INTRODUCTION
The antimicrobial agent methicillin was introduced into clinical practice in 1960 and methicillin-resistant Staphylococcus aureus (MRSA) was described 1 year later [Reference Jevons1]. Worldwide, MRSA is now responsible for considerable mortality, morbidity and health-care expenditure both in hospital and community settings [Reference Cosgrove2, Reference Kluytmans-Vandenbergh and Kluytmans3]. In The Netherlands MRSA has been controlled effectively in hospitals by a search and destroy strategy [Reference Wertheim4]. In 2006, 2% of the S. aureus invasive isolates in The Netherlands were resistant to methicillin, compared to 20–40% in surrounding countries [5].
The Netherlands was the first country to report patients with a specific MRSA variant associated with pigs [Reference Voss6]. This strain was not typable by pulsed-field gel electrophoresis (PFGE) and was therefore referred to as NT-MRSA [Reference Bens, Voss and Klaassen7]. Since then an increasing number of Dutch studies have reported NT-MRSA in people in contact with pigs, i.e. pig farmers and family, co-workers and veterinarians [Reference Huijsdens8–Reference van Duijkeren11]. In a pilot survey, Voss et al. [Reference Voss6] found a 23% prevalence of NT-MRSA in 26 pig farmers. A study of pigs in slaughterhouses revealed that more than one third of them carried NT-MRSA [Reference de Neeling12]. Cattle (veal) farming was also identified as a risk factor for NT-MRSA carriage in humans [Reference Van Loo13, Reference Mooij14].
Since mid-2006, pig farmers and their family members are screened for MRSA at ambulatory care or before hospitalization. As a result thereof, the proportion of NT-MRSA in the national surveillance increased by 20% in the second half of 2006 and by 30% in 2007 [Reference Van Loo13] (unpublished data, RIVM) and even a threefold increase was reported from a hospital in the south of the country [Reference Van Rijen, Van Keulen and Kluytmans15]. Up to now, NT-MRSA has been found in France, Germany, Austria, Denmark, Belgium and Canada [Reference Armand-Lefevre, Ruimy and Andremont16–Reference Khanna21].
Despite published reports, no representative estimate of the prevalence of NT-MRSA on Dutch farms is available. Our objective was to obtain further insight into the prevalence and determinants of human carriage of NT-MRSA in relation to the presence of NT-MRSA in pigs on the farm.
MATERIALS AND METHODS
Selection of farms
A cross-sectional prevalence survey was conducted during the period January–October 2007. Farms were randomly selected from a complete list of pig farms in The Netherlands. On 50 farms we expected to include 140 persons, i.e. 50 farmers and their family (average Dutch family size 2·8), sufficient to estimate the MRSA point-prevalence, assuming 23% positivity [Reference Voss6], accepting a 5% risk of type I error with a precision of 10% and a design effect of 2 [anticipating clustering of cases; calculation with Epi-Info 6.04 (CDC, Atlanta, GA, USA)]. The study protocol was approved by the medical ethical committee of the Therapeutic Drug Evaluation Foundation (STEG, Almere, The Netherlands).
Sampling and questionnaires at pig farms
Procedures for human subjects
Written informed consent to participate in the research was obtained; for children aged <18 years parental consent was requested. A short questionnaire and nasal swabs (from both anterior nares) were taken by the research assistant visiting the farm. The questionnaire addressed individual factors such as age, sex, intensity of contact with pigs or other animals, potential other risk factors for MRSA carriage and self-reported medical history related to MRSA infection or (skin) problems.
Research assistants took their own nasal swabs just before they visited the farm, immediately after and the morning following the visit.
Procedures for animal data
Data on farm-related factors included size and type of farm, age groups of pigs present (sows, suckling piglets, weaned piglets, gilts and finishing pigs), measures of hygiene implemented, feeding method and housing characteristics. Pigs were sampled by nasal swabs and 60 samples were collected per farm, representative for the age group(s) of pigs present. These swabs were pooled into 10 pools of six swabs. Additionally, at each farm five environmental samples of dust were collected by wiping the top of the pen separations in different compartments of the pighouses. Visits were separated by at least 1 day and limited to two farms per week.
Laboratory analysis
Samples were cultured at the hospital laboratory in Breda (human samples) and the RIVM laboratory (pig and dust samples). Samples were first enriched in Mueller–Hinton broth with 6·5% NaCl, followed by selective enrichment in Phenol Red mannitol broth with 75 mg/l aztreonam and 4 mg/l oxacillin or ceftizoxime. After culturing on sheep blood agar and MRSA screen agar (Oxoid, Basingstoke, Hants, UK), suspected colonies were confirmed by polymerase chain reaction (PCR) for the S. aureus-specific DNA fragment [Reference Martineau22], the mecA gene [Reference de Neeling23] and the Panton Valentine leukocidin toxin genes [Reference Lina24].
For the human isolates, methicillin resistance was screened by a disk diffusion test using a cefoxitin disk and confirmed by the presence of the mecA gene by PCR. All MRSA strains were typed by spa typing [Reference Harmsen25] and strains in human samples not yet identified as NT-MRSA were checked for typability by PFGE [Reference Murchan26]. Furthermore, in human samples, susceptibility was determined for 21 antimicrobial agents with the VITEK system (bioMérieux SA, Craponne, France) according to the manufacturer's instructions.
Data analysis
Data were entered into Access, double-checked and verified with questionnaires and analysed with SPSS version 15.0 (SPSS Inc., Chicago, IL, USA) and SAS version 9.1 (SAS Institute, Cary, NC, USA). A dataset was created with individual human results and aggregated animal and farm data. Summary variables were created, such as a score for ‘personal hygiene in the stable’ based on the following hygiene measures: separate entrance and exit, changing room, showers, water, soap, boots, overalls and disinfection footbath.
The relationship between MRSA presence in human and animal and/or dust samples was investigated by χ2 test. Risk factors for MRSA carriage were first identified by univariate logistic regression analysis. For further analyses we considered exposure to MRSA-positive pigs or dust a prerequisite or ‘necessary cause’ for MRSA positivity in humans and therefore further analyses were limited to data from farms where pigs or dust tested positive. Multivariate regression analysis by stepwise, forward entry included factors influencing MRSA positivity in humans (P<0·2 in univariate analysis) and logical interaction factors thereof. A random cluster effect was included in the model to adjust for the fact that observations of humans on the same farm might not be independent.
Spa-types of human and animal MRSA samples were compared for each farm, including the results of the samples taken from the sample collectors pre- and post-farm visit. Antibiotic resistance patterns of human MRSA strains were compared for each spa-type by χ2 analysis.
RESULTS
Altogether 106 farms were contacted until 50 farmers (47%) agreed to participate in the research. The response rate was similar in different regions and types of pig farms. The most common reasons for non-participation were no interest or time, or retirement from farming. A total of 232 people were sampled on 50 farms: 50 farmers, 171 family members and 11 co-workers. The individual participation on the selected farms was high: we collected swabs and questionnaires from 221/231 reported household members (96%).
MRSA status per farm
On 28/50 (56%) of the farms pig or dust samples tested positive (hereafter referred to as ‘MRSA-positive farms’) and on 15/50 (30%) of the farms one or more MRSA-positive persons were found. MRSA in humans was only found on MRSA-positive farms (Fig. 1). On the 15 farms with MRSA-positive people, pigs were also positive with the exception of one farm; on this farm, however, dust samples were positive. The prevalence of MRSA in farm residents was the highest on farms with both sows and finisher pigs. Finishing farms were more often MRSA positive than farms with sows only, but only part of the positive finishing farms (38%) housed MRSA-positive people while on all MRSA-positive farms where sows were present, MRSA was also found in farm residents (see Fig. 2).
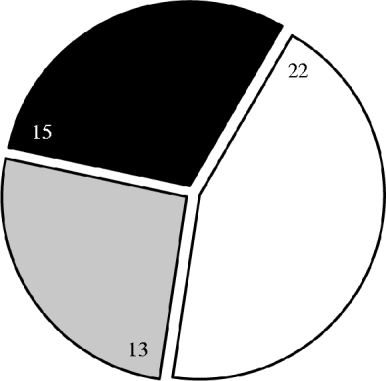
Fig. 1. Prevalence of methicillin-resistant Staphylococcus aureus (MRSA) per farm. Number of farms (total 50) indicated in segments. Farms with one or more MRSA-positive persons and MRSA in pigs or dust (black area); farms with MRSA in pigs or dust only (grey); farms completely MRSA negative (white).
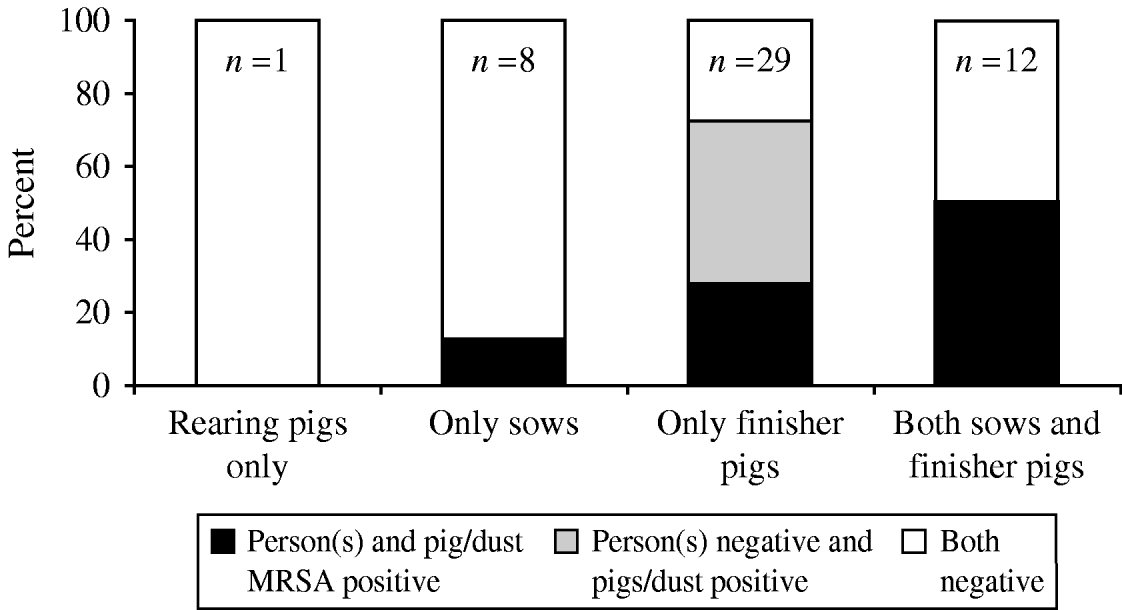
Fig. 2. Proportion of farms with methicillin-resistant Staphylococcus aureus (MRSA)-positive people, pigs and dust compared to farms with or without sows and finisher pigs. Number of farms (total 50) indicated in bars.
MRSA status per person – univariate analysis
MRSA was identified in 33/232 people (14%). Table 1 shows the relationship between MRSA positivity and the main potential risk factors (univariate analysis). At the individual level, living on a MRSA-positive farm was the most important risk factor. The higher the proportion of positive samples from pigs or dust per farm, the higher the positivity rate in people. The intensity of contact with pigs was another important determinant. In persons working with pigs on a regular basis, 29% (95% CI 20–38) carried MRSA whereas 12% (95% CI 3–29) of persons who did not work with pigs but entered the pighouse(s) at least once per week were positive. Only 2% (95% CI 0–6) of those who reported no contact with pigs were positive. On MRSA-positive farms, 49% of the people working with pigs harboured MRSA (95% CI 36–62).
Table 1. Human MRSA carriage of persons living on pig farms in relation to individual and farm-related characteristics on 50 pig farms in The Netherlands (left) and the on subgroup of 28 MRSA-positive farms (right), January–October 2007; univariate logistic regression analysis
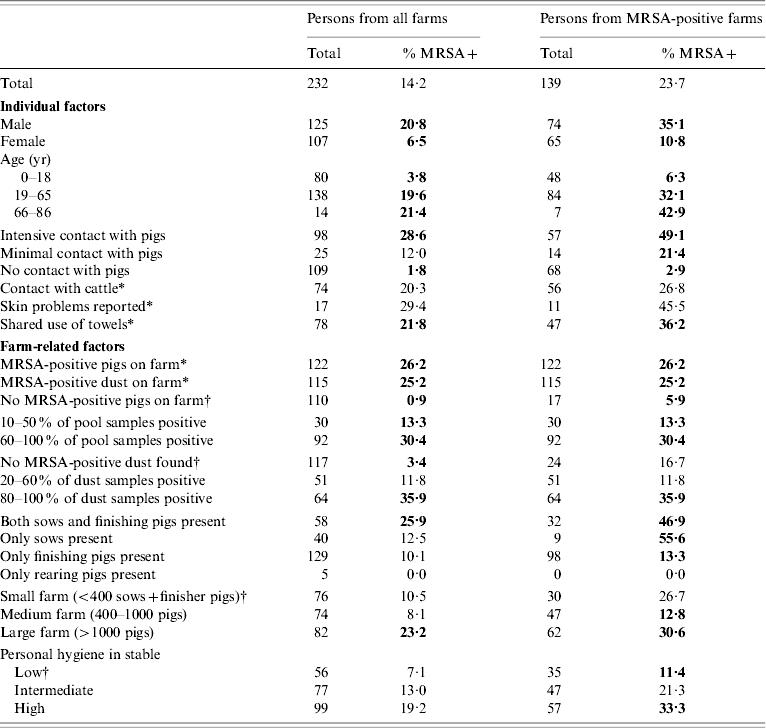
Percentages in bold are considered significant at P<0·05 level.
* MRSA rate where factor present (shown) compared to factor absent (not shown).
† Divided into equal groups for optimal comparison.
At the farm level, the presence of sows related to a higher rate of MRSA in people. Several other factors were associated with MRSA positivity in humans, i.e. age, gender, sharing of towels, type of farm and number of pigs on farm. The presence of finisher pigs, number of new pig-batches recently received, and cleaning and disinfection measures in the pighouse did not influence MRSA rates.
No indication for other potential causes of MRSA was found. Four persons (on two farms) who were diagnosed with MRSA previously, proved negative at this sampling. Twelve family members worked in a hospital or nursing home but none of them carried MRSA. We did not find a relationship with recent hospitalization, team sports or presence of horses, cattle or other animals on the farm. MRSA appeared to be more frequent in people with skin problems (P=0·06), but these were only reported by a small number (n=17).
MRSA per person on positive farms – multivariate analysis
On the 28 positive farms 139 people were sampled and the incidence of MRSA was 24%. Multivariate regression analysis of the results from the positive farms determined two significant risk factors for MRSA carriage out of 12 factors identified as potential risk factors in the univariate analysis. These were ‘intensity of contact with pigs on the farm’ and ‘presence of sows and finishing pigs’ (Table 2). The random cluster effect of ‘farm’ was not significant.
Table 2. Risk factors for human MRSA identified by unilevel multivariate analysis, based on persons sampled at the 28 farms where pigs or dust samples were MRSA positive (n=139)
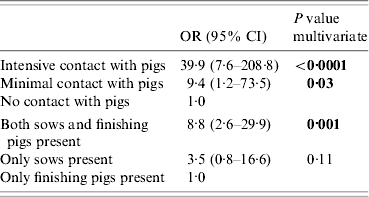
OR, Odds ratio, CI, Confidence interval.
P values in bold are considered significant at P<0·05 level.
Genotyping MRSA
All MRSA isolates from human samples were Panton–Valentine leukocidin (PVL) negative. Seven different but related spa-types were found, most commonly t011 (45%), t567 (21%) and t108 (15%) which are all known NT-MRSA strains. Furthermore, two cases of t899 (known NT-MRSA) were found and also two t2330, one t2741 and one t588. The latter three spa-types were consequently submitted for PFGE analyses and also identified as NT-MRSA. In the pig and dust samples, t011 and t108 spa-types were most prevalent, as well as t567, t899 and t2330. Two spa-types, t588 and t2741, were found in people but not in pigs. Some spa-types found in pigs were not present in people.
On all 15 farms with MRSA-positive people, the spa-types in human samples matched with the types found in pigs on the same farm (30/33 persons). These were of spa-types t011 (eight farms), t108 (three farms), t567 (two farms), t2330 (one farm) and t899 (one farm). On 13/15 farms the type found in the pigs was the only spa-type found in people. However, on two farms persons were carrying another spa-type than found in the pigs. On one farm two co-workers harboured t108 and t2741 while the farmer and pigs carried t011; this farm also cared for other animals (sheep and horses). On the other farm, a household member yielded t588 while two other household members and the pigs had t108; on this farm a child had been previously diagnosed with MRSA.
Antimicrobial resistance patterns
All human MRSA isolates were resistant to tetracycline and all isolates were fully susceptible to vancomycin, teicoplanin, nitrofurantoin, fusidic acid and rifampin. Other antimicrobials showed variable resistance (Table 3).
Table 3. Susceptibility to antibiotics of human MRSA isolates (farm residents and collectors), by spa-type
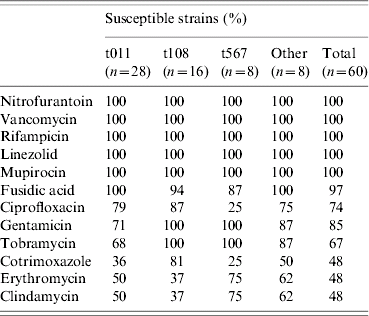
The levels of resistance to ciprofloxacin, fosfomycin, tobramycin and cotrimoxazole were dependent on spa-types (χ2, P<0·05). Of the predominant spa-types, t011 showed higher levels of resistance to tobramycin and cotrimoxazole than the other spa-types, while t567 was more frequently resistant to fosfomycin and cotrimoxazole and less sensitive to ciprofloxacin.
Effect of short-term exposure of human and animal sample collectors
The research assistant who collected the human samples on 50 farms remained negative for MRSA throughout the research period, whereas 13/32 veterinary assistants collecting samples from pigs and stable dust had MRSA-positive samples on one or more occasions. On 13 farm visits (10 assistants), the collector was MRSA positive directly after sampling but negative by the next day. Two collectors were still positive the day after the visit; a sample from one of them 1 month later was negative, no repeat sample was available from the other. The spa-types identified from 11/12 collectors corresponded with the types found in the pighouses. One collector was already carrying MRSA before his first farm visit: he visited three farms and remained MRSA positive with spa-types not corresponding to the MRSA types on these farms.
DISCUSSION
This study shows that 30% of farms have MRSA-positive farm residents and 56% have MRSA present in pighouses. In all farm residents the incidence of MRSA was 14% and this doubled (29%) in persons working with pigs. The farms investigated were a representative sample of The Netherlands by region and type of farm, although the response rate (47%) may have caused a bias for farms cooperating because of previous knowledge on MRSA (and an interest in participating) or no knowledge (and no fear of participating). Nasal sampling is known to identify the majority of MRSA carriers [Reference Mertz27]. Visits were spaced and samples carefully handled to avoid cross-contamination from one farm to another.
The transmission route of MRSA is probably from pigs to people. People were found to be positive only on farms with MRSA in pigs or dust, increasingly so with higher positivity rates in pigs. Spa-typing showed that 91% of people were colonized with similar strain(s) as the pigs on their farm. All MRSA strains were of NT-MRSA type and closely related. Most strains were of spa-types identified previously as NT-MRSA and of the animal-related MLST type ST398 [Reference van Duijkeren11, Reference van Duijkeren28] and additional PFGE typing confirmed that all other spa-types were NT-MRSA. Two spa-types, t588 and t2741, were recovered from people but not pigs; more spa-types were found in pigs than in human samples.
Intensive and repeated exposure to positive pigs appears to be an important factor in MRSA colonization. MRSA positivity was common in persons working with pigs and also persons with less intensive but regular contact (weekly) with the animals. The MRSA transmission from pigs to humans was higher on farms with sows than on finisher farms, while MRSA in pigs circulated more frequently on finisher farms. Management of (breeding) sows (with regular deliveries, care of piglets) requires closer contact with pigs, especially with piglets, as well as longer working hours in the pighouse than management of finisher pigs and this may lead to a higher rate of MRSA transmission from pigs to humans. The high prevalence of NT-MRSA in dust samples from pighouses implies that MRSA could be spread by inanimate material as well, as has been postulated for hospital ICUs [Reference Dansby29, Reference Wilson, Huang and McLean30].
The persistence of MRSA carriage is not determined in our point-prevalence survey. Assuming a continuous exposure, farm residents can be expected to remain MRSA positive, however, distinguishing persistent carriers would need repeated sampling [Reference Kluytmans, van Belkum and Verbrugh31, Reference Nouwen32]. The results from repeated samples of our research teams imply that the risk of acquiring MRSA during short farm visits is limited to transient carriage, even when exposure to pigs is intense. It would be interesting to follow NT-MRSA carriage of pig farmers when they are no longer in contact with pigs (i.e. when on holiday or retiring).
The low MRSA rate (2%) in persons with no contact with pigs suggests a low level of human-to-human transmission of NT-MRSA. Hospital screening activities after detection of a MRSA carrier showed that animal-related ST398 MRSA led to fewer secondary cases (three from 24 index patients) than other MRSA genotypes (62 cases from 56 index patients [Reference Nouwen32]).
Impact of NT-MRSA in Dutch health-care system
The 14% MRSA carriage of pig-farm residents is much higher than the 0·12% prevalence in the open population and at primary care (studied in 2005–2006 [Reference Degener, Groningen and de Neeling33]) and 0·03% at routine hospital admission (data from 1999–2000 [Reference Wertheim4]). Countrywide, there are about 9700 pig farms (National database CBS, 2005). Extrapolation of our results indicates that nearly 6000 MRSA carriers (range 4000–9500) may be expected in pig-farm residents. This justifies the hospital strategy to screen people having contact with pigs in Dutch hospitals since 2006 and agrees with a high proportion of NT-MRSA cases in the national MRSA surveillance database. Moreover, a recent report from hospitals showed that 30% of index patients carried animal-related MRSA [Reference Wassenberg34].
The increase in MRSA-positive patients, identified by hospital screening, attributable to farm-animal MRSA will cause increased costs due to care in isolation, longer stay in hospital, specific diagnostics and medication [Reference Mooij14]. However, the clinical relevance for the people concerned still needs further investigation. Transmissibility from human to human appears to be low and few symptomatic cases of NT-MRSA infections have been found [Reference van Belkum35].
Use of antimicrobials in pig farming and consequences for MRSA in humans
Antimicrobial selection pressure in general is one of the probable factors that have facilitated the emergence and spread of veterinary MRSA [Reference van Duijkeren28]. Antimicrobial consumption in pig farming in The Netherlands is substantial compared to other livestock farming [Reference Mevius and van Pelt36]. Tetracyclines and trimethoprim–sulfonamide (trimsulfa) are most widely used; we found levels of resistance to these antibiotics of 100% and 52%, respectively. In other recent Dutch studies resistance to trimethoprim–sulfamethoxazole was not or hardly ever found in MRSA in pigs and humans [Reference van Duijkeren11, Reference de Neeling12], hence this resistance might be currently emerging. The pattern of resistance of the MRSA samples in our study was otherwise comparable to that in other recent studies [Reference van Duijkeren11–Reference Van Loo13].
Implications for health care and future research
Since 2007, adjusted guidelines for hospitals in The Netherlands require screening for MRSA and care in isolation for all people professionally in contact with live pigs or veal calves. Our results, however, have shown that the frequency and intensity of contact with pigs, whether professional or not, are a determinant for MRSA risk and hence the terminology ‘contact’ should be refined further. The persistency of NT-MRSA carriage in humans after different types of exposure should also be studied further.
NT-MRSA appears to be frequently transmitted from pigs to people, but less so from person to person (the present study [Reference Van Rijen, Van Keulen and Kluytmans15, Reference Degener, Groningen and de Neeling33]). The need for strict management of patients (isolated care) might be reviewed if human-to-human transmission of NT-MRSA is indeed as limited as shown.
Although the antimicrobial resistance pattern found here has no consequences yet for current treatment options of MRSA, further spread of NT-MRSA and selection of resistant strains by the high use of antimicrobials in pig farming, may impede the usefulness of antimicrobials in the future or necessitate differential treatment of MRSA and NT-MRSA (and thus T/NT-typing before treatment). The use of antimicrobials in pig farming should be studied in relation to MRSA prevalence and possible alternative treatment strategies investigated.
We found no association between personal hygiene and MRSA carriage, possibly because personal hygiene at the level that we investigated was not an important factor in pig-to-human MRSA transmission, and transmission of MRSA between humans did not seem to play an important role. Other factors, such as intensity of contact with animals and actual use of cleaning and protection methods may be more important. Protection methods might need to be adjusted to the type of pigs and activities in the stables. The role of hygienic measures in transmission reduction also requires further study.
NT-MRSA will be no doubt studied more extensively in other countries in Europe in the future. The pig-farming sector involves a wide European network of farms and the accompanying meat industry. Pig farms in other countries are probably facing a similar problem as the Dutch farms, although this has as yet not been reported as extensively. Action is needed at a European level to assess the situation and design appropriate measures to prevent further spread of NT-MRSA.
ACKNOWLEDGEMENTS
We thank T. Bosch, C. Deisz, P. Hengeveld, J. Hordijk and P. Willemse, for their contributions to the laboratory analyses and data entry. The veterinary assistants of the Animal Health Service and the Food and Consumer Product Safety Authority are acknowledged for the collection of samples in the pighouses. Furthermore, we are grateful to Dr L. Graat, Dr H. de Neeling and Dr M. van der Sande for useful comments on the research protocol and earlier versions of the paper. This research received funding from VWS, the Dutch Ministry of Health, Welfare and Sport and LNV, the Ministry of Agriculture, Nature and Food Quality.
DECLARATION OF INTEREST
None.







