To the Editor—A growing body of evidence has highlighted the role of novel decontamination techniques in augmenting routine cleaning to reduce surface bacterial counts in hospitals. Most studies undertaken are quasi-experimental or have a ‘before-and-after’ design, which limits objective critical appraisal and meta-analysis. Reference Marra, Schweizer and Edmond1,Reference McDonald and Arduino2 Our research group designed a protocol for a 2-treatment, repeated crossover study over 2 years targeting near-patient sites in an intensive care unit (ICU) to evaluate a novel intervention, namely a custom-built handheld cold-air plasma (CAP) device. Herein, we share our experience developing the research methodology and the initial challenges we encountered.
After discussions with staff, it was clear that for this study to be sustainable, it would need to be minimally disruptive to patient care, it could not impede professional activities, and it could not rely in any way on busy ICU staff. Patients in ICUs are known to receive more hands-on interventions from healthcare workers than patients in other areas of the hospital, Reference Schenk, Schleyer, Jones, Fincham, Daratha and Monsen3 and this was evident to the researchers observing the workings of the unit. Identifying consistent windows of time when the intervention could be tested and when surface sampling could be undertaken was paramount to the study design.
Previous work in vitro has shown that a CAP device can deliver a 6-log reduction in surface bioburden in 90 seconds. Reference Cahill, Claro and O’Connor4 The objectives of our study were to show, among other things, a 2-log reduction in surface bioburden with the plasma, delivered over 30 seconds. Surface sampling was undertaken using Petrifilm Aerobic Count Plates (3M, St Paul, MN), and the results expressed in colony forming units (CFU). The sampling regimen required us to have access to patient rooms for ~5 minutes for sampling, with an additional 15 minutes to deliver the CAP intervention, the prototype of which is currently under development.
Our critical care unit has 12 beds, 4 of which are in isolation rooms rather than the open multibed area (Fig. 1). These 4 rooms have been shown to be more contaminated than surfaces in the open-ward area Reference McDermott, Skally, O’Rourke, Humphreys and Fitzgerald-Hughes5 and are also designated care areas for patients colonized with multidrug-resistant organisms. The focus of our sampling was the bed frame Reference Boyle, Kearney, Carling and Humphreys6 within these 4 isolation rooms, which was divided into zones of separate sampling sites (Fig. 2).

Fig. 1. Diagram indicating location of study rooms within our intensive care unit. Rooms included in the study are within the rectangle outlined by the dashed red line.
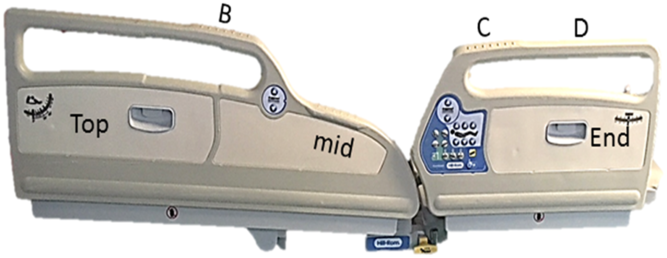
Fig. 2. Zoned sampling sites on bed frame.
A 7-day pilot of the sampling regime was undertaken by a research nurse and a translational postdoctoral microbiologist to evaluate the feasibility of our protocol and to build rapport with clinical staff in the ICU. Each day, we attempted to access all 4 isolation rooms at 09:00, 11:00, and/or 14:00 hours. We made 79 separate room-access attempts that generated 256 samples. The median contamination level observed at all sites was 1.10 CFU/cm2 (interquartile range, 0.35–3.1 CFU/cm2). A hygiene standard has been proposed such that the recovery of >2.5 CFU/cm2 from a hospital surface constitutes a hygiene failure. Reference Mulvey, Redding and Robertson7 Applying this standard to our results, 71% of bedrail surfaces of occupied beds sampled harbored >2.5 CFU/cm2 and thus failed to meet the standard. Thus, targeting such sites with a cleaning intervention was appropriate and worthwhile. Contamination levels did not differ significantly over sites of different texture or based on whether the rail sampled was on the left of right of the patient, so our target sampling sites did not change following this exercise. However, integrating our study into the workings of an ICU required flexibility in our sampling approach.
In 13 of 79 access attempts (16%), we were unable to enter isolation rooms or undertake sampling due to the absence of a bed due to transfer to the operating theatre or radiology (n = 7) or a bedside procedure being undertaken (n = 3). We were advised by nursing staff on 3 occasions that sampling was not appropriate at that time because the patient was in the final hours of life, when clinical priority shifted to comfort and facilitating time with loved ones. Although the highest levels of surface contamination were measured at 14:00 hours, room access at this time was challenging because visiting hours occurred from 13:00 to 15:30, even though we were able to reduce the time spent sampling from 5 to 3 minutes.
Critical care patients have considerable healthcare needs with rapid and or unpredictable requirements for intervention from healthcare workers, none of which can be impeded by research. Admission to the ICU is a significant life event for patients and is distressing for their loved ones. Undertaking research in any clinical area such as in an ICU requires cognizance of the delicate task of meeting research objectives without adversely impacting or interrupting clinical care, interfering with patient rest or visiting time, or impeding the professional activities of healthcare colleagues. Despite the sincere aims of researchers to positively impact on patient care, they must develop and implement sustainable study protocols that do not inconvenience staff or patients. Researchers must consider consultation with ICU staff, honest assessments of feasibility, and an awareness of the unmeasurable interruptions to the clinical environment when designing studies set in ICUs or other critical care environments. By viewing patients, their families, and hospital staff as stakeholders to research undertaken in the space where care is provided, realistic and feasible studies can be optimized to reflect real-life conditions.
Acknowledgments
We thank the patients and staff for their cooperation, and patience.
Financial support
This work was supported by a grant from the Health Research Board, Ireland (grant no. HRA-D12015-1141).
Conflicts of interest
H.H. is in receipt of research funds from Astellas and Pfizer and has received a consultancy fee from Pfizer (Ireland) in the past 5 years. R.S. taught a course that received funding from Daiichi Sankyo, with no personal renumeration. P.C. has issued and licensed patents with Ecolab. All other authors have no conflicts of interest to declare.




