The consumption of high-energy diets rich in fat and sugars has increased worldwide, paralleling the worldwide rise in obesity and metabolic conditions such as type 2 diabetes mellitus and CVD( Reference Astrup, Dyerberg and Selleck 1 , Reference Danaei, Lu and Singh 2 ). Despite high inter-individual variability in sensitivity to gains in weight and adiposity under high-fat (HF) diets in both humans( Reference Blundell, Stubbs and Golding 3 – Reference Stoger 5 ) and rodents( Reference Azzout-Marniche, Chaumontet and Nadkarni 6 , Reference Levin, DunnMeynell and Balkan 7 ), HF over-feeding is known to favour the development of adiposity( Reference Bray and Popkin 8 ) and metabolic disorders( Reference Poudyal, Panchal and Ward 9 ) involving insulin resistance and ultimately leading to chronic diseases( Reference Hardy, Czech and Corvera 10 , Reference Nagao, Asai and Sugihara 11 ). Moreover, early perinatal HF exposure, by maternal HF nutrition during pregnancy and lactation, very likely contributes to the predisposition of offspring to HF-induced obesity and insulin resistance in later life( Reference Ainge, Thompson and Ozanne 12 – Reference Li, Sloboda and Vickers 14 ).
Although numerous studies have investigated how HF feeding affects carbohydrate and lipid metabolism, little is still known about its potential impact on protein and amino acid (AA) metabolism( Reference Guillet, Masgrau and Boirie 15 , Reference Katsanos and Mandarino 16 ). Several studies have shown that high plasma concentrations of several AA, including branched-chain AA, are associated with obesity and the early onset of insulin resistance, as well as a higher risk of developing the metabolic syndrome or type 2 diabetes( Reference Lotta, Scott and Sharp 17 – Reference Yamakado, Nagao and Imaizumi 22 ). Elevated circulating AA levels suggest an impaired metabolism, which may arise from either decreased AA catabolism and/or increased AA release as a result of increased protein degradation in some tissues, in line with the initiation of insulin resistance. However, the causes and underlying mechanisms are still poorly understood, and this is because of several reasons( Reference Lotta, Scott and Sharp 17 , Reference Lynch and Adams 18 ). One reason is the diversity of the phenotypes reported with tissue-specific alterations in AA catabolism, with a modulation of catabolic enzyme activities being either similarly or oppositely altered, depending on the tissue( Reference Lynch and Adams 18 , Reference She, Van Horn and Reid 23 ). The other reason is the unresolved debate about the possible alterations of the tissue kinetics of protein synthesis and degradation, with only few and conflicting findings about how these fluxes are affected by obesity and insulin resistance( Reference Guillet, Masgrau and Boirie 15 , Reference Katsanos and Mandarino 16 ). For instance, proteolysis fluxes have mostly been measured at the whole-body level but seldom at the muscle level, with contradictory results, which generally reported no effects of obesity or insulin resistance, or in some cases a proteolysis increase at the whole-body level( Reference Chevalier, Marliss and Morais 24 – Reference Gougeon, Morais and Chevalier 26 ) or a proteolysis decrease at the muscle level( Reference Patterson, Horowitz and Wu 27 ). Such discrepancies probably reflect the limitations of classic tracer methods when investigating protein kinetics and their alterations( Reference Fouillet, Bos and Gaudichon 28 ). In this context, new and alternative approaches are required in order to gain a more integrated insight into the partitioning and potential reallocation of AA between different anabolic and catabolic fluxes in tissues, so as to ultimately decipher the effects of obesity and insulin resistance on AA and protein metabolism.
To this end, the natural abundances of stable N isotopes in tissue proteins constitute new, relevant and promising biomarkers of AA metabolism in tissues. Dietary protein N is the sole source of body protein N, but body proteins are naturally enriched in the heavier stable N isotope (15N) over dietary protein. This 15N bioaccumulation (Δ15N, also known as the trophic step or discrimination factor) is globally due to discrimination against this heavy isotope during N waste formation and particularly during urea production( Reference Cantalapiedra-Hijar, Ortigues-Marty and Sepchat 29 – Reference Poupin, Mariotti and Huneau 31 ). More precisely, Δ15N values vary among tissues as a result of differences in AA composition( Reference O’Brien 32 ), but also and mostly as a result of the particular AA metabolism in the tissue, because of the existence of several isotope effects associated with different metabolic pathways such as AA oxidation, as well as protein turnover in tissues( Reference Poupin, Mariotti and Huneau 31 , Reference Martinez del Rio and Wolf 33 ). Consequently, variations in different body fluxes of the catabolic or anabolic use of AA can result in Δ15N variations, and tissue Δ15N measurements are integrative biomarkers for the tissue-specific metabolic impact of nutritional and/or physiopathological conditions. For instance, in humans, variations in hair 15N natural abundance values have been shown to reflect changes in N metabolism during pregnancy( Reference Fuller, Fuller and Sage 34 ), nutritional stress( Reference Fuller, Fuller and Sage 35 ), anorexia( Reference Hatch, Crawford and Kunz 36 , Reference Mekota, Grupe and Ufer 37 ) and cirrhosis( Reference Petzke, Feist and Fleig 38 ), and Δ15N values in some animal tissues have been shown to reflect dietary protein quality and anabolic use efficiency( Reference Cantalapiedra-Hijar, Ortigues-Marty and Sepchat 29 , Reference Poupin, Bos and Mariotti 30 , Reference Martinez del Rio and Wolf 33 , Reference Cheng, Sheahan and Gibbs 39 – Reference Robbins, Felicetti and Sponheimer 41 ). More precisely, we have previously shown, by analysing simultaneous multi-tissue Δ15N measurements using a multi-compartment model, that tissue protein Δ15N values constitute integrated and interpretable biomarkers of tissue AA partitioning between protein synthesis and oxidation( Reference Poupin, Mariotti and Huneau 31 ).
In addition, the natural abundances of stable C isotopes in tissue proteins are also informative about the dietary origin of tissue AA. In contrast with N, the heavier, stable C isotope (13C) only modestly bioaccumulates( Reference O’Brien 32 , Reference McCutchan, Lewis and Kendall 42 ), and if the C found in tissue proteins preferentially originates from dietary proteins it can also derive from dietary lipids and carbohydrates (CHO) that are used for non-indispensable AA synthesis( Reference Fernandes, Nadeau and Grootes 43 – Reference Wolf, Newsome and Peters 46 ). Because dietary macronutrients generally differ in their natural 13C abundances, those in tissue proteins may reflect macronutrient use for AA synthesis and consequent routing to tissue proteins, which can vary in line with nutritional and physiopathological conditions( Reference Hatch, Crawford and Kunz 36 , Reference Fernandes, Nadeau and Grootes 43 , Reference Kurle, Koch and Tershy 44 , Reference Wolf, Newsome and Peters 46 – Reference Hobbie 49 ).
In this study, we used these new C and N isotopic biomarkers in order to better characterise the tissue-specific dysregulations of AA metabolism induced by exposure to an HF diet. C isotopic values were used as indicators of the relative utilisation of dietary macronutrients for tissue AA synthesis, and N isotopic values as indicators for the preferential metabolic use of tissue AA for anabolic v. catabolic purposes. The study was carried out in rats exposed to an HF diet during the perinatal and/or post-weaning periods, as a model of early exposure to HF that is known to increase the risk for obesity and insulin resistance.
Methods
Animals and diets
The animal protocol formed part of a larger study designed to explore the combined effects of diet and exposure to contaminants, whose initial results have already been presented at the 9th Copenhagen Workshop on Endocrine Disrupters – COW2017( Reference Zalko, Canlet and Tremblay-Franco 50 ). In this ancillary study, specific tissues were sampled for isotopic analyses of a subset of animals. Sprague–Dawley dams were pair-fed either a normal-fat (NF) or HF diet during gestation and lactation, from gestational day 8 onwards, while maintaining an equal N intake. After weaning, from postnatal day 21 onwards, male pups were pair-fed with either the NF or HF diet to include all combinations of perinatal and post-weaning dietary exposures (NF-NF, n 9; NF-HF, n 7; HF-NF, n 8; HF-HF, n 8). Dietary macronutrient compositions were adjusted daily according to the recommendations of Reeves et al.( Reference Reeves, Nielsen and Fahey 51 ) in order to meet the protein and lipid requirements of gestation, lactation and early postnatal growth. The macronutrient composition of the diets following the stabilisation of food intake (from postnatal day 35) is detailed in Table 1. Compared with the NF diet, the HF diet had the same protein content (16·7 wt%), a higher lipid content (35 v. 6 wt%) owing to CHO substitution and a different CHO profile (67 % maltodextrin and 33 % sucrose v. 100 % maltodextrin) for its potential effect on obesity and insulin resistance. Food intake was measured daily throughout the study. On postnatal day 115, the body composition of the animals was measured using NMR-MRI-based technology (EchoMRITM 700) and oral glucose tolerance tests (OGTT) were performed in order to determine insulin sensitivity. In brief, a glucose bolus (1 g/kg) was administered orally after an overnight fast, and blood samples were taken from the tail vein before and 15, 30, 60 and 120 min after glucose administration. The blood glucose (OneTouch Ultra Easy glucometer; LifeScan) area under the concentration–time curve (AUC) was calculated using the trapezoid method. The rats were euthanised under pentobarbital anaesthesia on postnatal day 120 after an overnight fast. After an abdominal incision, blood was drawn from the abdominal aorta using a citrate-coated syringe. After centrifugation (12 000 g , 2 min), plasma and erythrocytes were rapidly aliquoted, frozen in liquid N2 and stored at −80°C. Plasma insulin levels were measured by ELISA (Millipore). Different tissues were also rapidly collected (liver, small intestine, heart; tibialis anterior, gastrocnemius, soleus and extensor digitorum longus muscles; and subcutaneous adipose tissue), weighed, freeze-clamped in liquid N2 and stored at −80°C until further analyses. Faeces were also collected and stored under the same conditions. The animals were handled according to the recommendations of the Regional Ethics Committee (accreditation number issued by the Ministry for Research: 00837·01).
Table 1 Nutrient and isotopic compositions of normal- and high-fat diets
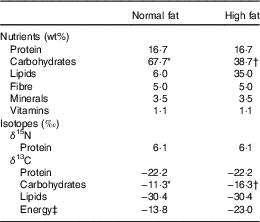
* 100 % maltodextrin.
† 67 % maltodextrin, 33 % sucrose.
‡ Weighted average of lipids and carbohydrates according to their C content and wt% (Equation (2)).
Feed efficiency and liver protein anabolism efficiency
Feed efficiency was defined as the ratio of body weight at postnatal day 120 relative to cumulative food intake. Liver samples were homogenised and centrifuged (20 000 g , 10 min) and the protein content was measured using a bicinchoninic acid assay kit (Bio-Rad). Liver protein anabolism efficiency (%) was defined as the ratio of liver protein content at the time of killing relative to cumulative protein intake over the post-weaning period.
Tissue protein extraction
Frozen tissues were cooled with liquid N2 and pulverised with a pestle and mortar. Tissue proteins were precipitated from pulverised tissues (100 mg) with 5-sulfosalicylic acid (10 %, 7 μl/mg of tissue). Twice, after centrifugation (10 000 g , 4°C, 20 min), the supernatant was removed and the protein pellets were washed with 5-sulfosalicylic acid (10 %) and freeze-dried. Subcutaneous adipose tissue (1 g) was first homogenised in 0·9 % NaCl using an Ultra-Turrax homogenizer (T25 digital; IKA). After centrifugation (2000 g , 2°C, 20 min), the infranatant containing the proteins was collected using a syringe and needle, and the floating fat layer containing the lipids was stored at −20°C until isotopic analysis. Adipose tissue proteins were further precipitated with 100 μl of 5-sulfosalicylic acid (100 %) and thereafter treated as described for other tissues. Plasma proteins were precipitated from 0·5 ml of plasma with 5-sulfosalicylic acid (100 %, 50 μl/ml of plasma) and the samples were centrifuged (2000 g , 4°C, 20 min) after 1 h of incubation at 4°C. The supernatant was removed and the protein pellets were washed with H2O, centrifuged again as described above and freeze-dried. The protein pellets from all matrices were delipidated using 1:1 ethanol–diethyl ether. Twice, the proteins were mixed for 1 h with the solvent mixture using a tube rotator and the supernatant was removed after a brief centrifugation (1000 g , 20°C, 5 min). The remaining solvents were eliminated by centrifugal evaporation. The faeces were freeze-dried and pulverised, and then the resulting powder was analysed without any further treatment.
Isotopic analysis
The natural abundances of N and C stable isotopes in samples were expressed relative to standards (atmospheric N2 for 15N/14N and Vienna Pee Dee Belemnite for 13C/12C, respectively) using the delta notation:
where δ is the parts-per-thousand difference in ratio of heavy to light isotopes (15N/14N and 13C/12C) in the samples (R sample) and standards (R standard, 0·0112372 for C and 0·0036765 for N). We measured δ 15N and δ 13C in the proteins in the different tissues sampled, in the faeces, in lipids from adipose tissue and in dietary proteins, CHO and lipids. Isotopic measurements were performed with an isotope-ratio mass spectrometer (Isoprime; VG Instruments) coupled to an elemental analyser (EA Vario Micro Cube; Elementar). Tyrosine was used for calibration and drift correction. δ 15N and δ 13C values for tissue proteins and faeces were expressed relative to the δ value of dietary proteins using the capital delta notation (∆tissue proteins=δ tissue proteins−δ dietary proteins). The δ 13C of adipose tissue lipids was similarly expressed but relative to dietary lipid δ 13C. For each diet, δ 13C of non-protein energy sources was calculated as the average δ 13C of lipids and CHO weighted by their C content and dietary occurrence as follows( Reference Fernandes, Nadeau and Grootes 43 , Reference Kurle, Koch and Tershy 44 ):
where δ 13CC, δ 13CL and δ 13CE are, respectively, the δ 13C values of dietary lipids, CHO and non-protein energy sources, %C and %L are dietary mass fractions of CHO and lipids and 0·444 and 0·768 are the reference C mass fractions of CHO and lipids, respectively.
Assessment of the contribution of dietary macronutrients to amino acid synthesis
The C in tissue proteins has various origins. Indeed, even though dietary proteins are preferentially routed to tissue proteins as AA, dietary lipids and CHO are used for the biosynthesis of non-indispensable AA and thus routed to protein synthesis, the degree of which varies according to the dietary macronutrient content( Reference Fernandes, Nadeau and Grootes 43 , Reference Kurle, Koch and Tershy 44 , Reference Wolf, Newsome and Peters 46 ). Therefore, by hypothesising an isotopic steady state owing to the entire renewal of tissue proteins since weaning, the δ 13C of tissue proteins represents the sum of dietary macronutrient δ 13C values weighted by their respective contributions to C in tissue proteins (routing coefficients), in addition to the difference in δ 13C between diet and tissue proteins because of isotopic fractionation along metabolic pathways (the trophic step). In our study, the three dietary macronutrients differed in terms of their δ 13C and the two diets in terms of their δ 13CE, because of the different CHO and lipid contents (Table 1). These δ 13C signatures of macronutrients and diets allowed us to estimate the routing coefficients for each macronutrient and diet.
In a first step, the contributions of dietary proteins were derived from the following equation:
where δ 13CTd , δ 13CP and δ 13CEd are, respectively, the δ 13C values of proteins in a tissue, of dietary proteins and of dietary non-protein energy sources in the post-weaning diet d (d=NF or HF). p d and 1−p d are the proportions of protein and non-protein dietary macronutrients routed to C in tissue proteins (the protein and energy routing coefficients, respectively). TSP and TSEd are the specific trophic steps on dietary protein and energy, and TSd is the global trophic step (TSd=p d×TSP+(1−p d) TSEd ). To solve Equation (3), p and TS were assumed to be equal across our diets having an identical and adequate protein content, as p and TS have previously been shown to be extremely consistent over a spectrum of dietary compositions, except for deficient or excessive protein contents( Reference Fernandes, Nadeau and Grootes 43 ). By solving Equation (3) simultaneously for each post-weaning diet (NF and HF), we obtained an identifiable system of two linear equations and two unknowns, p and TS, whose solution was as follows:
The contributions of dietary CHO and lipids to C in tissue proteins were then estimated using the following equation:
Here, cd and 1−p−c d are the proportions of CHO and lipids routed, respectively, to C in tissue proteins (the CHO and lipid routing coefficients) and δ 13C C d and δ 13CL are their respective δ 13C values in diet d. Equation (6) was solved for c d as follows:
Assessment of tissue amino acid allocation between anabolic and catabolic pathways
We had previously investigated the causes and significance of ∆15N variations across tissues and diets using a multi-compartmental analysis of multi-tissue ∆15N measurements in rats, and showed that the ∆15N of a tissue protein varies as a function of pox, the proportion of tissue AA that is directed towards oxidation rather than used for protein synthesis( Reference Poupin, Mariotti and Huneau 31 ). In particular, according to model predictions, a rise in ∆15N levels indicates an increase in pox in all tissues except muscle, where in contrast it is indicative of a reduction in pox because of its opposite sign of isotopic fractionation associated with AA catabolism. Indeed, in most tissues, AA losses for catabolic purposes favour 14N elimination and hence 15N retention owing to the isotope effects associated with transamination and liver deamination, whereas under catabolic conditions muscle releases some of the most 15N-enriched AA (such as Ala and Gln) into the bloodstream, which may favour 15N elimination from the muscle as previously discussed( Reference Poupin, Mariotti and Huneau 31 ). Our model prediction for the ∆15N and pox relationships in splanchnic tissues was further validated experimentally by combining AA tracers and arteriovenous balance methods in ruminants, where we showed that the ∆15N of plasma proteins, which are synthesised in the splanchnic bed and mainly in liver, linearly increases with the splanchnic AA metabolism and oxidation and the liver urea production( Reference Cantalapiedra-Hijar, Ortigues-Marty and Sepchat 29 ). In the present study, the observed diet-induced ∆15N variations were interpreted in the light of these previous modelling( Reference Poupin, Mariotti and Huneau 31 ) and experimental( Reference Cantalapiedra-Hijar, Ortigues-Marty and Sepchat 29 ) results: a higher ∆15N was interpreted as indicative of an higher pox in all tissues except the muscle, where it was interpreted as indicative of a lower pox.
Statistical analyses
Because this was an ancillary study during which thirty-two animals received the four dietary treatments studied here, the sample size was fixed. However, we reasoned that the study would be powerful enough with eight rats in each group, because this sample size allowed minimally detectable effect sizes of 0·3 ‰ using a t test on group means for both ∆15N and ∆13C values, assuming a level of significance of 0·05, a power level of 0·80 and a standard deviation of 0·2 ‰( Reference Dell, Holleran and Ramakrishnan 52 ). Such a 0·3 ‰ effect size was small enough to enable the detection of diet-induced changes in (i) tissue AA metabolism using ∆15N values, according to our previous model predictions( Reference Poupin, Mariotti and Huneau 31 ), and (ii) nutrient routing to tissue protein using ∆13C values, because 0·3 ‰ is <2 % of the difference between dietary CHO and lipid ∆13C values.
ANOVA using Tukey’s post hoc test was run under SAS (version 21) in order to determine differences between the groups in terms of body composition, metabolic parameters and ∆15N and ∆13C values (α=0·05). Pearson’s correlation coefficients between ∆15N and metabolic and body composition parameters of interest were evaluated for their significance using the Benjamini–Hochberg false discovery rate (FDR)( Reference Benjamini and Hochberg 53 ). The FDR correction was used to control the rate of false discoveries among significant correlations, and correlations with an FDR≤15 % were considered as significant. The data are expressed as means and standard deviations.
Results
Body composition, insulin sensitivity and feed efficiency
Post-weaning nutrition was the only factor that affected the body composition and metabolic parameters of the animals. Compared with the NF post-weaning diet, the HF post-weaning diet (i.e. NF-HF and HF-HF v. NF-NF and HF-NF) induced a higher body weight (+28 %, 529 (sd 50) v. 412 (sd 27) g, P<0·0001), with a higher fat mass (+178 %, 121 (sd 38) v. 44 (sd 16) g, P<0·0001) and a higher lean mass (+10 %, 354 (sd 33) v. 322 (sd 36) g, P<0·05). The following metabolic parameters were also increased under the HF post-weaning diet: plasma insulin levels (+100 %, 74·8 (sd 48·6) v. 37·5 (sd 12·3) pM, P<0·01), glucose OGTT AUC (+19 %, 824 (sd 105) v. 695 (sd 54) mM×120min, P<0·01), feed efficiency (+27 %, 66·4 (sd 4·8) v. 52·2 (sd 3·4) %, P<0·0001) and liver protein anabolism efficiency (+18 %, 1·98 (sd 0·45) v. 1·69 (sd 0·16) %, P<0·05).
Natural abundances of stable carbon isotopes (∆13C) in tissue proteins and dietary macronutrient use for amino acid synthesis
Only post-weaning nutrition affected ∆13C values in tissue proteins, faeces and adipose tissue lipids, as presented in Table 2. ∆13C values after HF post-weaning feeding were lower in all the pools sampled, regardless of perinatal nutrition (NF-HF and HF-HF were lower than NF-NF and HF-NF, all P<0·0001). This indicates a more marked routing of dietary lipids, the macronutrient with the lowest δ 13C, to adipose tissue lipids and tissue proteins when the animals consumed the HF diet.
Table 2 Natural abundances of carbon stable isotopes in tissue proteins, faeces and adipose tissue lipids (∆13C) Footnote *(Mean values and standard deviations)
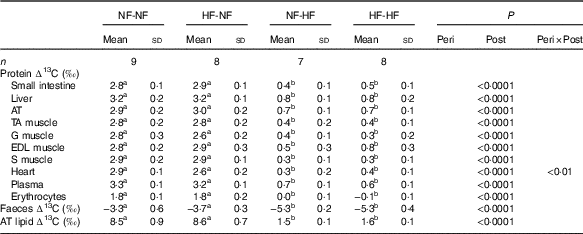
NF-NF, rats exposed to the normal-fat (NF) diet during the perinatal and post-weaning periods; NF-HF, rats exposed to the NF diet during the perinatal period and the high-fat (HF) diet during the post-weaning period; HF-NF, rats exposed to the HF diet during the perinatal period and the NF diet during the post-weaning period; HF-HF, rats exposed to the HF diet during both the perinatal and post-weaning periods; peri, perinatal diet; post, post-weaning diet; AT, adipose tissue; TA, tibialis anterior; G, gastronemius; EDL, extensor digitorum longus; S, soleus.
a,b Mean values with unlike superscript letters were significantly different between groups (P<0·0001).
* Protein and faeces ∆13C values are the differences between protein or faeces δ 13C and dietary protein δ 13C. AT Lipid ∆13C is the difference between AT lipid δ 13C and dietary lipid δ 13C.
Given the lack of effects of perinatal nutrition on ∆13C values, the contributions of dietary macronutrients (protein, CHO and lipid) to C in tissue proteins (i.e. nutrient routing) were calculated for post-weaning diets only (HF: n 15, NF: n 17), for each sampled tissue (intestine, liver, plasma, erythrocytes, heart and four muscles). In each tissue, for identifiability reasons, protein routing (but not CHO and lipid routings) was assumed to be equal across the diets given their identical protein (but not energy) content. These nutrient routing results are presented in Table 3 (mean values across tissues) and in online Supplementary Table S2 (values for each tissue). For all tissues, proteins were preferentially sourced from dietary proteins, with protein routing that averaged 74·2 (sd 2·4) % across the tissues (Table 3). The routing of CHO and lipids was found to be largely dependent on diet, with a higher routing of lipid under HF feeding, but overall a higher routing of CHO than lipid for all tissues and diets. On average, in all the tissues sampled, lipid routing indeed reached 3·4 (sd 0·3) % and 12·1 (sd 1·1) % with the NF and HF diets, respectively, whereas CHO routing was 22·4 (sd 2·1) % and 13·7 (sd 1·3) %, respectively (Table 3). Therefore, in rats fed the NF and HF diets, where dietary lipids contributed to 13 and 61 % of C in non-protein sources (CHO+lipids), dietary lipids contributed to 13 % and only 47 % of non-protein C routed to tissue proteins (energy routing, see the ‘Methods’ section). It should be noted that, among the tissues studied, erythrocytes were outliers for all nutrient routing, with the highest value for protein routing and the lowest values for CHO and lipid routing (online Supplementary Table S2).
Table 3 Routing coefficients of dietary macronutrients to tissue proteins in rats fed a normal- or high-fat diet (Mean values and standard deviations and minimum and maximum values across tissues)

CHO, carbohydrates.
The protein, CHO and lipid routings are the proportions of dietary protein, CHO and lipid C used for amino acid synthesis and consequently routed to C in tissue proteins, and the trophic step is the difference in δ 13C between dietary and tissue proteins owing to isotopic fractionation along metabolic pathways (i.e. the coefficients p, c, 1−p−c and TS of Equation (6), see the ‘Methods’ section). The protein routing and trophic step were assumed to be equal across the diets, whereas the CHO and lipid routings differed in rats fed the normal-fat or high-fat diet.
Natural abundances of stable nitrogen isotopes (∆15N) in tissue proteins and amino acid allocation between anabolic and catabolic pathways
Fig. 1 shows ∆15N values in faeces and proteins of the small intestine, liver, tibialis anterior and gastrocnemius muscles, and adipose tissue. In adipose tissue proteins, ∆15N values were up to 0·5 ‰ higher in rats fed the HF diet after weaning than in those never exposed to the HF diet (NF-HF and HF-HF v. NF-NF; P<0·001 and P<0·05, respectively), and also tended to be 0·2 ‰ higher following perinatal exposure alone (HF-NF v. NF-NF; P<0·08). In faeces and proteins of the small intestine and tibialis anterior muscle, combined HF exposure during the perinatal and post-weaning periods (HF-HF) resulted in 0·7, 0·4 and 0·3 ‰ higher ∆15N values, respectively, compared with all the other conditions (all P<0·05). These diet-induced ∆15N variations indicated diet-induced differences in pox, the AA allocation between catabolic and anabolic pathways, in the corresponding tissues. By contrast, ∆15N did not differ between the diet groups in proteins of the liver (3·7 (sd 0·2) ‰), heart (4·9 (sd 0·3) ‰), plasma (4·6 (sd 0·2) ‰), erythrocytes (2·2 (sd 0·1) ‰) and the gastrocnemius (3·5 (sd 0·2) ‰), soleus (4·1 (sd 0·2) ‰) and extensor digitorum longus muscles (3·7 (sd 0·2) ‰). Detailed ∆15N values are shown in the online Supplementary Table S1.
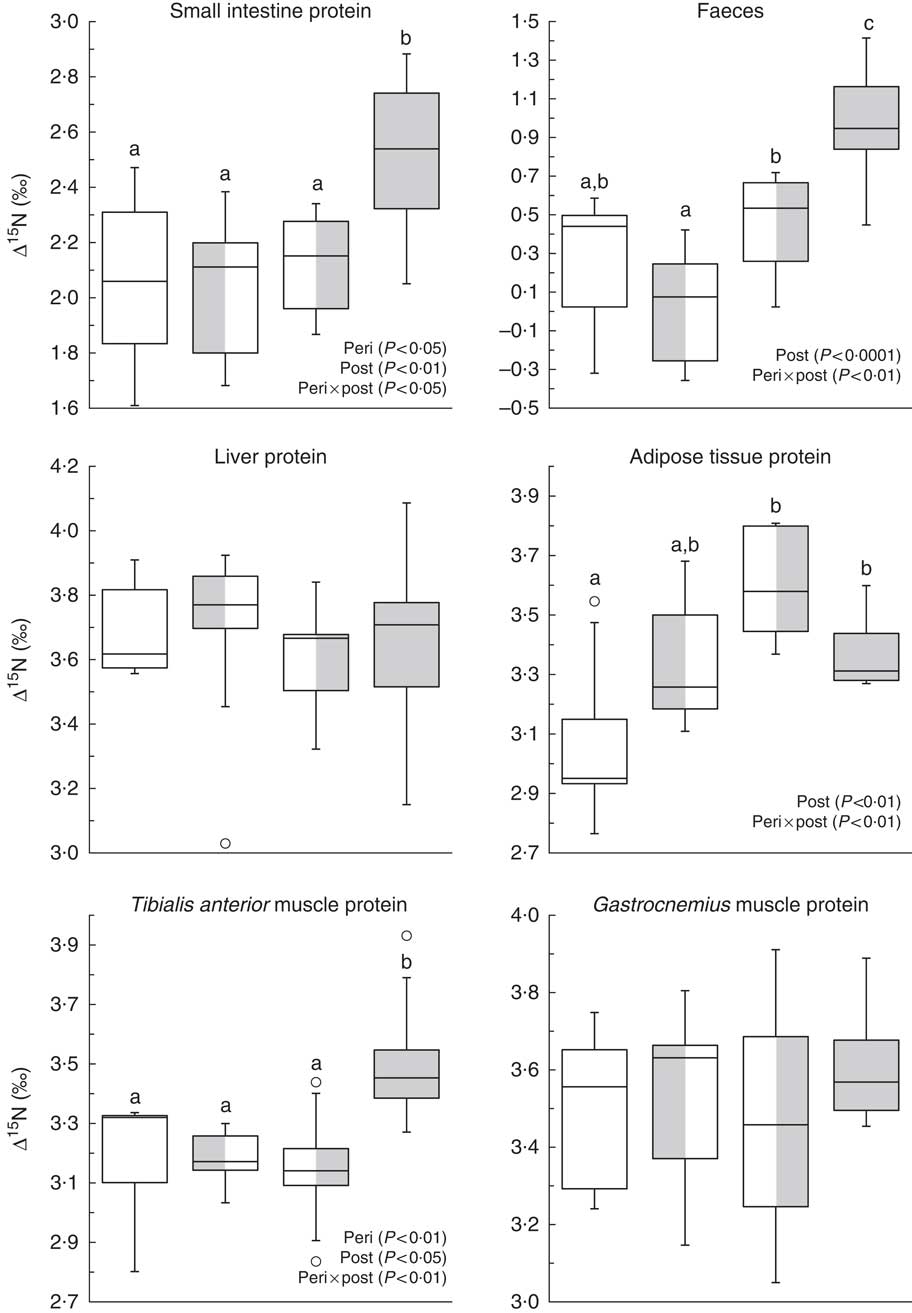
Fig. 1 Natural abundances of N stable isotopes in tissue proteins (Δ15N, ‰) in rats exposed to a normal-fat (NF) diet or a high-fat (HF) diet at different periods. Boxes represent the interquartile range (IQR, the difference between the 75th and the 25th percentiles), the line inside the box corresponds to the medians and 50th percentile and whiskers extend to maximum and minimum values or 1·5 times the IQR if outliers (![]() ) exceed that distance.
) exceed that distance. ![]() , NF-NF;
, NF-NF; ![]() , HF-NF;
, HF-NF; ![]() , NF-HF;
, NF-HF; ![]() , HF-HF. Peri, perinatal diet; post, post-weaning diet. a,b,c Values with unlike letters were significantly different between groups (P<0·05). Values concern the protein fraction of tissues except for faeces (bulk) that were only freeze-dried and homogenised before analysis.
, HF-HF. Peri, perinatal diet; post, post-weaning diet. a,b,c Values with unlike letters were significantly different between groups (P<0·05). Values concern the protein fraction of tissues except for faeces (bulk) that were only freeze-dried and homogenised before analysis.
Pearson’s correlation coefficients of the significant correlations seen between ∆15N and body composition or metabolic parameters are listed in Table 4. The ∆15N values of intestinal proteins, faeces and adipose tissue proteins were all positively associated with body weight, fat mass and feed efficiency, and ∆15N values of faeces were also positively associated with glucose OGTT AUC (0·37≤r≤0·47). In contrast, the ∆15N values of proteins in the gastrocnemius muscle were positively associated with lean mass (r 0·45), whereas the ∆15N values of liver proteins were negatively associated with liver protein anabolism efficiency (r −0·56), glucose OGTT AUC (r −0·46) and feed efficiency (r −0·40).
Table 4 Pearson’s correlation coefficients of significant correlations between natural abundances of stable nitrogen isotopes in tissue proteins (Δ15N) and body composition and metabolic parametersFootnote †
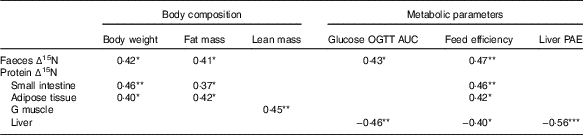
OGTT AUC, area under the concentration–time curve during oral glucose tolerance test; G, gastrocnemius; PAE, protein anabolism efficiency.
*P<0·05, **P<0·01, ***P<0·001, n 28–32.
† All correlations were significant at a false discovery rate of 15 %.
Discussion
During this animal study performed in a carefully controlled dietary environment, we were able to reveal some of the tissue-specific changes that affect protein and AA metabolism in response to HF feeding depending on the exposure window (perinatal and/or post-weaning). This was made possible by the use of a new isotopic approach that consisted in measuring the natural abundances of 13C and 15N (δ 13C and δ 15N) in proteins from a large set of tissues. Using δ 13C, we were able to determine the contribution of each dietary macronutrient to AA renewal in the different tissues (i.e. nutrient routing), whereas the use of δ 15N enabled us to characterise the partitioning of AA between oxidation and protein synthesis pathways in the different tissues (i.e. pox, the proportion of tissue AA that are directed towards oxidation rather than used for protein synthesis). More specifically, our tissue protein δ 13C measurements were analysed with a new routing model, which allowed quantitative estimation of nutrient routing for each studied diet, whereas our tissue protein δ 15N measurements were interpreted in the light of previous modelling( Reference Poupin, Mariotti and Huneau 31 ) and experimental( Reference Cantalapiedra-Hijar, Ortigues-Marty and Sepchat 29 ) results, which allowed qualitative estimation of the diet-induced differences in pox. A necessary condition to validate our interpretation of these δ 13C and δ 15N data was that sufficient time had elapsed since the last diet-shift – that is, δ 13C and δ 15N were no longer evolving but had reached their final equilibrium values with the post-weaning diet. In our study, nearly all body proteins had very likely reached their isotopic equilibrium at the end of the 100-d post-weaning period. Indeed, according to findings available in the literature, the times necessary to reach δ 13C and δ 15N equilibrium in rat tissues range from less than or about 50 d in small intestine, liver and plasma to about 100 d in muscles( Reference MacAvoy, Macko and Arneson 45 , Reference Arneson and MacAvoy 54 – Reference Kraeer, Arneson and MacAvoy 57 ). The only exception was erythrocytes, for which a longer period (130–180 d) has been reported to be necessary to achieve isotopic equilibrium( Reference Poupin, Mariotti and Huneau 31 , Reference Kurle 55 ).
Dietary macronutrient use for amino acid synthesis
Because the three dietary macronutrients generally differ in terms of their δ 13C values and can all be used for AA synthesis, δ 13C in tissue proteins reflects macronutrient use for AA synthesis and subsequent routing to proteins, the extent of which may vary in line with the macronutrient composition of the diet( Reference Fernandes, Nadeau and Grootes 43 , Reference Wolf, Newsome and Peters 46 , Reference MacAvoy, Arneson and Bassett 56 ). Compiled data from studies involving a wide variety of dietary conditions suggest a contribution of about 75 % of dietary protein to C in tissue proteins( Reference Fernandes, Nadeau and Grootes 43 , Reference Kurle, Koch and Tershy 44 , Reference MacAvoy, Arneson and Bassett 56 ) with, for instance, protein routing of 74 % to bone collagen under a broad range of dietary compositions except for diets markedly deficient or excessive in protein( Reference Fernandes, Nadeau and Grootes 43 ). Here, protein routing was estimated to reach 74·2 (sd 2·4) % across all tissues, which is consistent with the mean values and minor inter-tissue variations recorded by the studies in the literature( Reference Fernandes, Nadeau and Grootes 43 , Reference Kurle, Koch and Tershy 44 , Reference Hobbie 49 , Reference MacAvoy, Arneson and Bassett 56 ). Furthermore, taking advantage of the different δ 13C signatures of dietary CHO and lipid, we were able to distinguish their respective routings for the first time. In the same way as for protein routing, we found few inter-tissue variations regarding the routing of CHO and lipid under each diet, although erythrocyte was an outlier with the lowest values for both CHO and lipid routing. This limited inter-tissue variability probably reflects the fact that almost all the tissues studied are reliant on a mix of energy fuels during the postprandial period and more on lipids during the post-absorptive period. The only exception is erythrocytes, which are strictly dependent on glucose to fuel their energy metabolism because they lack mitochondria. However, as erythrocytes do not synthesise protein, the lipid and CHO routing calculated in erythrocytes will tend to reflect the mixed utilisation of lipid and CHO in bone marrow. Notwithstanding this, and as discussed above, our routing estimates for erythrocytes may have been partially biased because erythrocytes was the tissue most likely to be influenced by incomplete isotopic turnover.
Interestingly, we found that during NF feeding lipid contributed to the routing of non-protein nutrients in proportion with their dietary occurrence in non-protein nutrients (13 %), whereas during HF feeding lipid contributed more to the routing of non-protein nutrients (47 %) but less than in proportion with their dietary occurrence in non-protein nutrients (67 %). One likely explanation is that dietary lipids cannot enter all the pathways of non-protein C routing to AA( Reference Fernandes, Nadeau and Grootes 43 ): some AA (Ala, Ser, Cys and to a lesser extent Gly) are synthesised from 3C precursors, which are mostly produced during glycolysis, whereas others (Asx, Glx, Pro and Arg) are derived from 4 and 5C precursors produced during the tricarboxylic acid cycle that is fuelled by both glucose and fatty acids via acetyl-CoA. Therefore, an increase in the proportion of lipid in the diet may lead to a proportional rise in lipid-derived C in Asx, Glx Pro and Arg, but not in Ala, Ser, Cys and Gly. In this context, HF-diet-induced changes to the routing of dietary CHO and lipid merely reflected differences in their dietary availability and possible access to the metabolic pathways of AA synthesis, rather than being the consequence of marked changes to the enzymatic activities that occur during adaptation to a new dietary environment.
In our young adult rats, the routing of dietary lipids to tissue proteins thus increased in line with recent, post-weaning, HF exposure, whereas we found no significant effect of earlier, perinatal, HF exposure. This suggests that perinatal HF exposure has no lasting effect on dietary nutrient routing to tissue proteins in later life. It does not preclude the possibility that HF exposure might have affected nutrient routing during the perinatal period in the same way as it did during the post-weaning period, but this could not be traced in tissue protein δ 13C at the end of the 100-d post-weaning period. After such a time delay, routing effects limited to the perinatal period are no longer detectable. As discussed above, 100 d were indeed sufficient to reach isotopic equilibrium with the post-weaning diet in nearly all tissues by complete isotopic turnover since the perinatal period( Reference MacAvoy, Macko and Arneson 45 , Reference Arneson and MacAvoy 54 – Reference Kraeer, Arneson and MacAvoy 57 ), so that the isotopic footprints of earlier effects on nutrient routing had been cleared.
Tissue amino acid allocation between anabolic and catabolic pathways
Although all dietary macronutrients contain C, only dietary proteins contain N and are the almost exclusive sources of the N found in tissue proteins. Because bioaccumulation of the heavy isotope 15N is known to occur, our tissue protein δ 15N measurements were expressed as ∆15N in order to represent 15N enrichment over dietary protein. Our tissue protein ∆15N measurements ranged from 2 to 5 ‰; these values were in line with the few findings available in the literature on rodents( Reference Poupin, Bos and Mariotti 30 , Reference Poupin, Mariotti and Huneau 31 , Reference MacAvoy, Macko and Arneson 45 , Reference Arneson and MacAvoy 54 – Reference MacAvoy, Arneson and Bassett 56 , Reference Yoneyama, Ohta and Ohtani 58 ). Furthermore, the diet-induced ∆15N differences that we observed in some tissue proteins point to diet-induced differences in pox, the relative proportion of AA entering the tissue that is directed toward catabolism. Indeed, according to our previous experimental and modelling results( Reference Cantalapiedra-Hijar, Ortigues-Marty and Sepchat 29 , Reference Poupin, Mariotti and Huneau 31 ), a higher δ 15N difference between tissue and dietary proteins (i.e. a higher ∆15N) indicates a higher pox in all tissues except the muscle, where it indicates a lower pox. Although we found that HF feeding systematically favoured AA synthesis from dietary fat in all tissues, we saw no associated changes in ∆15N, and thus in pox, in most of the tissues studied (seven out of eleven), namely three skeletal muscles (gastrocnemius, soleus and extensor digitorum longus), the heart, plasma, erythrocytes and liver. In contrast, the present results suggest that HF nutrition affected pox in three other tissues, namely the small intestine (and consequently faeces), tibialis anterior muscle and adipose tissue. In the protein of these three tissues, we observed an HF-induced increase in ∆15N, but according to different patterns: prolonged HF exposure covering both the perinatal and post-weaning periods was required for the small intestine and tibialis anterior muscle, whereas for adipose tissue post-weaning exposure alone was sufficient, and perinatal exposure alone tended to be sufficient, to attain a significant effect. According to our previous works( Reference Cantalapiedra-Hijar, Ortigues-Marty and Sepchat 29 , Reference Poupin, Mariotti and Huneau 31 ), the HF-induced increase in protein ∆15N indicated with confidence a more preferential catabolic use of AA (i.e. a higher pox) in the small intestine and the opposite (i.e. a lower pox) in the tibialis anterior. In contrast, as adipose tissue had not been considered in our previous works, a metabolic interpretation of its increase in ∆15N levels was not straightforward. Because a compelling body of evidence supports the reduced expression and activity of enzymes involved in branched-chain AA oxidation (i.e. branched-chain aminotransferase and α-ketoacid dehydrogenase) in adipose tissue in the context of both genetic and diet-induced obesity and insulin resistance( Reference Lynch and Adams 18 , Reference Estrada-Alcalde, Tenorio-Guzman and Tovar 59 , Reference Polakof, Rémond and David 60 ), higher ∆15N levels likely indicate less preferential catabolic orientation of AA metabolism in adipose tissue (i.e. a lower pox), as is the case in muscle.
Regarding the tibialis anterior muscle, the rise in its ∆15N level after prolonged HF nutrition – during both the perinatal and post-weaning periods – indicated a lower pox and thus a stronger preferential anabolic orientation of its AA metabolism. Higher protein synthesis in some muscles was likely in the specific context of our study, as it encompassed the early phase of the development of obesity where the gain in fat mass is accompanied by a concomitant gain in lean and muscle masses, as observed here and reported elsewhere( Reference Masgrau, Mishellany-Dutour and Murakami 61 ). During this early phase, the gain in muscle mass has been shown to be associated with an increased fractional protein synthesis rate in the tibialis anterior muscle but not in the soleus muscle( Reference Masgrau, Mishellany-Dutour and Murakami 61 ), which is exactly in line with our observation of a ∆15N increase in the former but not in the latter. Furthermore, limited evidence has suggested that branched-chain α-ketoacid dehydrogenase activity, the rate-limiting step in branched-chain AA oxidation, is reduced in the muscle of obese rodents( Reference Adams 62 ). The rise in ∆15N levels in the protein of the tibialis anterior muscle after prolonged HF exposure could therefore result from an increased anabolic use and/or a decreased catabolic use of AA in this tissue. Such a reduction in the relative allocation of AA to oxidation in specific muscles may contribute to the elevation of circulating levels of branched-chain AA and some other AA during obesity and insulin resistance.
Regarding the small intestine, its increased ∆15N levels after prolonged HF nutrition – during both the perinatal and post-weaning periods – indicated a higher pox and thus a weaker preferential anabolic orientation of its AA metabolism. There is a paucity of data regarding the effect of HF feeding on intestinal protein metabolism. In a mouse model of obesity, some cellular changes have recently been observed in the small intestine epithelium that might impair the synthesis of proteins, and particularly those involved in the gut barrier function( Reference Nakadate, Motojima and Hirakawa 63 ). Interestingly, the similarity between the ∆15N profiles of proteins in the small intestine and faeces reflected the important contribution of enterocyte shedding and intestinal mucins to the N flux entering the colon, and suggests that faeces might be considered as a more accessible proxy for the small intestine when seeking to determine isotopic biomarkers of diet-induced metabolic orientations.
Apart from the above-mentioned effects of the dietary group on ∆15N values in some tissues, we found significant correlations between the ∆15N values in other tissues and certain indicators of their protein anabolism efficiency, which depends on their efficiency of AA anabolic use for protein synthesis v. catabolic use for oxidation (pox). In particular, Δ15N levels in liver protein were negatively correlated with liver protein anabolism efficiency, and Δ15N in gastrocnemius muscle protein was positively correlated with lean mass, which is known to increase in line with muscle mass and muscle protein anabolism efficiency. Such inverse associations between protein Δ15N and efficiency of AA anabolic (v. catabolic) use in liver and muscle were in line with our previous model predictions, and resulted from the opposite signs of isotopic fractionation associated with AA catabolism in liver and muscle( Reference Poupin, Mariotti and Huneau 31 ). The negative association between liver protein ∆15N and anabolism efficiency had previously been confirmed experimentally in a metabolic tracer study in ruminants, where we have shown that the ∆15N of liver-synthesised plasma proteins increases linearly with liver AA oxidation and urea production( Reference Cantalapiedra-Hijar, Ortigues-Marty and Sepchat 29 ), but the present study is the first to have demonstrated a positive association between muscle protein ∆15N levels and lean mass. Our results therefore indicate that in different splanchnic and peripheral tissues Δ15N could be used as a valuable biomarker of protein anabolism in the considered tissue.
Strengths and limitations of the present study
By using a new isotopic approach consisting in analysing the natural abundances of 13C and 15N in tissue proteins, we were able to unveil the effects of HF feeding on the nutrient routing to tissue AA and on the relative metabolic use of tissue AA for oxidation v. protein synthesis (pox), respectively. The change in lipid content between diets was confounded with a change in CHO content and profile, so that the HF diet was enriched in fat but also in sucrose compared with the NF diet, a classic dietary pattern for mimicking the contrasted profiles of energy intake in humans while being able to induce obesity and insulin resistance with the fat- and sucrose-enriched diet( Reference Yang, Miyahara and Takeo 64 , Reference Wong, Chin and Suhaimi 65 ). During our nutrient routing estimation by 13C data analysis, for identifiability reasons, the protein routing and trophic steps were assumed to be equal across the diets despite their different compositions. An identical protein routing between diets is very likely given their identical protein content, inasmuch as protein routing has been found notably stable – about 75 % – under a broad range of dietary compositions, and our present estimate for protein routing was indeed strictly in line with these previous estimates( Reference Fernandes, Nadeau and Grootes 43 , Reference Kurle, Koch and Tershy 44 , Reference Hobbie 49 , Reference MacAvoy, Arneson and Bassett 56 ). In contrast, an identical trophic step between diets of different CHO and lipid contents and profiles is a more questionable simplifying assumption that could have biased our estimates for the CHO and lipid routings, but probably only marginally. Indeed, numerically, our estimate for trophic step only marginally affected the CHO and lipid routings calculated in Equations (7) and (8) (e.g. a 25 % variation in the trophic step value used in these equations resulted in an absolute difference of <1 % in the calculated CHO and lipid routing values). Our routing estimates for erythrocytes may have also been biased because of incomplete isotopic turnover in this tissue, but only marginally as the erythrocytes isotopic values should have reached 80–90 % of their final equilibrium values at 100 d, when considering that 130–180 d are required for complete isotopic turnover in this tissue( Reference Poupin, Mariotti and Huneau 31 , Reference Kurle 55 ). A last limitation relates to the interpretation of the diet-induced ∆15N differences in terms of pox, which was based on previous modelling and experimental findings rather than by a devoted modelling analysis of the present data set, because the data set at hand was insufficient for being analysed by such a complex multi-compartmental model as the one we previously developed( Reference Poupin, Mariotti and Huneau 31 ).
In conclusion, our results show that HF feeding increases both dietary fat deposition in adipose tissues and dietary fat routing to the protein of all tissues via AA synthesis from tricarboxylic acid cycle intermediates. In addition, our ∆15N data also support HF-induced changes to the relative orientation of AA metabolism towards oxidation v. protein synthesis in three tissues, with more oxidation in the small intestine but less in the tibialis anterior muscle and adipose tissue.
Acknowledgements
The authors thank N. Khodorova for her technical assistance in isotopic measurements, and Dr M. Sebilo and V. Vaury (UMR 7618 BIOEMCO, Université Pierre et Marie Curie, Paris, France) for supplying the internal standard for isotopic measurements.
O. L. M. was supported by a PhD grant from the doctoral school ABIES (ED 581).
The authors’ contributions are as follows: H. F. and J.-F. H. formulated the research question in this ancillary study derived from a larger experiment designed by S. P. and D. Z. S. P. conducted the animal protocol and tissue sampling, O. L. M. conducted the tissue preparation and isotopic analysis and H. F. and N. P. conducted the isotopic data modelling and analysis in collaboration with J.-F. H. and F. M. H. F., J.-F. H., O. L. M. and F. M. analysed and interpreted the data. All authors were involved in the preparation of the manuscript and approved the final manuscript. H. F. had primary responsibility for the final content.
The authors declare that there are no conflicts of interest.
Supplementary Material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518000326








