Introduction
The inherent economic, aesthetic and food values of ungulates and their ability to influence forest structure and composition, and dispersal of seeds, nutrient cycling, soil structure and succession (McNaughton, Reference McNaughton, Sinclair and Norton-Griffiths1979; Crawley, Reference Crawley1983), make them an important component of any ecosystem in which they occur. For example, in Asia, ungulates such as the gaur Bos gaurus, sambar Cervus unicolor, chital Axis axis and wild pig Sus scrofa are known to provide 60–70% of biomass consumed by tigers Panthera tigris, leopards Panthera pardus and dholes Cuon alpinus (Johnsingh, Reference Johnsingh1992; Venkataraman et al., Reference Venkataraman, Arumugam and Sukumar1995).
Because ungulates form a major part of carnivore diets (Schaller, Reference Schaller1967; Seidensticker, Reference Seidensticker1976; Johnsingh, Reference Johnsingh1983; Karanth & Sunquist, Reference Karanth and Sunquist1995; Karanth & Nichols, Reference Karanth and Nichols1998; Biswas & Sankar, Reference Biswas and Sankar2002; Bagchi et al., Reference Bagchi, Goyal and Sankar2003; Jathanna et al., Reference Jathanna, Karanth and Johnsingh2003) their conservation is essential for sustaining populations of predators. Declines in tiger populations have been attributed to low prey populations (Karanth & Stith, Reference Karanth, Stith, Seidensticker, Christie and Jackson1999), and Schaller (Reference Schaller1967), Sunquist (Reference Sunquist1981), Seidensticker & McDougal (Reference Seidensticker, McDougal, Dunstone and Gorman1993) and Karanth (Reference Karanth1995) established correlations between tiger and prey densities. Karanth & Sunquist (Reference Karanth and Sunquist1995), Miquelle et al. (Reference Miquelle, Smirnov, Quigley, Hornocker, Nikalaev and Matyushkin1996), Karanth & Nichols (Reference Karanth and Nichols1998), Carbone & Gittleman (Reference Carbone and Gittleman2002) and Karanth et al. (Reference Karanth, Nichols, Kumar, Link and Hines2004) also proposed that abundance of carnivores is largely dependent on densities of a variety of sizes of ungulate prey and biomass. Tiger populations can thrive, even with low levels of poaching, if their prey base is protected and maintained at an adequate density (Karanth & Stith, Reference Karanth, Stith, Seidensticker, Christie and Jackson1999).
If declining prey populations are a threat to the survival of predators then conservation strategies need to focus on protection of key prey populations. Although data on densities, biomass and distribution are thus important for monitoring prey populations (Karanth, Reference Karanth1995), their relatively low densities, unique habitat requirements, crop raiding habits, consumption by local people and lack of scientific studies hinders their conservation (Karanth & Sunquist, Reference Karanth and Sunquist1992).
Several studies in the sub-tropical forests of Asia have estimated population parameters for ungulates in India (Schaller, Reference Schaller1967; Berwick, Reference Berwick1974; Johnsingh, Reference Johnsingh1983; Karanth & Sunquist, Reference Karanth and Sunquist1992, Reference Karanth1995; Khan et al., Reference Khan, Chellam, Rodgers and Johnsingh1996; Biswas & Sankar, Reference Biswas and Sankar2002; Bagchi et al., Reference Bagchi, Goyal and Sankar2003; Jathanna et al., Reference Jathanna, Karanth and Johnsingh2003), Sri Lanka (Eisenberg & Lockhart, Reference Eisenberg and Lockhart1972) and Nepal (Seidensticker, Reference Seidensticker1976; Dinerstein, Reference Dinerstein1979; Tamang, Reference Tamang1982). However, these studies relied on non-standard methods and failed to address important questions relating to probabilities of detection and representative sampling (Karanth & Sunquist, Reference Karanth and Sunquist1992; Buckland et al., Reference Buckland, Anderson, Burnham and Laake1993; Karanth & Stith, Reference Karanth, Stith, Seidensticker, Christie and Jackson1999). After Karanth (Reference Karanth1987) emphasized the importance of using statistically and biologically valid methods, only a few studies, in tropical India (Karanth & Sunquist, Reference Karanth and Sunquist1992; Varman & Sukumar, Reference Varman and Sukumar1995; Khan et al., Reference Khan, Chellam, Rodgers and Johnsingh1996; Karanth & Nichols, Reference Karanth and Nichols1998; Biswas & Sankar, Reference Biswas and Sankar2002; Jathanna et al., Reference Jathanna, Karanth and Johnsingh2003), have generated estimates of herbivore densities based on distance sampling. Although these studies have advanced our understanding of ungulate ecology in tropical ecosystems in Asia, similar studies are required in the temperate forest ecosystems of Asia, especially in human-dominated landscapes.
Protected populations of wild ungulates sharing resources with livestock and herders in Bhutan’s temperate forests provide an opportunity to evaluate ungulate population dynamics and their interaction with livestock and other anthropogenic factors. Such data, if obtained using established, robust methodology such as distance sampling (Eberhardt, Reference Eberhardt1978; Burnham et al., Reference Burnham, Anderson and Laake1980; Buckland et al., Reference Buckland, Anderson, Burnham and Laake1993, Reference Buckland, Anderson, Burnham and Laake2001), can help formulate management strategies to protect predators and prey and reduce wildlife conflict with farmers. The study described here was designed to estimate population densities and biomass of the major prey species of tiger, leopard and dhole, and recommend measures for reducing crop damage whilst simultaneously protecting ungulate populations.
Study area
This study was conducted in five warden jurisdictions (Phobji, Athang, Langthel, Korphu and Trong) of the 1,750 km2 Jigme Singye Wangchuck National Park in central Bhutan (Fig. 1). The Park is bordered to the east and west, respectively, by the rivers Mangde chu and Sunkosh chu. The Park’s diverse physical features, temperature and rainfall have created climatic zones ranging from wet sub-tropical in the south to permanent alpine pastures and glaciers in the north (Wang, Reference Wang2001). Elevation rises from 150 m in Tingtibi, in the south, to > 4,900 m at the peak of Mt Jo-Durshing la. This steep altitudinal gradient makes c. 20% of the Park inaccessible for surveys, especially during summer and winter.

Fig. 1 (a) The locations of Jigme Singye Wangchuck National Park and the other protected areas of Bhutan and the biological corridors connecting them, and (b) the locations of the survey transects in the Park.
The Park has large areas of mature fir Abies spp. and pine Pinus spp. forests, ranging from sub-alpine to temperate, and large areas of climax broadleaf forest, ranging from temperate to subtropical. Along with Manas National Park in India and the Royal Manas National Park in Bhutan, this area is one of the largest tiger conservation areas in South Asia, includes the full range of vegetation types from the plains to the alpine zone, and has a high floral diversity. These diverse habitats host important populations of predators (tiger, leopard and dhole) and their prey (gaur, sambar, muntjac Munticus muntjac, wild pig, serow Capricornis sumatraensis, langur Trachypithecus geei and macaque Macaca mulatta). The Park is believed to be an important link between the northern and southern tiger populations of Bhutan, supporting up to 10% of Bhutan’s total tiger population (Wang, Reference Wang2001).
Almost 6,000 farmers live in 34 villages across the Park. Most of the farmers are either settled in the river valleys, on the gentle shoulders of the hills or in the glacier valleys. Agriculture (Wang et al., Reference Wang, Curtis and Lassoie2006) and livestock rearing (Wang & Macdonald, Reference Wang and Macdonald2006) are the major sources of livelihood. Farmers residing at lower altitudes keep cattle, whereas farmers in alpine regions prefer yak.
Methods
Line transect methods (Eberhardt, Reference Eberhardt1978; Burnham et al., Reference Burnham, Anderson and Laake1980; Buckland et al., Reference Buckland, Anderson, Burnham and Laake1993, Reference Buckland, Anderson, Burnham and Laake2001) were used to sight wild ungulates. Transects provide credible results (Anderson et al., Reference Anderson, Laake, Crain and Burnham1979) and have been successfully used to estimate animal densities in south Asia (Karanth & Sunquist, Reference Karanth and Sunquist1992, Reference Karanth and Sunquist1995; Varman & Sukumar, Reference Varman and Sukumar1995; Khan et al., Reference Khan, Chellam, Rodgers and Johnsingh1996; Biswas & Sankar, Reference Biswas and Sankar2002; Bagchi et al., Reference Bagchi, Goyal and Sankar2003; Jathanna et al., Reference Jathanna, Karanth and Johnsingh2003). Thirty-two line transects (mean length 5.2 km) were chosen to represent the five warden jurisdictions and different habitat types, elevations and proximity to waterholes and human settlements. The location and length of the transects were limited by accessibility on foot and by season. Field crews of 3–4 (usually but not always including SWW) walked the 32 transects 4–5 times each from September 2005 to July 2006, covering a total length of 849 km. Transects were mostly walked early in the morning (06.00–10.00) or in the evening (15.00–18.00) and animals were sighted on either sides of the transect line. All observers were appropriately trained in mammal observation and identification and distance sampling. For each transect total length walked, the number of animal clusters detected, cluster size, sighting distance (measured with a range finder) and sighting angle (measured with compass) were recorded for every prey species encountered. For species occurring in clusters (e.g. wild pigs and primates) the distances and angles were recorded to the centre of the cluster. Cattle populations occur in large clusters confined to certain areas and hence population estimation by the line transect method was inappropriate. Instead, cattle population data were obtained from village livestock officers.
Using software Distance v. 5.0 (Thomas et al., Reference Thomas, Laake, Strindberg, Marques, Buckland and Borchers2006) analyses were carried out for each species in each of the warden jurisdictions. Data were checked for errors before importing into Distance (Jathanna et al., Reference Jathanna, Karanth and Johnsingh2003). Prior to generating final results using Distance, exploratory analyses were carried out (Buckland et al., Reference Buckland, Anderson, Burnham and Laake2001) to check for any evidence of evasive movement before detection (‘rounding’ and ‘heaping’ of data) and to truncate outlier observations, if necessary, to improve model fitting (Jathanna et al., Reference Jathanna, Karanth and Johnsingh2003). The fit of potential models to each data set was judged using Akaike’s information criteria (AIC; Buckland et al., Reference Buckland, Anderson, Burnham and Laake2001). AIC was computed as AIC = -2ln(k) + 2q; where ln(k) is the log likelihood function evaluated as the maximum likelihood estimates of the model parameters and q is the number of parameters in the model (Buckland et al., Reference Buckland, Anderson, Burnham and Laake1993, Reference Buckland, Anderson, Burnham and Laake2001; Burnham & Anderson, Reference Burnham and Anderson1998). The selected model was used in Distance to estimate the following model parameters: encounter rate (n/L; n = number of detections, L = transect length), effective strip width, average probability of detection, cluster density, cluster size and density of individuals (Burnham et al., Reference Burnham, Anderson and Laake1980; Buckland et al., Reference Buckland, Anderson, Burnham and Laake1993). I used a global detection function with transect-wise encounter rates to obtain transect-wise density estimates, and used these estimates in an analysis of variance to test for differences in density estimates of prey species across the five areas. Density estimates and average body sizes of prey species obtained from the Field Guide to the Mammals of Bhutan (Royal Government of Bhutan, 2004) were then used to estimate the biomass of prey species in the study area.
Results
Along the 849 km of transects a total of 285 sightings were made of 13 species: muntjac (n = 102), wild pig (n = 54), sambar (n = 48), langurs (n = 24), macaque (n = 15), serow (n = 6), goral Nemorhaedus goral (n = 4), musk deer Moschus chrysogaster (n = 1), Himalayan black bear Slenarctos thibetanus (n = 2), Kalij pheasant Lophura leucomelanos melanota (n = 12), hornbill Aceros nepalensis (n = 9), white bellied heron Andrea insignis (n = 7) and monal pheasant Lophorus impejenus (n = 1). Preliminary analysis revealed low detection frequency and further analysis was therefore limited to prey species consumed by predators. Data for langur and macaque showed evidence of evasive movements before detection and ‘heaping’ of data (Table 1).
Table 1 Results of distance sampling for three carnivore prey species and primates (langur Trachypithecus geei and macaque Macaca mulatta, combined) in Jigme Singye Wangchuck National Park, Bhutan (Fig. 1), with number of detections of each species or taxa, sampling effort in km, Akiake information criteria (AIC), detection probability, mean cluster size and density and mean density of individuals (with 95% confidence interval, CI), and estimated biomass.

A half-normal key function with no adjustment terms best described the detection functions for muntjac, wild pig and sambar (Table 1). The estimated density of wild pig was the highest at 3.68 km-2. The estimated density of sambar, the preferred prey of tiger (Karanth & Sunquist, Reference Karanth and Sunquist1995), was 1.19 km-2. Using the detection function for sambar, the density of serow was estimated to be 0.36 km-2. A half-normal key function also provided the best fit to the primate data (2.37 km-2). Estimates of density by warden jurisdiction describes the distribution of prey species in more detail (Fig. 2). Of the five areas, Trong had the highest overall prey density followed by Phobji and Langthel. Athang and Korphu had < 4 animals km-2. Muntjac, wild pig and sambar were sighted in all the five areas. Primate density was highest in Langthel followed by Korphu and Trong. Athang had low ungulate and primate densities. Analysis of variance revealed that the densities of ungulates (F = 17.66, P < 0.001) and primates (F = 11, P < 0.001) were significantly different across the five areas.
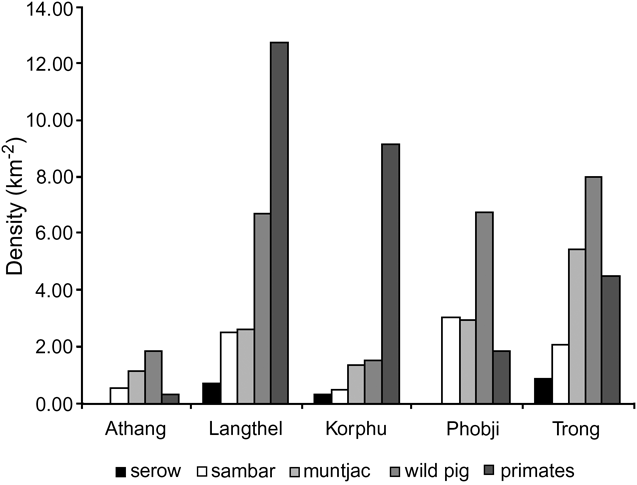
Fig. 2 Density of the five main prey species in five warden jurisdictions in Jigme Singye Wanghcuck National Park (Fig. 1).
Based on prey densities and average body sizes, the study area harboured a low ungulate biomass of 379 kg km-2. Muntjac (11%) and sambar (43%) together contributed 54% of the total biomass and wild pig 37%. Based on the figures obtained from the records maintained by village livestock officers, livestock density was estimated to be 6 km-2, with a biomass of 615 kg km-2.
Discussion
Although distance sampling has been widely used (Biswas & Sankar, Reference Biswas and Sankar2002; Bagchi et al., Reference Bagchi, Goyal and Sankar2003) for estimating prey densities and biomass (Karanth & Sunquist, Reference Karanth and Sunquist1995; Karanth & Nichols, Reference Karanth and Nichols1998), its applicability has been limited in areas with low visibility such as Bhutan because of the need for an adequate number of sightings (Burnham et al., Reference Burnham, Anderson and Laake1980; Buckland et al., Reference Buckland, Anderson, Burnham and Laake1993). The low detection probability in this study was because of Bhutan’s mountainous terrain combined with low prey densities, occasional foggy weather and dry litter on the forest floor, the noise of which may have alerted animals before we were able to see them. In spite of these limitations, with sufficient effort distance sampling can be an effective tool in studying prey populations in Bhutan’s difficult terrain.
The estimate of total ungulate density (7.4 km-2) is much lower than ungulate densities recorded in other areas in South Asia (Seidensticker, Reference Seidensticker1976; Dinerstein, Reference Dinerstein1979; Karanth & Nichols, Reference Karanth and Nichols1998; Biswas & Sankar, Reference Biswas and Sankar2002), which range from 16.3 km-2 (Karanth & Sunquist, Reference Karanth and Sunquist1995) to 90.8 km-2 (Harihar, Reference Harihar2005). In addition to climatic and topographical factors, other possible reasons for the low densities in Bhutan may include poaching of ungulates, both for consumption and in retaliation for crop damage, and competition with local livestock. Local farmers put out snares and traps to protect their crops from wild ungulates and also graze livestock at a mean density of 6 km-2. Previous studies in India (Sankar, Reference Sankar1994; Mathai, Reference Mathai1999) have reported decreasing ungulate populations in areas with high competition from livestock. Voluntary resettlement of residents and their stock from the Nagarahole (Karanth & Sunquist, Reference Karanth and Sunquist1992) and Gir (Khan, Reference Khan1996) protected areas in India allowed prey populations to increase. Long-term temporal and spatial monitoring of ungulate populations, in habitats both with and without cattle grazing and human pressures, are required to elucidate the factors limiting ungulate populations in Bhutan.
The estimated density of wild pigs in Bhutan (3.7 km-2) is similar to densities reported for other areas in Asia, ranging from 2.5 km-2 in Kanha, India (Karanth & Nichols, Reference Karanth and Nichols1998) to 6.1 km-2 in the Chilla Range, India (Harihar, Reference Harihar2005). Wild pigs were often sighted because they frequent open marshy areas where visibility is high, whereas other ungulate species are less easily sighted because of thick forest cover. Muntjac and sambar are also widely distributed across the study area. The muntjac density of 2.2 km-2 is lower than in Kanha and Nagarhole, India (6.0 km-2; Karanth & Nichols, Reference Karanth and Nichols1998), but higher than in Bandipur, India, (1.0 km-2; Johnsingh, Reference Johnsingh1983) and Bardia, Nepal (1.7 km-2; Dinerstein, Reference Dinerstein1980). The density of sambar (1.2 km-2), mostly sighted in hilly areas, is similar to that reported from Kanha (1.5 km-2; Karanth & Nichols, Reference Karanth and Nichols1998). The highest density of sambar is reported from the Chilla Range (24.3 km-2; Harihar, Reference Harihar2005).
Ungulate biomass in Bhutan is the lowest reported from studies in Asia (379 kg km-2). The lowest biomass reported previously was in Bardia National Park (2,842 kg km-2; Dinerstein, Reference Dinerstein1979). Large mammalian predators such as tigers and leopards have been known to respond to prey biomass and densities (Carbone & Gittleman, Reference Carbone and Gittleman2002; Karanth et al., Reference Karanth, Nichols, Kumar, Link and Hines2004). In Bhutan low ungulate densities may be responsible for low numbers of predators (Wang, Reference Wang2008). Given an annual prey intake of c. 3,000 kg per tiger (Karanth & Nichols, Reference Karanth and Nichols1998), a biomass of 379 kg km-2 could support only up to 1.2 tigers per 100 km-2. If we consider other predators such as leopards and dholes, which also share this ungulate biomass, then the population of tigers that could be supported is even lower.
To maintain viable predator populations and reduce predation on livestock, wild prey densities need to increase. Firstly, resource competition between livestock and wild ungulates needs to be reduced by reduction in grazing pressure of domestic livestock in the forests. Secondly, livestock populations need to be reduced in the forests so that wild ungulates may recolonize their natural habitats away from human settlements. However, livestock currently provides a major proportion of predator diets, especially for tigers and leopards (Wang & Macdonald, Reference Wang and Macdonald2009). Any abrupt reductions in livestock could lead to food scarcity for predators. Habitat management could attract native ungulates into forests from marginal lands near settlements and then a programme could be initiated to reduce livestock grazing pressure. A livestock intensification programme to reduce livestock numbers, and in particular stray grazing in forest, is now being implemented across national parks in Bhutan. If this proves successful, managers could gradually ban grazing in areas of high ungulate density and biomass. This would provide multiple benefits to conservation: reduction in crop damage, lower predation rates on livestock and, potentially, improved support amongst farmers for conservation of large carnivores. Where such programmes are in place the frequency of crop damage is declining, and wild ungulates are being sighted more frequently in the forest.
Acknowledgements
I would like to thank my field assistants (Kinzang Lham, Kuenzang Dorji, Kesang Wangchuk, Sonam Dorji, K.B. Gurung, Jigme Wangchuk, Karma and Ngawang Tenzin) for their hard work, and Drs James P. Lassoie, Paul Curtis, Milo E. Richmond, Ullas Karanth, A.J.T. Johnsingh and Koustubh Sharma for reviewing this article. I greatly appreciate the financial support of Save the Tiger Fund/NFWF, Whitley Fund for Nature and Disney Conservation.
Biographical sketch
Sonam Wangyel Wang’s research interests include understanding the relationships between predators, prey and people in the mountainous region of Bhutan, with a particular focus on the ecology of tigers and leopards. He is currently using camera-trapping and distance sampling to estimate populations of tigers, leopards and their prey species in Bhutan’s rugged terrain.





