INTRODUCTION
Volumetric modulated arc therapy (VMAT) is now being employed more commonly to treat patients with head and neck cancers. The main attractive characteristic of VMAT is its ability to deliver treatment in a relatively short period of time and less monitor units compared to static beam intensity modulated radiation therapy (IMRT) or conventional three-dimensional radiotherapy (3DCRT).Reference Verbakel, Johan and Suresh1, Reference Lu, Cheng and Kuo2 Furthermore, VMAT produces slightly superior plan quality in terms of both target volume coverage and sparing of organs at risk (OARs).Reference Lu, Cheng and Kuo2
Several studies have reported clinical and dosimetric advantages of VMAT over IMRT.Reference Verbakel, Johan and Suresh1, Reference Suresh3–Reference Quan, Li and Li5 The analysis from these studies is based on anatomical characteristics from the pre-treatment computerised tomography (CT) images, which could change throughout the duration of treatment due to weight loss/gain or internal organ motion. Thus, the impact of these changes on VMAT plans for patients with locally advanced head and neck cancer is not well known. In particular, a common occurrence for these patients is weight loss due to dysphagia as a result of treatment toxicities. The impact of weight loss on VMAT has not been well established. Wei Wang et al.Reference Wang Wei, Yang and Hu6 investigated the need for adaptive radiotherapy before the 25th fraction for patients with nasopharyngeal carcinoma. Their results indicate that adaptive radiotherapy can prevent potential under-dosage of the target volumes. Furthermore, dose to the OARs was significantly reduced with adaptive re-plan. Other studies have observed large variations of dose to the spinal cord during a course of radiotherapy.Reference Hen, Chen, Liu, Schultheiss and Wong7 One of the main limitations of the article by Wei Wang et al.Reference Wang Wei, Yang and Hu6 is that a single repeat CT scan at fraction 25 was used to evaluate the impact of weight loss. It is possible that the impact of weight loss is significant prior to that. Bhide et al.Reference Bhide, Davies and Burke8 suggests that significant weight loss can be observed during the first week of treatment. Thus, routine imaging using image-guided radiation therapy (IGRT) and more frequent re-scan is more feasible and can be used to analyse data so that an accurate dose is delivered to the planning target volumes (PTVs).
Weight loss can also impact clinical outcome. Chen et al.Reference Chen, Daly and Cui9 evaluated 317 patients treated using IMRT with or without adaptive radiotherapy. Online correction using IGRT before each fraction was used and significance of any weight loss was evaluated by the clinician. The results indicate that the survival rates were 73 and 79% among patients treated with and without adaptive radiotherapy respectively. Also, loco-regional control was achieved better with adaptive radiotherapy. Based on the evidence from previous studies on adaptive radiotherapy, it may be beneficial investigating the impact of weight loss in modalities such as VMAT.
In this study, we evaluated the dosimetric impact of weight loss on VMAT and IMRT plans. Our analysis focused on three OARs; the spinal cord, brainstem and bilateral parotid glands. In addition, the feasibility of adaptive radiotherapy using cone beam computed tomography (CBCT) and its impact on the patient, as well as the departmental workflow were investigated.
MATERIALS AND METHODS
Patient selection and treatment planning
Previously treated IMRT plans for ten patients with locally advanced head/neck cancers were analysed. The diagnosis for these patients was oropharynx, larynx, base of tongue and tonsil. All of these patients had bilateral neck nodal involvement and experienced some weight loss during treatment. All patients were scanned on a Siemens Somatom Sensation 16 (Siemens Medical Solutions, Forchheim, Germany) and treated with Elekta Infinity with an agility head (Elekta, UK).
The spinal cord was contoured from the level of the foramen magnum superiorly at the base of brainstem to 1·2 cm inferior to the PTV. Other structures contoured include the oral cavity and the larynx. A VMAT plan was produced for all of the previous IMRT plans using Pinnacle planning software version 9·0 (Philips, Fitchburg, WI, USA). The dosimetric data that were collected and analysed include; max point dose to planning at risk volumes for spinal cord and brainstem (SC+0·5 cm and BS+0·5 cm, respectively) as well as mean dose to the right and left parotid glands. All the patients were planned using both techniques and compared to the cone beam scans that was taken at verification on the treatment machines. Table 1 shows the departmental tolerance doses for the critical structures analysed during VMAT optimisation for all head and neck. The tolerances used based on the departmental protocol were similar to the QUANTEC recommendations.Reference Kirkpatrick, van der Kogel and Schultheiss10
Table 1 Organs at risk dose constraints used in treatment planning

Abbreviations: SC, spinal cord; BS, brainstem; Max, maximum; cc, cubic centimeters.
* QUANTEC recommendations.
Imaging protocol and re-planning
The imaging protocol in the department was to perform CBCT for the first three fractions then weekly, provided that the set up errors are within tolerance. Occurrence of weight loss is investigated when there is a discrepancy between the external contour of the CBCT and the pre-treatment CT. If the cone beam structure sets were more than 2% out of tolerance compared to the primary dataset structures then a re-plan was warranted based on the departmental protocol. Images are then sent to the planning software where automatic segmentation is used to fuse the CBCT images with the pre-treatment CT. In this study, only one re-plan was done for each patient selected. The external contour of the CBCT is used as the new external and tissue outside of this contour is given a density of 0. Beams are then computed and data collected accordingly.
Statistical analysis
Descriptive statistics were used to describe the differences in dose to the SC, BS and parotid glands for the IMRT and VMAT plans. A paired student t-test was used to determine the mean percentage changes between the IMRT and VMAT. The significance level used was 5% for the two-tailed test conducted using Excel 2010.
RESULTS
Spinal cord doses
The DVH indices were analysed for the spinal cord planning risk volume (SC+0·5 cm), brainstem planning risk volume (BS+0·5 cm), and bilateral parotid glands. Table 2 shows the percentage change in max point dose to SC+0·5cm and BS+0·5 cm for IMRT and VMAT. In addition, Figure 1a shows the percentage change data for the spinal cord planning risk volumes. The mean difference in dose to the SC+0·5 cm was 1·03 and 1·19% for the IMRT and VMAT plans respectively (p=0·135).

Figure 1 (a) Percentage change in max point dose to SC+0.5cm for IMRT and VMAT. (b) Percentage change in max point dose to BS+0.5cm for IMRT and VMAT.Abbreviations: IMRT, intensity modulated radiation therapy; VMAT, volumetric arc therapy; SC, spinal cord; BS, brainstem.
Table 2 Percentage change in max point dose to SC+0.5 cm and BS+0.5 cm for IMRT and VMAT
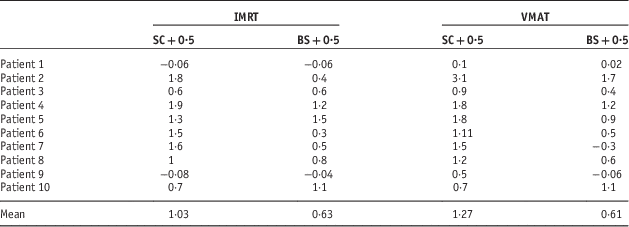
Abbreviations: IMRT, intensity modulated radiation therapy; VMAT, volumetric arc therapy; SC, spinal cord; BS, brainstem.
Patient 2 had particularly interesting results. The weight loss was so severe that a new scan (new immobilisation mask as well) and a re-plan was needed. The extent of weight loss was clearly visible on the CBCT. The concern for this patient was the increase dose to the spinal cord which was 1·9% for the IMRT. If this patient was treated with VMAT, the increase in dose to the spinal cord would have been higher at 3·1%.
Brainstem doses
Figure 1b shows percentage difference in dose to the BS+0·5 cm in both IMRT and VMAT plans for all ten patients. As for dose to the BS+0·5, the mean percentage difference was 0·63% for the IMRT plans and 0·61% for the VMAT plans (p=0·895). There were variations in the percentage changes in the doses in all the patients. For patient number 2 VMAT plan showed a significant increase in the dose to the brainstem.
The parotid gland doses
Figures 2a and 2b show the results from the analysis of the mean parotid doses for all the ten patients. For patient number 5, the disease had invaded the left parotid and, therefore, was not contoured. The results show that the percentage change in mean dose to right parotid was −8·0% whereas that of the left parotid was −6·4% for the IMRT treatment plans. The results for IMRT and VMAT were comparable in both the right and left parotid glands depending on the site of the primary tumour (p=0·0266 and 0·605, respectively).

Figure 2 (a) Percentage change in mean dose to the Lt Parotid for IMRT and VMAT. (b) Percentage change in mean dose to the Rt Parotid for IMRT and VMAT.Abbreviations: IMRT, intensity modulated radiation therapy; VMAT, volumetric arc therapy.
In the VMAT plans, the mean percentages change for the left and the right parotid glands were −6·7 and −6·6%, respectively. Patient number 6 showed significant change (35%) in the mean dose and this was consistent for both IMRT and VMAT. Similarly, patient 2 had significant changes in the dose to the right parotid gland.
DISCUSSION
Impact of the adaptive process on the patient
The impact of adaptive radiotherapy on clinical outcomes is not well known and only a few studies have provided some analysis on this.Reference Schwartz, Garden and Thomas11 The results in this study show the importance of imaging and immobilisation in ensuring that accurate doses are delivered and that the OARs receive doses that closely resemble the planned doses. In addition, they show the impact of weight loss on the predicted doses to the OARs in treatment planning. In most cases weight loss will result in increased dose to the OARs which might impact the quality of life for the patient if a re-plan is not done. Differences noted in the doses received by the OARs after re-planning with VMAT and IMRT demonstrate the importance of adaptive head and neck radiotherapy. Clinically significant changes in the doses to the parotid glands were noted in patient 2 and patient 6. This is possible in tumors close to the parotid gland where significant response to radiation may occur. Adaptive radiotherapy will benefit patients with gross lymphadenopathy compared to those without.
The perceived benefits of adaptive radiotherapy in ensuring accurate dose delivery to the tumour and improved OAR sparing need to be complemented by accurate treatment set-ups.Reference Schwartz, Garden and Thomas11 Daily CBCT protocols may be necessary to detect time-trend errors in head and neck radiotherapy. However, there is concern that daily CBCT protocols may increase radiation dose for the patient.Reference Kan, Leung and Wong12 This is a somewhat controversial issue since the need for precise set-up and accurate delivery of the treatment needs to be weighed against radiation dose associated with frequent imaging.Reference Al-Wassia and Constantinescu13
Clinical implications
The technical advances from 3D conformal to IMRT and VMAT have resulted in increased normal tissue sparing, especially the spinal cord and the parotid glands.Reference Jeong, Lee and Kwak14 The results in this study show that there is potential to reduce parotid gland doses in adaptive radiotherapy. The most common side effect in head and neck patients, especially from 3DCRT is xerostomia. It is prudent to utilise the full benefit of VMAT and IMRT to spare these OARs during radiotherapy.
In addition to the need to keep the OARs dose low. There is a need to ensure an accurate set-up is achieved. Weight loss in head and neck patients can change the location of the isocentre within the patient and may result in increased dose to the OARs. A large set-up variation has been reported for head and neck patients and the use of multiple regions of interest for image matching is advised.Reference van Kranen, van Beek and Rasch15 Also, correction of rotational errors may play a significant part in reducing OAR dose. Den et al.Reference Den, Doemer and Kuibiek16 reported a statistically significant difference for rotational errors between inter-fraction and residual errors. Therefore, frequent use of CBCT IGRT is feasible and required for accurate analysis of weight loss and is essential for a successful implementation of adaptive radiotherapy.
Impact on workflow
A successful implementation of adaptive radiotherapy requires a rigorous process and well established policy to assess the need for a re-plan. The main impact of the adaptive process on the clinical department is the significant increase of workload. The basic workflow for image-guided adaptive radiotherapy is to co-register the CBCT from the treatment with the original CT and then monitor any changes to the anatomy which may warrant a re-plan. Using manual segmentation for fusing the CBCT images with the pre-treatment CT is time consuming and may be susceptible to inter-observer variation. Furthermore, manual contouring by the physician is time consuming and puts a strain on practical application of adaptive radiotherapy.Reference Chao, Bhide and Chen17 As such, deformable auto-segmentation registration of images has been mentioned as an effective alternative.
Several algorithms have been written to achieve a correct auto-segmentation of CBCT with the planning images. For example, Zhen et al.Reference Zhen, Gu, Yan, Zhou, Jia and Jiang18 proposed an algorithm called deformation with intensity simultaneously corrected. The algorithm basically applies an intensity correction step on the CBCT at every iteration of the registration process. The authors show that this algorithm is robust against CBCT image artefacts and improves the registration accuracy. This article is mainly a theoretical one and as such the dosimetric impact of this algorithm is not investigated. In addition, only six clinical patient data was used to evaluate the performance of this algorithm.
Alternatively, Tsuji et al.Reference Tsuji, Hwang and Weinberg19 conducted a dosimetric evaluation of auto-segmentation using intensity based free form registration algorithm. This study used 16 clinical plans and dose to the target volume as well as OARs structures were evaluated. The authors conclude that their registration method is not robust enough to replace physician drawn volumes for the target structures. However, OAR contours of a sufficient accuracy were produced when assessed by dosimetric end points. One of the limitations of the algorithm mentioned in the article is that it does not accurately register images when there is disparate patient position. Also, the cohort of patients used in the study exhibit large anatomical changes and is not representative of a typical patient. Most patients experience subtle changes to their anatomy. Other methods of image registration have also been mentioned, but no gold standard has yet to be found.
Recommendations
1. A well-defined protocol for a re-plan will provide some order to the adaptive process.
2. In-house studies in adaptive radiotherapy may assist in the development and implementation of future protocols for head and neck adaptive radiotherapy.
3. The authors advice that staffs are properly trained and quality assurance checklist along each step in the workflow is implemented to minimise the occurrence of deviations in both IMRT and VMAT techniques.
Limitations
The dosimetric analysis was limited to analysis of the OAR doses without analysis of impact on the planning target volumes (PTV). In addition to the small number of patients and other methodological limitations mentioned in the discussion, the analysis in this article was based on self reporting of deviations which may be inexact and could result in significant under-reporting.
CONCLUSION
This study shows clinically significant impact of weight loss on DVH indices analysed in head and neck OARs. The impact of weight loss is greater on VMAT plans compared to IMRT with regards to the spinal cord dose. One of the major impacts of the adaptive process on the patient is the increased dose resulting from more frequent imaging. Literature shows that the advantages of adaptive radiotherapy outweigh the dose contribution from CBCT. Some authors have reported the potential benefit of adaptive radiotherapy with respect to clinical outcome, and they have shown favourable results.
Therefore, clinical departments may need to consider adaptive radiotherapy to improve the quality of life in head and neck patients. In addition, there is a need to establish robust process and quality assurance mechanisms in order to cope with the increased workload demand as well as minimise errors. The use of automated independent checks as well as proper training of staff members would lessen the burden of the increased workload and potentially lead to a decrease in errors.
Acknowledgements
The author acknowledges the manager at the Christie NHS foundation trust and colleagues for their support.
Financial support
This research received no grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.






