Introduction
The cultivation of vannamei shrimp in intensive ponds is an aquaculture activity that has witnessed significant growth in various countries, particularly Indonesia (Supono, Reference Supono2021). The increasing demand for vannamei shrimp has driven an increase in its production, facilitated by the intensification of cultivation systems (Bosman et al., Reference Bosman, Soesilo and Rahardjo2021). Furthermore, intensive cultivation of this aquatic animal is characterized by high stocking densities and the extensive use of high-protein artificial feeds (Emerenciano et al., Reference Emerenciano, Rombenso, Vieira, Martins, Coman, Truong, Noble and Simon2022). Several studies have shown that feeds serve as a nutrient source, containing essential components, such as proteins, fats, carbohydrates, vitamins, and minerals required for the maximum growth and enhanced performance of vannamei shrimp (Ayisi et al., Reference Ayisi, Hua, Apraku, Afriyie and Kyei2017). The protein requirement of this animal ranges from 32.2 to 35.6% (Lee and Lee, Reference Lee and Lee2018).
The main objective of the production process of vannamei shrimp in intensive aquaculture systems is to maximize the productivity of ponds by increasing stocking density and optimizing input provision, coupled with adequate technological support. Cultivation at high stocking density comprises the use of commercial artificial feed and the operation of large-scale water aerators, complemented by the addition of probiotic bacteria. This process aims to stimulate growth while maintaining water quality suitable for the maximum growth of the animal (Tantu et al., Reference Tantu, Salam and Ishak2020).
Intensive cultivation of vannamei shrimp often leads to increased production yields and the generation of a significant amount of solid and dissolved waste in the form of sludge and effluent (Prasetiyono et al., Reference Prasetiyono, Bidayani, Robin and Syaputra2022). The solid waste primarily originates from uneaten feed, metabolic residues, undigested or unabsorbed feed, and dead microorganism colonies. Meanwhile, the dissolved waste is generated from the decomposition of organic feed materials in water, leading to the production of various compounds, including ammonia, urea, carbon, nitrogen, and phosphorus (Dauda et al., Reference Dauda, Ajadi, Tola-Fabunmi and Akinwole2019; Iber and Kasan, Reference Iber and Kasan2021; Jasmin et al., Reference Jasmin, Syukri, Kamarudin and Karim2020). According to Patil et al. (Reference Patil, Antony, Avunje, Viswanathan, Lalitha, Jangam, Kumar, Solanki, Reddy and Vinayakarao2021), aquatic animals typically retain 20–25% of the consumed protein and release the rest into the ponds as total ammonia nitrogen and organic nitrogen. The organic sediment accumulating at the bottom of the ponds becomes a source of nitrogen and phosphorus in shrimp cultivation systems (Syah et al., Reference Syah, Fahrur, Suwoyo and Makmur2017).
Waste generated from vannamei shrimp farming activities can lead to changes in environmental quality. The presence of inorganic compounds originating from the decomposition of organic materials containing nutrient components often leads to elevated levels of nutrient elements in the water, including ammonium (NH4), nitrate (NO3), and orthophosphate (PO43−). According to previous studies, the total nitrogen and total phosphorus content in the discharged water from farming activities represent approximately 10–30% and 30–80% of the organic matter released into the water (Dauda et al., Reference Dauda, Ajadi, Tola-Fabunmi and Akinwole2019). Furthermore, the presence of these elements at high concentrations typically leads to increased growth of microalgae or phytoplankton, potentially disrupting aquatic ecosystems (Cui et al., Reference Cui, Jin, Wang, Gao, Fu, Yang and Wang2021). The organic waste from aquaculture activities often undergoes demineralization processes, converting the macro- and micronutrient content into mineral elements (Chiquito-Contreras et al., Reference Chiquito-Contreras, Hernandez-Adame, Alvarado-Castillo, Martínez-Hernández, Sanchez-Viveros, Chiquito-Contreras and Hernandez-Montiel2022). These mineral elements are known to play an essential role in supporting the abundance of phytoplankton (Ramos et al., Reference Ramos, Schulz, Voss, Narciso, Muller, Reis, Cachao and Azevedo2017).
Vannamei shrimp farmers discharge a small amount of water from the production ponds during cultivation activities and replace it with fresh water (Iber and Kasan, Reference Iber and Kasan2021). This practice is often carried out to maintain water quality throughout the production process. Furthermore, the accumulated sediment sludge is removed through siphoning, and at the end of each cycle, it is completely discharged from the ponds (Hossain et al., Reference Hossain, Sarker, Amin, Hossain and Miah2016). The waste generated from vannamei shrimp production activities is typically collected and processed in specialized locations, known as wastewater treatment ponds (WTPs) before it is discharged into the general water body (Zein et al., Reference Zein, Nurdin, Rianto and Ramadhani2023).
WTPs play an essential role in the cultivation of vannamei shrimp, standing as the main requirement for farming activities. The presence of these ponds aims to ensure the sustainability of the pond business and the environmental ecosystem (Melian, Reference Melian2020). The implementation of WTPs is a proactive measure to minimize the burden of shrimp farming waste. The objective is to ensure that the waste meets environmental quality standards, leading to sustainable farming activities with increased environmental awareness (Syah et al., Reference Syah, Fahrur, Suwoyo and Makmur2017). When constructing shrimp ponds, farmers are required to also build WTPs for treatment of waste before discharge into public waters. This imperative arises from the significant potential for waste generation and its associated environmental impacts (Mohd et al., Reference Mohd, Gafur, Hartanto, Sirajuddin, Anshar, Sabar, Arsyad and Zaenal2019).
The dynamics of environmental quality conditions, including phytoplankton abundance in pond effluents, can vary across different locations. This variation depends on several factors, including production capacity, production cycles, feed quantity, stocking density, the use of probiotics, and the treatment of waste before discharge (Prasetiyono et al., Reference Prasetiyono, Bidayani, Robin and Syaputra2022). The northern coastal area of Bangka Island is a region on the island where intensive-scale vannamei shrimp farming activities using high-density polyethylene (HDPE) ponds are prevalent. Mustafa et al. (Reference Mustafa, Estim, Shaleh and Shapawi2018) stated that intensive shrimp farming ponds in Indonesia typically use HDPE sheets as the lining material. The use of these materials has been reported to increase production and it is suitable for application in regions characterized by high soil porosity, sandy soil types, and soils containing mineral acids. HDPE-lined ponds also exhibit a greater abundance of plankton when compared to those with soil-lining. This difference can be attributed to the erosion of pond walls or bottoms in soil-lined ponds, which is caused by aeration and rainfall.
Cultivation in this area uses seawater as a water source, and the effluent undergoes treatment before being discharged. The study of phytoplankton abundance and the quality of waste from vannamei shrimp ponds is an important undertaking for cultivating other organisms. Therefore, this study aims to evaluate the abundance of phytoplankton and environmental quality in the waste of intensively cultured vannamei shrimp ponds in the northern coastal area of Bangka Island, as well as the factors supporting the abundance of phytoplankton. The results are expected to serve as a basis for the cultivation of plankton feeder and detritus feeder biota using this waste.
Materials and methods
This study was conducted at the intensive shrimp ponds located in the Belinyu District, Bangka Regency, from February to March 2023. A total of four ponds were selected as samples for the experimental procedures. Furthermore, the coordinates of the four pond locations are shown in Table 1, and a map of these locations is presented in Figure 1.
Table 1. Research location coordinates

Figure 1. Map of vannamei shrimp research locations in the northern coastal area of Bangka Island.
Sampling of water and sediment in each pond area was conducted during the operation of the containment pond and waste treatment from shrimp production activities on days 50–90. Days 50–90 were the periods with the highest concentrations of nitrate and phosphate in the sampling locations (Rachmansyah et al., Reference Rachmansyah, Taukhid, Tampangallo, Tahe and Undu2021). Nitrate and phosphate were compounds derived from the decomposition of organic waste in the ponds and were essential nutrients for phytoplankton growth. The samples were collected in the morning from 09:00 to 11:00 AM local time under overcast weather conditions with an air temperature of 29°C. Sampling of water and sediment for the analysis of shrimp pond waste quality was conducted at four points within each shrimp pond waste treatment location (WTPs). The first point was located at the influent area where the water flowed from the production pond to the WTPs. The second and third points were in the middle of the WTPs, whereas the fourth point was near the effluent area where the water exited the WTPs. Water sampling for phytoplankton analysis was performed by collecting water using a 10 l volume container. The water in the container was filtered using a plankton net, specifically a number 25 net, for a total of ten times. Subsequently, the filtered samples were transferred into 100 ml bottles, and two–three drops of Lugol's solution were added for preservation. The samples were then observed under a microscope at magnifications ranging from 40× to 100× (Lestari et al., Reference Lestari, Samawi, Faizal, Moore and Jompa2021). The identification of phytoplankton was conducted using the phytoplankton identification book by Stafford (Reference Stafford1999). Based on the observation results, the abundance of phytoplankton, diversity index, evenness index, and dominance index were calculated. The calculation of phytoplankton abundance followed the guidelines provided by APHA (2017):
where N is the plankton abundance (cells ml−1), n is the number of counted plankton (cells), a is the area covered by the glass slide (mm2), A is the total field of view under the microscope (mm2), v is the volume of water sample in the collection bottle (ml), V c is the volume of concentrated plankton under the cover glass (ml), and V is the volume of filtered water sample (ml).
The structure of the phytoplankton community was analysed through the calculation of three ecological indices based on the equations outlined in Odum (Reference Odum1998). These indices included the Shannon–Wiener diversity index, Shannon–Wiener evenness index, and Simpson dominance index. Plankton diversity was calculated using the Shannon–Wiener index formula as follows:
where H′ is the Shannon–Wiener diversity index, S is the number of species, Pi is relative abundance (ni/N), ni is the number of individuals of species i, and N is the total number of individuals.
The calculation of phytoplankton evenness was performed using the following formula:
where E is the evenness index, H′ is the Shannon–Wiener diversity index, H′max is the natural logarithm (ln) of the number of species (S), and S is the number of species.
The dominance index of plankton was calculated using the following formula:
where D is the dominance index, ni is the number of individuals of species i, and N is the total number of individuals.
The analysis of nutrient characteristics in shrimp pond waste consisted of ammonium, nitrite, nitrate, orthophosphate, total organic matter (TOM) in water, and TOM in sediment. Furthermore, the analysis of ammonium (NH4), nitrite (NO2), nitrate (NO3), and orthophosphate (PO4) was carried out using a UV–Vis spectrophotometer, following the guidelines provided by APHA (2017). The evaluation of TOM in water was performed using volumetric methods, whereas the assessment of TOM in sediment was carried out with gravimetric methods.
The parameters salinity, temperature, pH, dissolved oxygen (DO), and oxidation reduction potential (ORP) were measured in situ using specific measuring instruments. These instruments included the Atago hand refractometer, TP101 digital thermometer, Hanna Digital HI 98107 pH meter, portable Luxtron DO meter, and digital ORP-169F ORP meter. The parameters of calcium (Ca) and magnesium (Mg) were analysed using an atomic absorption spectrophotometer, following the guidelines specified in APHA (2017). Furthermore, the total suspended solids (TSS) were analysed using the gravimetric method based on the procedures outlined in APHA (2017).
The data obtained in this study were analysed descriptively and quantitatively. A comparison of nutrient and organic matter characteristics, as well as environmental quality among the pond locations, was conducted using analysis of variance with a confidence level of 95%. To assess the strength of the relationship between phytoplankton abundance and nutrients with environmental quality parameters, principal component analysis (PCA) was performed using XLSTAT 2019 software (Thakar et al., Reference Thakar, Luthra and Khattar2018). Furthermore, the results were presented in the form of matrices and graphs.
Results
Shrimp ponds’ characteristics
The northern coastal area of Bangka Island was located adjacent to the Natuna Sea. Furthermore, this region had experienced a significant increase in intensive and extensive cultivation of vannamei shrimp in ponds since 2018. Based on field surveys and information from the Bangka Regency Fisheries Office in 2022, there were a total of 32 intensive aquaculture operators in the region. The shrimp farming operators consisted of both corporate entities (business consortia) and individual ownership. The total area of vannamei shrimp production ponds owned by each operator varied, ranging from a minimum of 10,000 m2 to a maximum of 200,000 m2, with an average of 60,000 m2. BL1, BL2, BL3, and BL4 were intensive farms located on the north coast of Bangka Island and their production characteristics are presented in Table 2.
Table 2. Characteristics of production activities of vaname shrimp farms on the north coast of Bangka Island at 60th day of culture

BL1, BL2, BL3, and BL4 were intensive shrimp ponds that had been in production for a total of 6, 5, 11, and 8 cycles since their establishment, respectively. The total area of each of them was 40,000, 55,000, 179,500, and 35,000 m2, respectively. These ponds used HDPE tarpaulins for vannamei shrimp production. During the production process, commercial artificial feed was used to enhance shrimp growth, while paddle wheels were extensively used to supply oxygen in the water. The protein content of the commercial feed provided to shrimp at all pond locations is relatively similar. At BL1, the protein content is 32% for 1–30 days of cultivation and 28% for cultivation periods exceeding 30 days. The protein content of the feed at BL1 is the same as that of BL2 and BL4. At BL3, the protein content provided to shrimp is 35% for 1–30 days of cultivation, 30–32% for 30–45 days of cultivation, and 28–30% for cultivation periods exceeding 45 days.
The application of probiotic bacteria, either mixed with the feed or directly introduced into the water (bioaugmentation), was also part of the production process. The addition of minerals to water or mixed with feed is applied at all pond locations. The added mineral content consists of macro and micro minerals. Minerals play a significant role in the physiological functions and are required for new shell formation and moulting in shrimp. Minerals are also required to cultivate phytoplankton as natural feed for shrimp. The presence of this mineral affects the presence of phytoplankton in ponds and pond waste.
In all study locations, each site had a pond that collected and treated the waste from vannamei shrimp production activities (WTPs) before being discharged into the natural waters. The waste treatment process in WTPs at each location used a sedimentation system. Furthermore, the sedimentation system was designed to settle organic matter from the waste generated in the production ponds. After the organic matter had settled, the wastewater was discharged into the natural water bodies. These WTPs were elongated in shape with a depth ranging from 50 to 100 cm, and the bottoms were made of soil without HDPE tarpaulin lining.
Phytoplankton abundance
The presence of phytoplankton was always observed in vannamei shrimp ponds due to the availability of nutrients from organic matter, which supported their growth. The abundance of phytoplankton in BL1, BL2, BL3, and BL4 ponds reflected their quantity and density in the water. Each pond exhibited a different level of phytoplankton abundance, as shown in Table 3.
Table 3. Phytoplankton abundance in the effluent of vannamei shrimp ponds in the northern coast of Bangka Island

Different superscript letters between the columns indicate significant differences in values (Duncan's test, P < 0.05).
Based on the results, there were differences in the phytoplankton groups found in each pond, as shown in Table 3. A total of six groups were found in BL1 and BL3 ponds, namely Chlorophyta, Cyanophyta, Chryptophyta, Bacillariophyta, Euglenophyta, and Pyrrophyta. In BL2 and BL4 ponds, five groups were found, including Chlorophyta, Cyanophyta, Chryptophyta, Bacillariophyta, and Pyrrophyta. Chlorophyta was the most abundant phytoplankton group found in each location compared to others. The results showed that the total abundance of phytoplankton in the effluent water of each pond was above 1,000,000 cells ml−1, with the highest abundance statically recorded in BL3 at 2,418,375 cells ml−1.
Certain species had been identified with higher abundance compared to others. The presence of phytoplankton varied among the ponds, with some species found in one pond but not in others. The identified samples in each pond are presented in Table 4. According to Table 4, several phytoplankton species were found in BL1, BL2, BL3, and BL4 ponds, with 13, 11, 18, and 14 genera, respectively. The images of the phytoplankton are presented in Figure 2. Some genera, such as Nanochloropsis, Chlorella, Microcystis, and Nitzschia, were consistently found in each location. Furthermore, there was a greater variation in the number of genera found in BL3.
Table 4. Identified phytoplankton in the effluent of vannamei shrimp ponds in the northern coast of Bangka Island


Figure 2. Phytoplankton in shrimp pond effluent: (A) Nanochloropsis, (B) Chlorella, (C) Golenkinia, (D) Oocystis, (E) Pandorina, (F) Radiosphaera, (G) Tetraselmis, (H) Microcystis, (I) Oscillatoria, (J) Spirulina, (K) Synechocystis, (L) Cryptomonas, (M) Chlamydomonas, (N) Hemiselmis, (O) Cyclotella, (P) Chrysochromulina, (Q) Navicula, (R) Nitzschia, (S) Euglena, (T) Trachelomonas, (U) Alexandrium, (V) Gymnodinium, (W) Gyrodinium, and (X) Protoperidinium.
The abundance of Nanochloropsis and Chlorella in BL1, BL2, BL3, and BL4 was significantly higher compared to other genera, as shown in Table 4. The findings showed that the abundance of Nanochloropsis in BL1, BL2, BL3, and BL4 ponds was 50.05, 51.21, 46.17, and 50.34%, respectively. The genus Chlorella showed a value of 21.45, 21.95, 16.13, and 21.57% in each respective location.
Phytoplankton diversity, evenness, and dominance
Phytoplankton diversity, evenness, and dominance described the richness in the structure of phytoplankton communities and the balance of individuals in a water body. The measurement indices were highly dependent on the presence and abundance of identified species. The values of diversity, evenness, and dominance indices in BL1, BL2, BL3, and BL4 ponds are presented in Table 5.
Table 5. Phytoplankton diversity, evenness, and dominance in the effluent of ponds in the northern coast of Bangka Island

The calculation results for the diversity index in BL1, BL2, BL3, and BL4 ponds showed that the values were above 1 and below 3, as shown in Table 4. According to the Shannon–Wiener diversity index criteria, values within the range of 1–3 showed a moderate level. This finding showed that the phytoplankton diversity in all ponds exhibited a moderate level.
The calculation of the evenness index in all ponds produced values above 0.6, as shown in Table 5. According to the Shannon–Wiener evenness index, values above 0.6 showed a high level of evenness in a water body. This signified a high level of uniformity in the distribution of individual phytoplankton species within the ponds.
The dominance index of phytoplankton in all ponds showed values below 0.5, as shown in Table 5. According to the Simpson dominance index criteria, values below 0.5 showed the absence of dominance of individual phytoplankton species over others in a water body. The effluent of BL1, BL2, BL3, and BL4 ponds was inhabited by species that did not dominate over each other.
Quality of pond effluent
The quality of pond effluent was described by nutrient profiles and the physicochemical quality of the water. The obtained values represented the load received by the effluent pond (WTPs) due to the activities conducted in the production pond. Furthermore, the magnitude of the values depended on the characteristics and capacity of the water body to receive or process the load entering the water body. The profiles of nutrient effluent and the physicochemical quality of the water in BL1, BL2, BL3, and BL4 are presented in Figure 3 and Table 6.
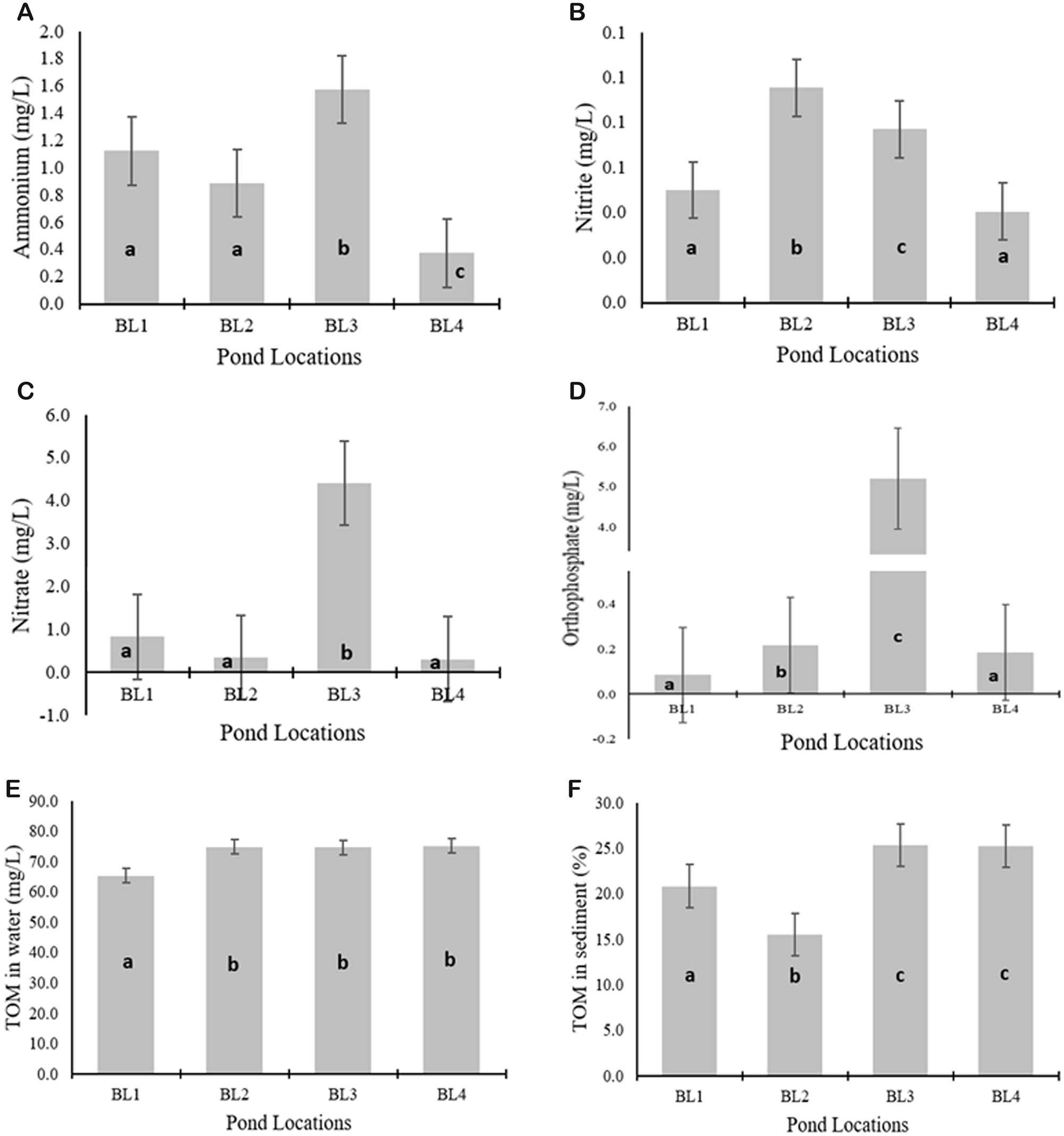
Figure 3. Characteristics of nutrient compounds and organic matter in shrimp pond effluent: (A) ammonium, (B) nitrite, (C) nitrate, (D) orthophosphate, (E) TOM in water, and (F) TOM in sediment. Error bars represent standard deviation; different letters within bars indicate significant differences (Duncan's test, P < 0.05).
Table 6. Characteristics of physical and chemical parameters in the effluent of vannamei shrimp ponds in the northern coast of Bangka Island

Different superscript letters between the columns indicate significant differences in values (Duncan's test, P < 0.05).
Nutrient compounds, such as ammonium, nitrite, nitrate, and phosphate were formed through the decomposition of organic matter. The values of these nutrient compounds varied significantly among all locations. The highest levels of ammonium, nitrate, and orthophosphate were found in the effluent of BL3, with values of 1.575 ± 0.161, 4.410 ± 1.072, and 5.203 ± 0.924 mg l−1, respectively. The concentration of TOM in BL3 and BL4 was not significantly different and higher than that in BL1 and BL2, with values of 74,750 ± 3304 and 75,208 ± 4317 mg l−1, respectively. Furthermore, the sediment TOM concentration was higher at BL3, measuring 25.39 ± 2.79, and was not significantly different from BL4 at 25.213 ± 2.167. This condition was influenced by the organic matter input originating from shrimp production activities in the production ponds.
Water quality parameters in the effluent of BL1, BL2, BL3, and BL4 showed variations among these locations, as shown in Table 6. These conditions were influenced by the organic matter load originating from production ponds, weather conditions, and air environment during measurements, as well as the waste management system in the waste treatment ponds. The salinity range was 18.25 ± 0.50–26.50 ± 2.38 ppt, and higher values were observed in BL3 at 26.50 ± 2.38, which was not significantly different from that in BL1 at 24.25 ± 2.87. The water temperature ranged between 27.60 ± 0.27 and 29.30 ± 0.32°C and did not significantly differ among all locations. The concentration of TSS ranged from 99.20 ± 50.13 to 262 ± 60.15 mg l−1. These TSS values were particularly high (>50 mg l−1), specifically in BL3, which was significantly higher compared to other locations. The fluctuation in surface pH ranged from 6.61 ± 0.56 to 7.14 ± 0.09. The DO level at the surface was relatively high (>4 mg l−1), with the highest DO value observed in BL1 at 6.83 ± 0.33 and the lowest in BL3 at 4.05 ± 1.52. The mineral values of calcium and magnesium in all pond locations did not significantly differ among the locations. The measured bottom ORP values in each location showed significant differences. In BL1 and BL2, ORP values were positive, with respective values of 185.75 ± 9.64 and 98.25 ± 4.50 mg l−1. This was inconsistent with BL3 and BL4 ponds, where the values were negative at −138.50 ± 26.41 and −108.75 ± 14.98 mg l−1, respectively.
The relationship between nutrient quality and environmental factors in shrimp pond effluents with phytoplankton abundance
Phytoplankton abundance was greatly influenced by the environmental conditions of the water. The strength of this relationship was analysed using PCA, and the graph illustrated the relationship between phytoplankton abundance and nutrients with environmental variables. Each pond location had its characteristic relationship among the factors as presented in Figure 4.
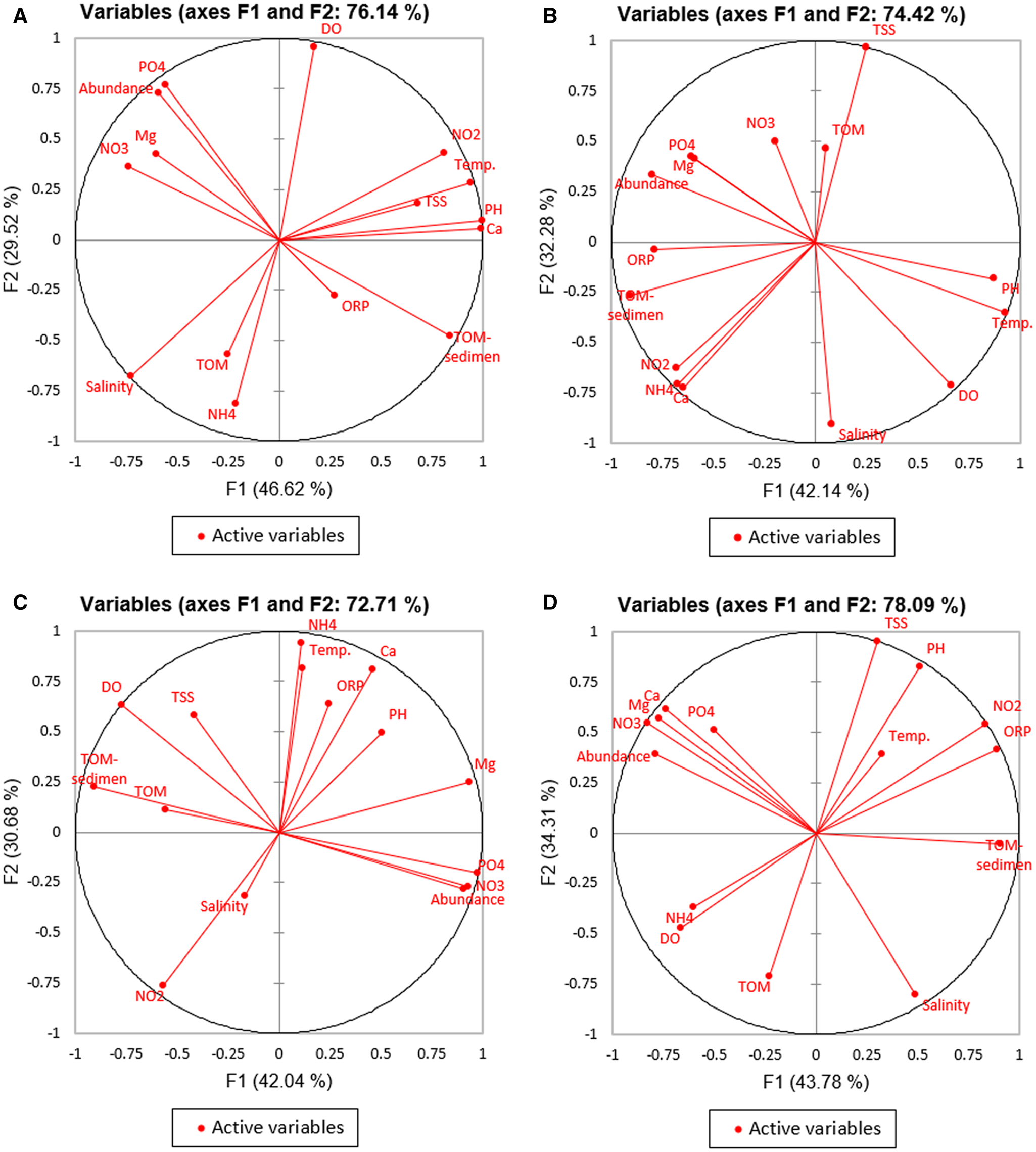
Figure 4. PCA biplot analysis of the relationship between phytoplankton abundance and environmental variables: (A) BL1 location, (B) BL2 location, (C) BL3 location, and (D) BL4 location.
PCA at BL1, BL2, BL3, and BL4 locations explained the data variability on two axes, with respective percentages of 76.14, 72.71, 74.42, and 78.09. The PCA results structured the cumulative variance of nutrient and environmental quality on the first and second axes as follows: 46.62% (F1) and 29.52% (F2) for BL1 location, 42.04% (F1) and 30.68% (F2) for BL2 location, 42.14% (F1) and 32.28% (F2) for BL3 location, and 43.78% (F1) and 34.31% (F2) for BL4 location.
Phytoplankton abundance at BL1, BL2, BL3, and BL4 was strongly influenced by orthophosphate (PO4), nitrate (NO3), and magnesium (Mg). At BL4, in addition to these factors, the abundance was also influenced by calcium. The Pearson correlation coefficients (n) derived from the relationship between phytoplankton abundance and orthophosphate, nitrate, and magnesium at BL1 location were 0.998, 0.513, and 0.897, respectively. At BL2, the correlation coefficients between phytoplankton abundance and orthophosphate, nitrate, and magnesium were 0.907, 0.844, and 0.856, respectively. In BL3 location, the coefficients obtained with orthophosphate, magnesium, and nitrate were 0.964, 0.957, and 0.747, respectively. Furthermore, when examining phytoplankton abundance at BL4 location, the correlation coefficients with orthophosphate, nitrate, magnesium, and calcium were 0.924, 0.925, 0.700, and 0.700, respectively. Positive correlation values exceeding 0.5 showed a robust positive relationship, as evidenced by the close association between these variables.
Discussion
The treatment of waste in the WTPs at BL1, BL2, BL3, and BL4 locations was still relatively simple and partial and had not comprehensively treated the wastewater generated from production ponds. Furthermore, WTPs in all locations were the only ponds with a sedimentation system. According to Syah et al. (Reference Syah, Fahrur, Suwoyo and Makmur2017), WTPs must consist of sedimentation, aeration, and equalization ponds. The ponds located at the study site exclusively used the system to minimize production expenses. Sedimentation ponds were reservoirs designed for the physical treatment of solid waste materials, to diminish the concentration of suspended solids through the process of settling. The underlying principle comprised of creating an obstruction to decelerate the movement of the wastewater, thereby prolonging the duration of travel and facilitating the deposition of solid particles.
The types and abundance of phytoplankton in each water location depended on the nutrient content present in the water. The abundance of phytoplankton in the ponds was influenced by the chemical and physical factors of the observed water quality and nutrient content. The presence and abundance of phytoplankton communities in aquatic environments were affected by the contributions made by individual phytoplankton species in the water body. Phytoplankton was expected to continue growing in response to external stimuli, such as light, temperature, and nutrient concentrations present in the aquatic environment (Palupi et al., Reference Palupi, Fitriadi, Kasprijo and Malfa2023).
The higher the presence of organic matter in the ponds, the higher the abundance of phytoplankton (Huang et al., Reference Huang, Luo, Zeng, Zhang, Peng, Jiang and Jiang2022). The high abundance at BL3 (Table 3) was attributed to the higher content of nutrient compounds in the form of ammonium, nitrate, and orthophosphate compared to other locations (Figure 3A, C, and D). The concentration of TOM in the water at BL3 was high and statistically not significantly different from that at BL2 and BL4 (Figure 3E). The results of this study also showed that the sediment TOM concentration was also higher, as shown in Figure 3F. This condition showed a greater production activity, capacity, and organic matter retention of vannamei shrimp production. Sediment TOM had a significant contribution to the presence of nitrogen and phosphorus in the water column (Rozpondek et al., Reference Rozpondek, Rozpondek and Pachura2017). High nutrient concentrations within a certain range could increase the abundance of phytoplankton (Miao et al., Reference Miao, Wang, Liu, Ma, Hu, Li and Chen2019). Nutrient compounds served as food sources and were the primary factors influencing the growth of these organisms. The effluent from vannamei shrimp production ponds contained high levels of organic matter (TOM), both in the water column and concentrated in the sediment (Jasmin et al., Reference Jasmin, Syukri, Kamarudin and Karim2020). The shrimp pond effluent in the form of nitrogen compounds (ammonium, nitrite, and nitrate) was formed from the decomposition of organic matter, containing proteins (Jimenez-Ojeda et al., Reference Jimenez-Ojeda, Collazos-Lasso and Arias-Castellanos2018).
High nitrogen (ammonium and nitrate) and phosphorus (orthophosphate) content in the wastewater had been reported to stimulate phytoplankton biomass production, with the proportions of these components playing an important role (Beuckels et al., Reference Beuckels, Smolders and Muylaert2015). Ammonium was an ionized form of ammonia that could be directly assimilated by phytoplankton (Klawonn et al., Reference Klawonn, Bonaglia, Whitehouse, Littmann, Tienken, Kuypers, Bruchert and Ploug2019). The source of ammonia in shrimp ponds came from protein metabolism in the body, faeces, unconsumed feed, and dead organisms that settled at the bottom (Setyastuti et al., Reference Setyastuti, Puspitasari, Sukamto and Asmarany2020). Ammonia could undergo oxidation and decomposition processes comprising nitrifying bacteria to form nitrite. Furthermore, nitrite was a transitional form in the conversion of ammonia to nitrate. According to previous studies, nitrate was a nutrient compound or essential element required by phytoplankton for growth (Wijaya and Elfiansyah, Reference Wijaya and Elfiansyah2022). The results showed that orthophosphate was the simplest form of dissolved phosphorus and could be directly absorbed by the organisms (Maslukah et al., Reference Maslukah, Zainuri, Wirasatriya and Widiaratih2020). Phosphorus was an important metabolite present in commercial shrimp feed, along with nitrogen. The presence of phosphorus in ponds was sourced from the decomposition of unconsumed feed and undigested phosphorus from shrimp faeces (Sari et al., Reference Sari, Tuwo, Rani, Saru and Prayitno2022).
The high nitrogen compounds in the wastewater could support phytoplankton biomass production because these compounds were macronutrients required for the synthesis of amino acids, proteins, nucleic acids, and other nitrogen-containing compounds (Hawrot-Paw et al., Reference Hawrot-Paw, Koniuszy, Gałczyńska, Zajac and Szyszlak-Bargłowicz2020). Apart from nitrogen, phosphorus was also a limiting factor for phytoplankton growth. The nutrient was required for cell metabolism, phospholipid, and nucleic acid production, as well as energy transfer (Han et al., Reference Han, Lu, Fan and Zhou2019). When nitrogen and phosphorus supplies were high, phytoplankton accumulated more nitrogen and phosphorus in its biomass, leading to increased cell division and higher biomass productivity (Beuckels et al., Reference Beuckels, Smolders and Muylaert2015).
The higher abundance of Chlorophyta compared to other phytoplankton groups at BL1, BL2, BL3, and BL4 (Table 3) was consistent with studies by Juliyanto et al. (Reference Juliyanto, Maftuch and Masithah2021), Kamilia et al. (Reference Kamilia, Sasmito and Masithah2021), and Palupi et al. (Reference Palupi, Fitriadi, Kasprijo and Malfa2023) who studied their abundance in intensive vannamei shrimp ponds at various locations in Indonesia. The use of commercial feed in aquaculture activities served as an important source of minerals in ponds, specifically phosphorus, which was a key nutrient for promoting the growth of the Chlorophyta group (Cremen et al., Reference Cremen, Martinez-Goss, Corre and Azanza2007). Chlorophyta was the most diverse group of algae with high reproductive capabilities compared to other groups (Adesalu, Reference Adesalu2015). This group could adapt physiologically and behaviourally, allowing them to tolerate environmental changes better than others (Palupi et al., Reference Palupi, Fitriadi, Kasprijo and Malfa2023).
Nanochloropsis and Chlorella were phytoplankton species in the Chlorophyta group that were consistently present and had a higher abundance compared to others at BL1, BL2, BL3, and BL4 (Table 4). These species were cosmopolitan, grew rapidly, and could thrive in a wide range of salinity levels (Rendon-Castrillon et al., Reference Rendon-Castrillon, Ramírez-Carmona, Ocampo-Lopez and Giraldo-Aristizabal2021; Santri et al., Reference Santri, Zulkifli, Lesbani, Hermansyah, Anwar, Ermayanti, Meylani and Fudholi2021). Plankton could also adapt to abnormal conditions in their habitats (Soeprapto et al., Reference Soeprapto, Ariadi and Badrudin2023). Cao et al. (Reference Cao, Wen, Li, Liu, Hu, Zhang and He2014) found that the use of probiotics in vannamei shrimp production ponds was a major factor supporting the growth of Chlorophyta, specifically the genera Nannochloropsis and Chlorella. Furthermore, probiotic supplementation could enhance the growth of Nannochloropsis and Chlorella species from the Chlorophyta group compared to others. Intensive ponds always used bioaugmentation by adding probiotic bacteria during the production process. Probiotic bacteria were regularly used in the production to enhance its efficiency. In addition to enhancing shrimp growth and immunity, the use of probiotics had positive effects on water quality and promoted the growth of beneficial phytoplankton (Amiin et al., Reference Amiin, Lahay, Putriani, Reza, Putri, Sumon, Jamal and Santanumurti2023).
The composition of phytoplankton was due to their adaptation to the aquatic environment. Different environmental conditions and nutrient availability lead to variations in composition, dominant species, density, and biomass (Miao et al., Reference Miao, Wang, Liu, Ma, Hu, Li and Chen2019). The diversity of these organisms was influenced by the biotic and abiotic factors within a pond, which affected the aquaculture ecosystem (Yang et al., Reference Yang, Zhu, Zheng, Lukwambe, Nicholaus, Lu and Zheng2020). Physical and chemical factors of the water, nutrient availability, and the use of different nutrients by individual phytoplankton could affect the diversity index value (Vesensia et al., Reference Vesensia, Arthana and Dewi2021). An environment experiencing pressure or disturbance was typically characterized by a moderate diversity index value (Sulihtia et al., Reference Sulihtia, Hasan, Hetiherawati and Arief2022). The moderate diversity index at BL1, BL2, BL3, and BL4 (Table 5) showed that the dispersion ability of individuals, nutrient use, and engagement in the ecological processes of each phytoplankton species was considered moderate. The stability of the formed phytoplankton community was also considered to be at the same level. Based on these findings, the water quality conditions, despite experiencing pressure or disturbance, were still capable of supporting phytoplankton growth.
The high evenness index at BL1, BL2, BL3, and BL4 (Table 5) showed that the abundance of each species was relatively equal, and there was no dominance of a particular group. The high evenness of phytoplankton in shrimp ponds was attributed to sufficient light intensity and water flow (Palupi et al., Reference Palupi, Fitriadi, Kasprijo and Malfa2023). The characteristics of the WTPs at all study locations showed a shallow depth (50–100 cm) with continuous water flow and current. This allowed the WTPs at these locations to receive sufficient sunlight intensity and adequate water flow. The high value of the evenness index showed that there was no dominance of one phytoplankton individual over others, despite the higher percentage of Nannochloropsis and Chlorella (Table 4). Furthermore, the distribution of individuals tended to be more uniform, and each of them had an equal opportunity to use the available nutrients (nitrate and phosphate) in the water, even when their quantity was limited (Odum, Reference Odum1998).
The results showed that the dominance of phytoplankton was not found at all locations (Table 5). This shows that the wastewater from shrimp ponds was at a moderate level of contamination and did not significantly impact the presence of phytoplankton. The low value of the dominance index suggested that the phytoplankton community structure was still relatively stable, the environment was sufficiently supportive, and the ecological pressure on biota in the habitat was not too high (Sari et al., Reference Sari, Tuwo, Rani, Saru and Prayitno2022). A value approaching zero showed that within the phytoplankton community structure, there were no species with significant dominance over others. All types of phytoplankton had equal abilities and opportunities to use resources in their environment, and the structure of the community remained relatively stable (Sulihtia et al., Reference Sulihtia, Hasan, Hetiherawati and Arief2022). The prominence of a certain species could show pollution or poor water quality, where only specific organisms could adapt to such conditions. Improper use of probiotics in intensive shrimp ponds often led to the prominence of these organisms in waste discharge. However, the appropriate use of probiotics in the correct amount and dosage suppressed their dominance in water bodies (Kawman et al., Reference Kawman, Uppabullung, Kaewtawee and Sangnoi2022).
All environmental factors influenced the abundance of phytoplankton, with some exerting a stronger impact compared to others. These conditions were influenced, in part, by location differences, characteristics, and concentrations of nutrients, as well as the overall environmental quality in each water location (Zhang et al., Reference Zhang, Gao, Li, Jiang, Chen, Yao, Messyasz, Yin, He and Chen2021). Pond waste referred to organic waste generated during aquaculture activities, including uneaten feed from shrimp farming, remained unabsorbed, and was expelled as faeces, as well as excretions during routine maintenance (Paena et al., Reference Paena, Syamsuddin, Rani and Tandipayuk2020). The decomposition of this organic solid waste led to the release of simple nutrient compounds, both macro and micro minerals, which served as essential elements facilitating the growth of microalgae in aquatic environments (Juliyanto et al., Reference Juliyanto, Maftuch and Masithah2021). Complex organic matter, comprising proteins, carbohydrates, fats, and minerals, underwent degradation by microorganisms into simpler inorganic compounds, such as nitrogen, phosphate, carbon, sulphur, calcium, magnesium, and various other minerals (Karyaningsih, Reference Karyaningsih2018). Among these compounds, nitrogen and phosphate were the primary factors with an essential role in stimulating phytoplankton growth (Klochenko et al., Reference Klochenko, Shevchenko, Nezbrytskaya and Bilous2019).
Based on the PCA in Figure 4, BL1, BL2, BL3, and BL4 exhibited similarities in nutrient parameters and environmental factors, which strongly influenced phytoplankton abundance, namely orthophosphate, nitrate, and magnesium. Nitrate and orthophosphate were nutrients (nitrogen and phosphorus) that could be directly absorbed and used by all species (Braga et al., Reference Braga, Berbel, Chiozzini and Andrade2017). Furthermore, these nutrients were major components that played an essential role in the growth and metabolism of phytoplankton (Rustiah et al., Reference Rustiah, Noor and Lukman2019). Each of these organisms required nitrogen and phosphorus. Nitrogen was an essential nutrient for phytoplankton and served as a major component of proteins, nucleic acids, chlorophyll, amino acids, N-containing osmolytes (such as glycine betaine), and chitin (Hasegawa et al., Reference Hasegawa, Rahman, Kato, Maki and Rahman2013). Phosphorus was an important nutritional component that participated in the formation of biomolecules, such as nucleic acids, proteins, and phospholipids. However, the most important role was in energy transfer mediated by adenosine triphosphate (ATP) and other high-energy compounds found in photosynthesis and respiration (Lin et al., Reference Lin, Litaker and Sunda2016). Magnesium was the most essential element required for the optimal growth of phytoplankton, and its efficiency could be maximized through complexation with nitrate. This nutrient was required for growth and development, and played a crucial role in chlorophyll molecules that affected the activity of photosynthesis enzymes (Carvalho et al., Reference Carvalho, Silva, Baptista and Malcata2011). The presence of nitrate and orthophosphate had a strong relationship with phytoplankton abundance in all shrimp ponds, owing to the crucial role of these compounds as the primary nutrient sources for phytoplankton growth. Magnesium also had an important contribution, particularly in relation to phytoplankton photosynthesis. A study by Encarnacao et al. (Reference Encarnacao, Burrows, Pais, Campos and Kremer2012) showed that metal ions, such as iron and magnesium, played an important role in photosynthesis. In particular, magnesium occupied a strategic position as a central element of the chlorophyll molecule, and all microalgal species had an absolute need for this element. Furthermore, it also had a function in the aggregation of ribosomes in functional units and the formation of catalase.
Conclusion
In conclusion, the abundance of phytoplankton in all discharge locations of vannamei shrimp farms in the northern coastal area of Bangka Island showed levels of abundance above 1,000,000 cells ml−1. Furthermore, the highest abundance was recorded at location BL3, reaching 2,418,375 cells ml−1. Several groups of phytoplankton were found in all shrimp ponds, including Chlorophyta, Cyanophyta, Chryptophyta, Bacillariophyta, and Pyrrophyta. The most abundant group was Chlorophyta, with the highest percentage of the genus Nanochloropsis. The values of diversity index, evenness, and dominance at all shrimp ponds show the same levels, with moderate diversity, high evenness, and no dominance. The nutrient characteristics (ammonium, nitrate, and orthophosphate) at BL3 showed the highest concentrations compared to other locations. The same parameters, namely nitrate, orthophosphate, and magnesium, strongly influenced the abundance of phytoplankton at all shrimp pond discharge locations.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Acknowledgements
The authors are grateful to Balai Pembiayaan Pendidikan Tinggi (BPPT), Kemendikbudristek, and Lembaga Pengelola Dana Pendidikan (LPDP). The authors are also grateful to IPB University, Bangka Belitung University, and individuals who participated conditionally or unconditionally in this study.
Author contributions
All authors have contributed equally to data collection, data analysis, writing, reviewing, editing, and finally, approving the final manuscript.
Financial support
This study was funded by Balai Pembiayaan Pendidikan Tinggi (BPPT), Kemendikbudristek (SK No. 1232/J5.2.3./BPI.06/10/2021).
Competing interests
None.












