveness ratios are $6,700 per QALY gained for hysterectomy, $45,000 for MRgFUS, and $58,000 for UAE.
Alternative Analyses
Due to the wide-ranging estimates of rates and costs of complications associated with hysterectomy, myomectomy, and UAE in the literature, in the first alternative analysis we assumed that the rates of complications with hysterectomy and myomectomy are each 6.2 percent, and the complication rate with UAE is equal 4 percent. In addition, we assumed additional costs of these complications equal to 50 percent of the procedure cost. Under these assumptions, the incremental cost-effectiveness ratio for hysterectomy relative to pharmacotherapy is $32,500 per QALY gained, the ratio for MRgFUS relative to hysterectomy is $55,800 per QALY gained, and the ratio for UAE relative to MRgFUS is $133,600 per QALY gained. As in the base case, myomectomy is dominated due to the fact that it is more costly and less effective than at least one other alternative.
If the 6-month recurrence rate with MRgFUS is assumed to be 3.6 percent, the incremental cost-effectiveness ratio for hysterectomy relative to pharmacotherapy is $21,800 per QALY gained, the ratio for MRgFUS relative to hysterectomy is $31,600 per QALY gained, and the ratio for UAE relative to MRgFUS is $248,000 per QALY gained. Again, myomectomy is a dominated alternative.
In the third alternative analysis, we vary the eligibility rate for MRgFUS to 50 percent and 90 percent. Should the eligibility rate with MRgFUS increase to 50 percent (from a base case of 35 percent), the cost per QALY gained for hysterectomy relative to pharmacotherapy is $21,800, the cost per QALY gained for MRgFUS relative to hysterectomy is $38,700, and the cost per QALY gained for UAE relative to MRgFUS is $64,300. Corresponding figures under the 90 percent eligibility assumption are $21,800, $30,100, and $78,400 per QALY gained. Under both alternative scenarios regarding eligibility, myomectomy remains dominated.
DISCUSSION
Our results suggest that MRgFUS is in the range of currently accepted criteria for cost-effectiveness, along with hysterectomy and UAE. Myomectomy was both more costly and less effective than MRgFUS and UAE. In the base-case analysis, incremental cost-effectiveness ratios were $21,800 per QALY gained for hysterectomy, $41,400 for MRgFUS, and $54,200 for UAE. Results are sensitive to changes in rates of fibroid recurrence with the different treatments, procedure costs, eligibility rate with MRgFUS, and assumptions regarding quality-of-life following hysterectomy.
Very few studies of the cost-effectiveness of uterine fibroids treatment exist, likely due in part to the scarcity of data on efficacy and safety of these procedures (1). Beinfeld and colleagues (Reference Beinfeld, Bosch, Isaacson and Gazelle5) compared the cost-effectiveness of UAE with hysterectomy and reported that UAE is cheaper and more effective than hysterectomy. In contrast, our results indicate that UAE is more costly and more effective (as measured by QALYs gained) relative to hysterectomy. Unlike Beinfeld et al., we included screening costs, treatment eligibility criteria, and multiple rounds of follow-up treatment for failure or recurrence. These components likely caused the average cost of UAE to be higher than hysterectomy in our analysis relative to Beinfeld.
The Agency for Healthcare Research and Quality compared costs and outcomes of hysterectomy and myomectomy, and concluded that hysterectomy results in favorable outcomes for up to 2 years (1). However, researchers concluded that there were insufficient data for a head-to-head comparison, and thus gave no recommendations for the most appropriate treatment strategy.
Most recently, Zowall and colleagues estimated the cost-effectiveness analysis of MRgFUS versus a “current practice” strategy (distribution of UAE, myomectomy, and hysterectomy) from the perspective of the National Health Service (NHS) in the UK (Reference Zowall, Carins and Brewer43). They report that MRgFUS is associated with cost savings and a small QALY gain relative to current practice. Our model differs from theirs in several major ways. First, while Zowall's model follows women until they reach menopause, ours is a lifetime model and includes physician monitoring costs and effects on quality-of-life that persist over a woman's lifetime. Notable among these lifelong effects in our model is the quality-of-life decrement attributable to having had hysterectomy. Second, in contrast to Zowall, we included costs of screening tests to determine eligibility (as they vary by treatment strategy); we also accounted for the fact that not everyone who is screened for UAE or MRgFUS is eligible to receive the procedure. In fact, many women who are initiated with UAE or MRgFUS end up receiving a different procedure altogether. The differences in relative prices for the various procedures is a third important factor. For example, the cost of hysterectomy in the UK study was estimated at £2,727 (approximately $5,500 at current exchange rates), while we estimated the cost of hysterectomy in the United States to be $10,155, and assumed that all women who failed two lines of treatment received a hysterectomy. A fourth difference from Zowall et al. is that we used recurrence rates for MRgFUS derived from the full clinical trial population, whereas they restricted their estimates to the subset of patients for whom the nonperfused fibroid volume was 60 percent or more of the total. In an alternative analyses in which we used their recurrence assumptions, the qualitative result that MRgFUS was cost-effective at a criterion of $50,000 per QALY was unchanged, but MRgFUS also became cost-effective at a criterion of $100,000 per QALY (because the incremental ratio for UAE compared to MRgFUS rose to $248,000 per QALY.) Finally, Zowall et al. compared MRgFUS to a single strategy that assumes 25 percent of women receive UAE, 25 percent receive myomectomy, and 50 percent receive hysterectomy. Our model includes a separate comparison with each of these strategies, to reflect the different outcomes and costs associated with each. The fact that the comparator to MRgFUS in the Zowall study is a composite that includes myomectomy (strongly dominated according to our model) is likely a large contributing factor to the differences between our results and those of Zowall et al.
Some important limitations of our study should be noted. Any cost-effectiveness study is subject to the limitations surrounding uncertainty in key model parameters. Because one-way sensitivity analyses revealed that the cost-effectiveness of three treatment options was very sensitive to several parameters and assumptions, we did not conduct a formal probabilistic sensitivity analysis. Such an analysis would have reinforced our conclusion that the current choice of treatment could easily go any of three different ways.
We assumed that 35 percent of women who present for treatment with MRgFUS will be clinically eligible to receive the procedure. While a recent study reported that 14 percent of women inquiring about participating in a trial of MRgFUS were eligible for the procedure, the authors acknowledged that their findings are likely to be lower than the reality due to restrictions imposed by their institution's Committee for Human Rights and the FDA (Reference Arleo, Khilnani, Ng and Min3). They conclude that once the procedure is used in clinical practice, eligibility rates will be higher. Our estimate is based on clinical input and communication from participating sites. We believe that the eligibility rate will increase as physicians become more comfortable with the technology and it is more widely assimilated into clinical practice.
Estimation of the probability of symptom relief with fibroid treatments posed some difficulty due to the scarcity of randomized controlled trials on fibroid treatments, measurement differences across studies, and varying lengths of follow-up. Estimating recurrence rates is also challenging. Recurrence can be defined in terms of symptoms or alternatively in terms of re-growth; due to the inconsistency in definitions, we used rates of retreatment to proxy for recurrence. We reviewed studies that reported retreatment rates regardless of the specific procedure (e.g., hysterectomy, myomectomy) received, standardized rates to 6-month risks using an exponential failure time model, and assumed a constant risk of recurrence in every 6-month period until menopause in the model. While recurrence is unlikely to be constant over time, at present no long-term data are available to contradict our assumption.
Quantitative data on the short- and long-term impact of hysterectomy on quality of life also are limited. With respect to short-term effects, we followed Beinfeld et al. (Reference Beinfeld, Bosch, Isaacson and Gazelle5), and assumed a 0.20 reduction in the utility in the cycle in which complications occur as well as a 0.20 reduction during recovery from hysterectomy. Regarding long-term effects, while some studies suggest that women experience an improvement in their quality of life following hysterectomy due to the fact that the condition for which they sought treatment is gone (Reference Rannestad28;Reference Spies, Cooper and Worthington-Kirsch34), other studies cite long-term negative effects such as an increase in cardiovascular risk, mental health issues, sexual problems, incontinence, and vaginal vault prolapse (Reference Hartmann, Birnbaum and Ben-Hamadi20;Reference Toaff39;Reference Volkers, Hehenkamp and Birnie41). We did not account for an increase in cardiovascular disease risk among women who had hysterectomy, as this is controversial and the quantitative impact is unclear. However, we also excluded the reduction in the risk of uterine cancer, so we believe that any bias from excluding long-term risks is small. In our model, the quality of life improvement due to elimination of fibroid symptoms following hysterectomy is captured in the health-state utility weights; long-term reductions in quality of life is captured by a lifetime decrement of 0.015 for women who received a hysterectomy. While we found similar estimates for quality of life decrements associated with comparable chronic conditions (Reference Sullivan, Lawrence and Ghushchyan38), data to support the long-term effects of hysterectomy on quality of life would strengthen our conclusions.
Our analysis does not include the impact of treatment on childbearing choices. Myomectomy is the only procedure currently recommended for women who desire future fertility. Although MRgFUS is the least invasive of the procedures studied here and there exist case studies of successful pregnancies among women who have received it (Reference Gavrilova-Jordan, Rose and Traynor16;Reference Morita, Ito and Ohashi25), MRgFUS is currently limited in its indication for use among women who do not desire future fertility. As the choice of treatment depends not only on cost and effectiveness but also on patient preferences, a study of patient preference (particularly with respect to future childbearing) for the various treatment strategies for uterine fibroids represents an area for future research.
Our study represents the first cost-effectiveness analysis to compare all of the major treatment options for women with uterine fibroids in the United States. Our findings suggest that along with UAE and hysterectomy, use of MRgFUS among premenopausal women with uterine fibroids is within the range of currently accepted criteria for cost-effectiveness. Until more data on the long-term efficacy of treatment options become available, any of the three could be the cost-effective choice, depending on patient preferences and resource availability. Although myomectomy was not found to be cost-effective in our analysis, it will remain an option for women planning future pregnancy until additional safety data on other uterine-sparing treatment strategies become available. Future cost-effectiveness studies would benefit from long-term studies that assess rates of recurrence and retreatment over time, as well as preferences for and quality-of-life impacts of the various treatments for uterine fibroids.
CONTACT INFORMATION
Amy K. O'Sullivan, PhD ([email protected]), Associate Director, David Thompson, PhD ([email protected]), Vice President, Paula Chu, BA ([email protected]), Research Associate, i3 Innovus, 10 Cabot Road, Suite 304, Medford, Massachusetts 02144
David W. Lee, PhD ([email protected]), Senior Director, Health Economics and Outcomes Research, 300 N. Grandview Blvd., W460, Waukesha, Wisconsin 53188
Elizabeth A. Stewart, MD ([email protected]), Obstetrics and Gynecology, Mayo Clinic and Mayo Medical School, Rochester, Minnesota 55901
Milton C. Weinstein, PhD ([email protected]), Department of Health Policy and Management, Harvard School of Public Health, 718 Huntington Avenue, Boston, Massachusetts 02115








