Stroke is relatively rare in children but has become increasingly recognized clinically. Hemiplegic migraine (HM) is a rare subtype of migraine, with attacks typically beginning in childhood or adolescence. Attacks are characterized by migraine headaches and motor weakness, which develop over several minutes. HM may therefore mimic acute stroke; however, symptoms last less than an hour and resolve spontaneously, often without sequela.Reference Harrison1–Reference Fedak, Zumberge and Heyer4 Distinction between these entities is important due to their different urgency and management. Neuroimaging is indispensible in working up patients presenting to the Emergency Department with stroke-like symptoms and can be used to distinguish between infarction and HM.
A 12-year-old boy presented with acute onset severe, left-sided headache with photophobia. After approximately 2 h of constant pain, the patient’s mother noticed a right facial droop, right facial paralysis, and right arm weakness. Two hours later, physical examination in the Emergency Department identified mild right-sided facial droop, but no other weakness; a severe headache persisted. Two hours afterward, the patient underwent magnetic resonance imaging (MRI) to exclude infarction.
Brain MRI was unremarkable on diffusion-weighted (Figure 1A), T1-weighted, T2-weighted, and post-contrast T1-weighted images. Fluid attenuated inversion recovery (FLAIR) images revealed subtle hyperintensity involving the cortex of the left hemisphere (Figure 1B). Susceptibility-weighted images demonstrated asymmetrically prominent veins throughout the left hemisphere (Figure 1C). Arterial spin-labeling perfusion images revealed hypoperfusion throughout the left hemisphere (Figure 1D). These findings were compatible with HM.
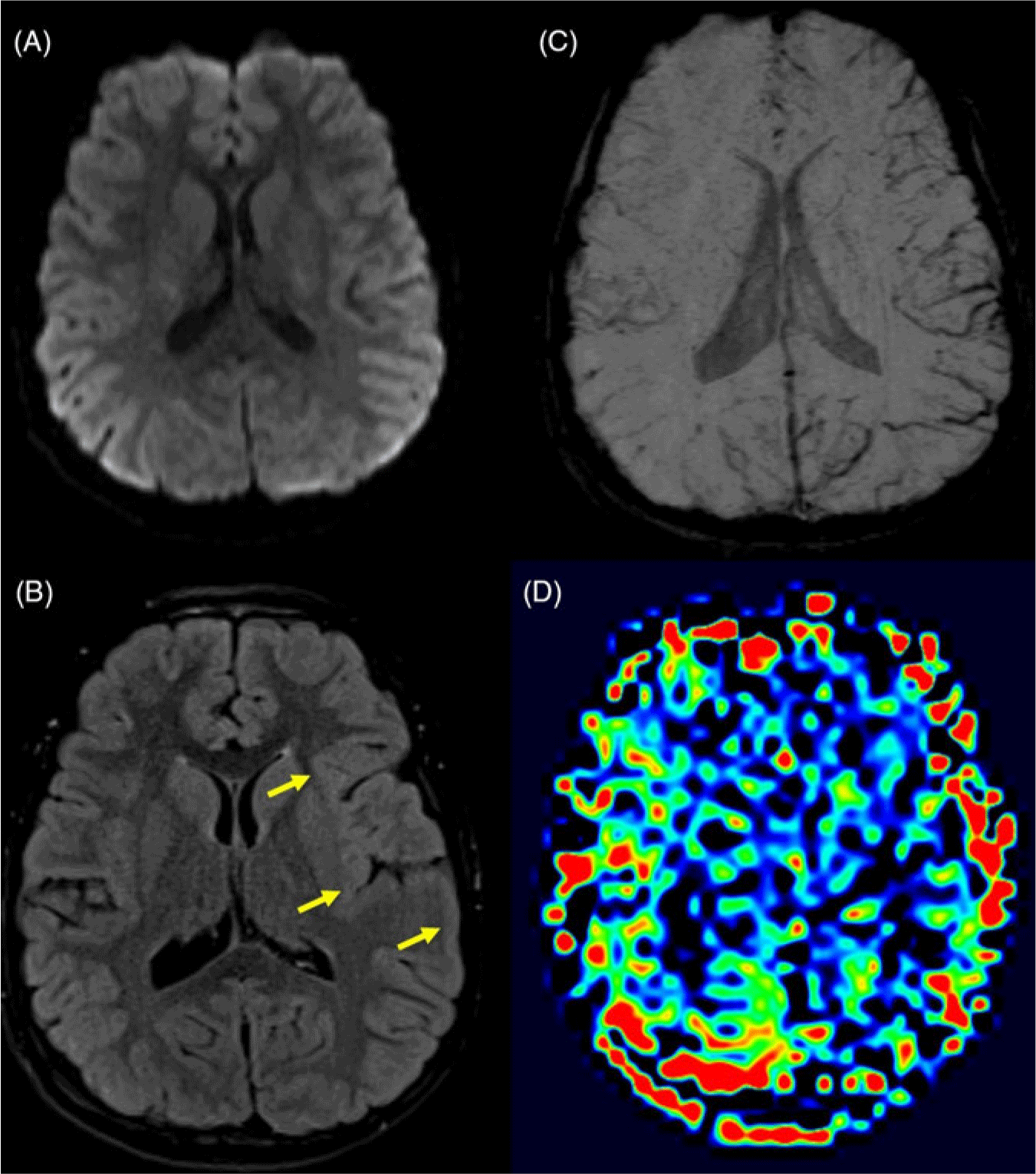
Figure 1: (A) Axial diffusion-weighted images show no areas of diffusion restriction, excluding stroke as a cause for the patients’ symptoms. (B) Axial FLAIR images show subtle but diffuse hyperintensity involving the cortex of the left frontal, temporal, and parietal lobes (yellow arrows) compared to the right side. (C) Axial susceptibility-weighted minimum intensity projection images show increased curvilinear susceptibility effect in the sulci throughout the left hemisphere, in keeping with venous prominence. (D) Axial arterial spin-labeling relative cerebral blood flow images show decreased blood flow to the left hemisphere.
HM has two forms, familial and sporadic, which occur with equal frequency and a prevalence of approximately 1/10,000.Reference Lykke, Kirchmann and Faerch5 With no associated family history, this is a case of sporadic HM. Three known genes are associated with familial HM: CACNA1A, ATP1A2, and SCNA1, with the proline-rich transmembrane protein PRRT2 gene also implicated.Reference Ducros2,Reference Riant, Roze and Barbance6 The pathogenesis of HM remains unclear, with conflicting theories between vascular or neuronal dysfunction suggested. The neurological deficit concomitant with both decreased and increased cerebral blood flow argues against a purely vascular mechanism.
HM may mimic an acute stroke, making clinical distinction difficult. Rapid diagnosis is vital to the urgent management of stroke patients, and neuroimaging, preferably with MRI, is essential in the workup. Brain MRI shortly after symptom onset in patients with HM demonstrates cerebral hypoperfusion,Reference Dreier7,Reference Mourand, de Champfleur and Carra-Dalliere8 with increased venous deoxyhemoglobin identified on susceptibility-weighted imaging by prominent cerebral veins within the cerebral sulci on the affected side.Reference Fedak, Zumberge and Heyer4 Although not fully understood, alterations in the blood–brain barrier within the affected region is believed to result in cortical swelling and vasogenic edema, both of which contribute to FLAIR hyperintensity.Reference Dreier7 As there is no cytotoxic edema, the diffusion-weighted images remain negative, thus excluding infarction. A shift to regional hyperemia and hyperperfusion typically occurs later,Reference Mourand, de Champfleur and Carra-Dalliere8,Reference Boulouis, Shotar and Dangouloff-Ros9 with subsequent near-normalization of cerebral blood flow and decreased prominence of cerebral veins, which correlates with symptom resolution.Reference Fedak, Zumberge and Heyer4 In one recent article, it was shown that patients scanned less than 14 h after aura onset consistently demonstrated decreased cerebral blood flow in symptom-related brain regions, while a constant increase in cerebral blood flow was seen in these regions 17 or more hours post-onset.Reference Boulouis, Shotar and Dangouloff-Ros9
Disclosures
The authors have no conflicts of interest to declare.
Statement of authorship
MTJ, AV, and ANP played equal roles in manuscript conception, composition, and revision.



