Introduction
Canine parvovirosis is a very contagious, severe and often lethal infectious disease which occurs in both domestic and wild dogs [Reference Kelly1, Reference Appel, Scott and Carmichael2]. Canine parvovirus type 2 (CPV-2), the aetiological agent of canine parvovirosis, belongs to the Parvoviridae family, Parvovirinae subfamily and it is included in Carnivore protoparvovirus 1 species together with Feline parvovirus, Mink enteritis virus and Raccons parvovirus [Reference Decaro and Buonavoglia3]. CPV-2 is a small (25 nm diameter) non-enveloped virus whose capsid is constituted by three major proteins surrounding a single-stranded linear DNA genome [Reference Decaro and Buonavoglia4]. Commonly, CPV-2 infects 4–12 weeks old pups, especially during the decline of maternally derived antibodies (MDA) [Reference Greene, Decaro and Greene5]. Generally, adults are resistant to CPV-2 infection due to reduced susceptibility or presence of specific immunity induced either by vaccination or previous infections [Reference Decaro and Buonavoglia4]. The main route of transmission is the oronasal through direct or indirect contact with the faeces of infected dogs or contaminated fomites; indirect contact is facilitated by the environmental resistance of the virus.
Using a very sensitive real-time PCR, the CPV-2 DNA was detected in faeces of infected pups for 46 days and this prolonged shedding contributes to environmental contamination [Reference Decaro6]. The virus is rapidly inactivated (1–2 min) at 100 °C while resists up to 7 h at 80 °C and 72 h at 56 °C; in addition, CPV-2 resists for 2 weeks at 37 °C and for more than 6 months at room temperature [Reference McGavin7].
CPV-2 is resistant to most disinfectants while is sensitive to formalin, β-propiolactone, hydroxylamine, oxidizing agents, halogens, aldehydes and sodium hydroxide [Reference McGavin7, Reference Scott8]. Among the halogens, sodium hypochlorite is often employed to decontaminate veterinary clinics and animal housing facilities, due to its broad spectrum of activity against several infectious agents [Reference Oie9]. Sodium hypochlorite is also easy to use and not expensive although several factors influence its efficacy.
Molecular biology techniques [Reference Decaro10] are able to detect low titres of CPV-2 in asymptomatic dogs shedding the virus in their faeces [Reference Decaro6]; this is useful in order to adopt adequate disinfection protocols in kennels and shelters where CPV-2 is responsible for severe outbreaks [Reference Decaro and Buonavoglia4]. On the other hand, tests that measure the infectivity of contaminating CPV-2 are also needed to evaluate the efficacy of disinfectants. The aim of the present study was to determine the effect of contact time, concentration and presence of organic matter on the virucidal activity of sodium hypochlorite solutions against several CPV-2 strains in vitro.
Methods
Cells
Virus propagation, antiviral assays and indirect immunofluorescence (IF) tests were performed on canine A-72 cell line grown at 37 °C with 5% CO2 in Dulbecco's minimal essential medium (DMEM) supplemented with 10% foetal calf serum.
Viruses and titration
Three CPV-2 strains were employed in the study: strain 2a 192/98 [Reference Cavalli11]; strain 2b 29/97 [Reference Buonavoglia12]; strain 2c 136/2000 [Reference Buonavoglia13]. The viruses were propagated on A-72 cells in order to obtain stock viruses for the subsequent experiments. Each stock virus was titrated on A-72 cells by an IF assay. Briefly, after an incubation period of 3 days at 37 °C, the infected cells were fixed with cold acetone and tested using CPV-2-specific canine antibodies and rabbit anti-dog IgG conjugated with fluorescein isothiocyanate (Sigma Aldrich Srl, Milan, Italy). Viral titre, determined on A-72 cells, was 105.5 tissue culture infectious doses (TCID50)/ml for strain 2a, 106 TCID50/ml for strain 2b and 105.25 TCID50/ml for strain 2c.
Sodium hypochlorite
A commercially available sodium hypochlorite stock solution 14–15% was used throughout the study and it was diluted with sterile-distilled water to the final concentration.
Citotoxicity assay
Cytotoxicity of the sodium hypochlorite solutions employed in the study was assessed using the In Vitro Toxicology Assay Kit (Sigma Aldrich Srl, Milan, Italy), based on 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (XTT). Toxicity was measured on fresh confluent A-72 cells in 96-well microtitre plates [Reference Lanave14]. Briefly, three sodium hypochlorite solutions (0.75%, 0.37% and 0.18%) were tested on A-72 cells in three separate experiments. A XTT stock solution was added and the plates were incubated at 37 °C for 72 h. The microtitre plates were read with an automatic spectrophotometer (microtitre plate reader Bio-Rad 680, Bio-Rad Laboratories S.r.l., Segrate, Italy) at 450 nm. Each experiment included blank wells containing complete medium without cells and control wells containing untreated cells.
Citotoxicity was calculated as follows:
Virucidal activity assays
The CPV-2 suspensions were mixed with an equal volume of sodium hypochlorite solution (1.5%, 0.74% and 0.36%) to obtain a final concentration of 0.75%, 0.37% and 0.18%. After 1, 5 and 15 min at room temperature, aliquots from the individual mixtures were titrated with the IF test. Preliminary experiments established that incubation times below 1 min (from 10 to 60 s) were too short to appreciate any virucidal activity of the three sodium hypochlorite solutions and contact times above 15 min were unable to increase the virucidal power any further. The IF test was performed as reported previously [Reference Desario15]. Briefly, 10-fold dilutions of the virus-disinfectant mixtures were inoculated, in quadruplicate, on freshly trypsinised A-72 cells, using 24-well plates containing slides, and then incubated for 72 h at 37 °C with 5% CO2. Controls wells consisted of cells inoculated with the viral suspension without sodium hypochlorite. Viral titres were expressed as the highest dilution resulting in positive IF on A-72 monolayers.
Virucidal activity in the presence of organic matter
Two types of experiments were performed to evaluate the interfering effect of organic matter on the virucidal activity of sodium hypochlorite. In the first set of experiments, DMEM containing 3% bovine albumin and 3% porcine red blood cells was employed to dilute the viral suspensions according to the European Regulations EN14476 [16]. For the second set of experiments, suspensions containing 6% of faeces (tested negative for CPV-2 by real-time PCR) and each strain of CPV-2, were employed to test the virucidal activity of sodium hypochlorite. In both settings, i.e. in the presence of organic matter or faeces, the virucidal effect of the three sodium hypochlorite solutions (0.75%, 0.37% and 0.18%) was evaluated after 1, 5 and 15 min as described above.
Data analysis
Three independent experiments were performed with each strain of CPV-2 and one representative set of data was shown for each strain. Student's t test was used to evaluate statistical differences which were considered significant when P was <0.05.
Results
Citotoxicity assay
The cytotoxic activity of the three sodium hypochlorite solutions was evaluated after 72 h incubation by microscopic examination of cell morphology and measurement of cell viability using the XTT colorimetric assay. The three solutions tested did not cause any morphological change nor reduced the viability of A-72 cells (data not shown).
Virucidal activity of sodium hypochlorite solutions
Results obtained with three different strains of CPV-2 are reported in Table 1 (strain 2a), Table 2 (strain 2b) and Table 3 (strain 2c). Data were similar for all strains employed, i.e. the highest concentration of sodium hypochlorite (0.75%) exhibited a significant virucidal activity when compared with control cultures regardless of the contact time (reduction of 5 log10 of virus titre, P < 0.05; Tables 1–3). On the other hand, the intermediate concentration (0.37%) showed a significant virucidal activity after 1 or 5 min (reduction of about 4 log10 of virus titre, P < 0.05), and after 15 min of contact, it was as efficient as the highest concentration (reduction of 5 log10 of virus titre, P < 0.05; Tables 1–3). The lowest concentration of disinfectant, i.e. 0.18%, did not exhibit any virucidal activity at any time point tested on any CPV-2 strain (Tables 1–3).
Table 1. Virucidal activity of sodium hypochlorite solutions against CPV-2a 192/98 in the presence or in the absence of organic matter
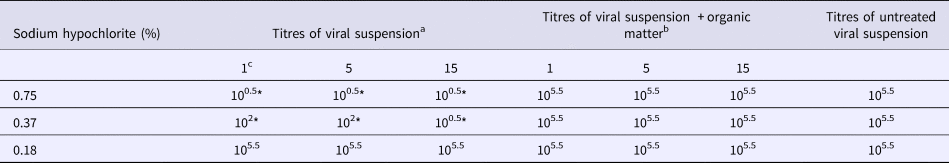
*P < 0.05 vs. untreated virus.
a TCID50/ml (log10).
b TCID50/ml (log10) in the presence of bovine albumin and porcine red blood cells.
c Contact time (minutes).
Table 2. Virucidal activity of sodium hypochlorite solutions against CPV-2b 29/97 in the presence or in the absence of organic matter
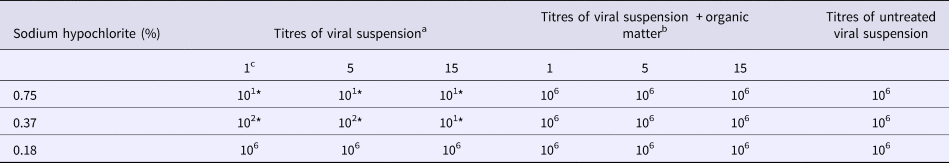
*P < 0.05 vs. untreated virus.
a TCID50/ml (log10).
b TCID50/ml (log10) in the presence of bovine albumin and porcine red blood cells.
c Contact time (minutes).
Table 3. Virucidal activity of sodium hypochlorite solutions against CPV-2c 136/00 in the presence or in the absence of organic matter

*P < 0.05 vs. untreated virus.
a TCID50/ml (log10).
b TCID50/ml (log10) in the presence of bovine albumin and porcine red blood cells.
c Contact time (minutes).
Effect of organic matter on the virucidal activity of sodium hypochlorite solutions
The interference played by organic matter, i.e. albumin and porcine red blood cells, on the virucidal activity of the three sodium hypochlorite solutions is reported in Tables 1–3. None of the three solutions showed virucidal activity against the three CPV-2 strains in the presence of organic matter. Indeed, viral titres in each experimental condition were identical to those of controls (i.e. 105.25–6 TCID50/ml; Tables 1–3).
Similar results were obtained when the efficacy of the three sodium hypochlorite solutions was tested in the presence of faecal suspensions containing each CPV-2 strain. No virucidal activity was observed when the sodium hypochlorite solutions were incubated (for 1, 5 or 15 min) with faecal suspensions containing CPV-2 strain 2a (virus titre was 105.5 TCID50/ml both in untreated and in sodium hypochlorite-treated wells), CPV-2 strain 2b (virus titre was 106 TCID50/ml both in untreated and in sodium hypochlorite-treated wells) or CPV-2 strain 2c (virus titre was 105.25 TCID50/ml both in untreated and in sodium hypochlorite-treated wells).
Discussion
Disinfection plans are cost-effective means to prevent and control infectious diseases. Veterinary clinics or animal housing facilities can passively harbour and diffuse infectious agents even for long periods and, if not properly disinfected, may contribute to spreading infectious pathogens.
In kennels and veterinary clinics, in order to maintain control of CPV-2 infections, both environmental disinfection and strict vaccination programmes are needed. The highest frequency of CPV-2 infections occurs in pups during the wane of MDA [Reference Greene, Decaro and Greene5], both in unvaccinated and in low responders to CPV-2 vaccination. Environmental contamination is still the main risk factor for CPV-2 infection to date, as shown by the occurrence of serious infectious outbreaks even in kennels where an appropriate vaccination programme is applied. Since the persistence of CPV-2 in the environment during interoutbreak periods is responsible for novel outbreaks in newborns, particular attention should be paid to the real efficacy of disinfection protocols. Among the halogens, sodium hypochlorite is useful against parvoviruses, which are resistant to several other disinfectants [Reference McGavin7, Reference Scott8], and it is commonly used to decontaminate clinics and kennels.
In the present study, an IF test was employed to measure CPV-2 titres after sodium hypochlorite treatment and the data indicate that a short contact time, 1 min at 0.75% or 15 min at 0.37%, can significantly reduce the viral load. It was previously reported that a contact time of 30 min was necessary for sodium hypochlorite solutions (0.15%) to inactivate CPV-2; however, haemagglutination test was employed to determine the efficacy of the disinfectant [Reference McGavin7]. The concentrations of sodium hypochlorite solutions employed in the present study were selected according to the previously published recommendations [Reference Dvorak17]; in addition, preliminary experiments were conducted to evaluate a large range of incubation times (from 10 s to 45 min) and to select the best timing for testing the virucidal activity (data not shown). The discrepancy in the optimal contact time and concentration of disinfectant reported in the present study and in previous experiments [Reference McGavin7] could be due to the different tests employed for CPV-2 titration. Since the IF test is capable of detecting even a few infectious viral particles in a sample, it is better suited than other assays to assess the power of a disinfectant. Reducing the contact time to 1–15 min and using concentrations of sodium hypochlorite still very low (0.75–0.37%), and within the recommended range [Reference Dvorak17], are great advantages in routine sanitisation of veterinary clinics and kennels.
Nature of surface, temperature, pH, humidity and water hardness are all environmental factors that can greatly impact the efficacy of a disinfectant [Reference Ewart and Smith18, Reference Greene and Greene19]. In addition, the amount of organic material (i.e. soil, bedding, litter, faeces, blood) on an item, or in areas to be disinfected, can greatly impact the efficacy of disinfectants since it provides a physical barrier that protects microorganisms from, or can even neutralise, the disinfectant. The data presented here confirm the importance of cleaning before disinfection since the presence of organic matter totally abrogated the virucidal activity of sodium hypochlorite solutions against the three CPV-2 strains. Future studies will determine if different temperature ranges, which may occur in kennels, could influence the virucidal activity reported here.
It should be underlined that high concentrations of sodium hypochlorite are corrosive and irritating for mucosae, eyes and skin [Reference Scott8]. In addition, an extended contact time, as well as high concentrations of halogens, increases by-product formation, environment pollution and toxicity for animals and humans. In conclusion, the use of effective concentrations of sodium hypochlorite for a short period of time may contribute to control the contamination with CPV-2 (and thus the frequency of CPV-2 outbreaks) minimizing the environmental impact.
Acknowledgements
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.






