Several recent studies suggest that the incidence of cancer in people with severe mental illness (SMI) may not be elevated, Reference Lawrence, Holman, Jablensky, Threlfall and Fuller1–Reference Kisely, Sadek, MacKenzie, Lawrence and Campbell3 and study results differ concerning cancer stage at presentation as some studies have found only few differences in stage at presentation Reference Chang, Hayes, Broadbent, Hotopf, Davies and M⊘ller4 and some report presentation at a later stage among people with SMI. Reference Kisely, Crowe and Lawrence2 Patients with a psychiatric condition have further been reported to have a reduced likelihood of surgery, and to have received less radiotherapy and fewer chemotherapy sessions. Reference Kisely, Crowe and Lawrence2 Additionally, most groups of people with SMI have been reported to have worse cancer survival not explained by stage at presentation. Reference Lawrence, Holman, Jablensky, Threlfall and Fuller1–Reference Batty, Whitley, Gale, Osborn, Tynelius and Rasmussen5 Similar results have been reported concerning specific cancers, including breast cancer, Reference Cunningham, Sarfati, Stanley, Peterson and Collings6,Reference Goodwin, Zhang and Ostir7 colorectal cancer, Reference Cunningham, Sarfati, Stanley, Peterson and Collings6 oral cancer, Reference Chang, Hou, Su, Chen, Ho and Lee8 pancreatic adenocarcinoma Reference Boyd, Benarroch-Gampel, Sheffield, Han, Kuo and Riall9 and prostate cancer. Reference Safdieh, Schwartz, Rineer, Weiner, Wong and Schreiber10 However, earlier studies are mainly based on regional samples and few of them examine both all cancers and history of psychosis, substance use disorders and mood disorders. The aim of the current study was to assess whether cancer stage at presentation, comorbidities or treatment received had an effect on differences in survival of people with cancer with and without history of mental illness taking into account gender, age and year of cancer diagnosis in Finland in 1990–2013. We hypothesised that (a) patients with SMI have higher case fatality from cancer than patients without SMI, (b) this is particularly true for patients with psychotic and substance use disorders but less pronounced in mood disorders, and (c) this is caused by both presentation at a more advanced stage and less intensive treatment.
Method
Population with a first cancer diagnosis in 1990–2013
The population with their first cancer diagnosis in 1990–2013 was obtained from the National Cancer Registry including all cancer patients in Finland. The data were examined back until 1969 to ensure that no earlier cancer diagnoses could be found. We focused on the four most prevalent cancers, namely cancer of the lung (C34), breast (C50) colon and rectum (C18, C19.8, C20-C21) and prostate (C61). Additionally, cancers were classified with the top ten cancers separately for men and women and other cancers (the rest of the C-diagnoses) were included as one group. Information concerning hospital admissions because of severe mental illness (SMI) before cancer diagnosis was individually linked to the study population from the Hospital Discharge Register for the years 1969–2013. We defined three main categories of mental disorders, namely substance use disorder (ICD-10 code F10–19 and corresponding ICD-9 and ICD-8 codes), 11–13 psychosis (F20, F22–29) and mood disorders (F30–33, F38–39) as the main diagnosis for hospital admission 1 year before the cancer diagnosis or earlier. If an individual had been admitted to hospital more than once and given a diagnosis belonging to more than one SMI category, we hierarchically allocated the individual to the psychosis subpopulation if diagnosed with psychosis at least once. Patients with substance use related and mood disorder related diagnoses were allocated to the substance use group. These individuals remained in the SMI population since their first admission to hospital as a result of the long natural course of these mental disorders. The data linkages were performed by the competent authorities and the study group received anonymised data.
Psychiatric and oncological services in Finland
The Finnish healthcare system is mainly public and financed by taxes collected by the state and the municipalities, including all psychiatric hospital services. Reference Lehtinen and Taipale14 A de-institutionalisation of psychiatric services has occurred in Finland since the beginning of 1980s, with a substantial reduction in psychiatric hospital beds and an increase in out-patient services. Municipalities are responsible for organising out-patient services, which are provided by public, private and third-sector actors. Reference Ala-Nikkola, Sadeniemi, Kaila, Saarni, Kontio and Pirkola15 Services for substance use disorders are provided within both social welfare and healthcare and by municipal, private and third-sector actors. Reference Varjonen16 Although the diagnostic process in cancers usually begins in primary healthcare, and first diagnostic tests are run in primary care, a preliminary diagnosis of cancer leads to referral to specialist care. Reference Hermanson, Vertio and Mattson17 Oncological services in Finland are mainly provided in specialist care organised in 20 hospital districts supplying specialist services for the residents. The hospital districts are managed and funded by the municipalities. The number of private clinics in oncological care is very small, for example in our data 0.5% of patients with cancer had been treated in private clinics.
Covariates in the models
Comorbidity was defined by admission to hospital for particular diseases (congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, liver disease, diabetes with or without chronic complications, hemiplegia or paraplegia, and renal disease) in the Hospital Discharge Register for 2 years preceding cancer incidence using a modified Charlson's comorbidity index. Reference Quan, Sundararajan, Halfon, Fong, Burnand and Luthi18 Cancer stage at presentation was categorised as (a) localised (51% of individuals, overall), (b) metastasised (regional or distant, 33%), and (c) unknown (16%). Cancer treatment was measured by six variables: (a) surgical therapy, (b) radiotherapy (both of which were further categorised as curative or palliative), (c) chemotherapy, (d) hormonal therapy, (e) other, and (f) treatment unknown. Age at presentation was categorised in 5-year age groups and year of cancer diagnosis as a continuous variable. Information concerning mortality was obtained from the Finnish Causes of Death statistics. We examined both cancer-specific and all-cause mortality. Ethical approval for the study was received from the Research Ethics Committee of the National Institute for Health and Welfare.
Statistical methods
We calculated proportions of SMI among incident cancer patients. We then calculated Kaplan–Meier estimates to examine survival differences between patient groups among men and women. Cox regression models were used to study survival differences in four steps to study the impact of covariates on patient group differences and survival in the whole study period. In the first step, we estimated hazard ratios for patient groups adjusting for age, year of incidence from 1990 and cancer type. In the following steps, we further adjusted in the second step for stage at presentation, in the third for cancer treatment, and in the fourth for Charlson's comorbidity index. Generalised R 2 was used as a measure of how well each set of covariates predicts survival. Reference Allison19 We conducted further Cox regression analysis to examine if patient group differences remained the same during the study period by adding the interaction term for incidence year and patient group and extracted average annual change estimates for each of the patient groups. In order to estimate hazard ratios for equivalent potential follow-up in different time points, we adjusted Cox regression models with 5-year follow-up in three cancer incidence periods (1990–1994, 1997–2001 and 2004–2008). SAS version 9.3 (SAS Institute, Cary, NC) was used in statistical analyses of the study.
Results
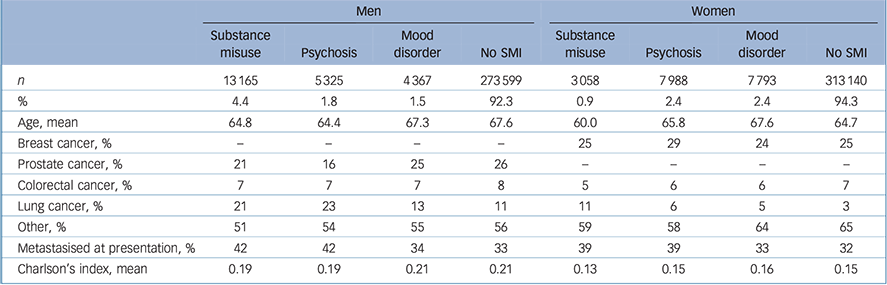
There were altogether 628 435 cases of first cancers in 1990–2013 in Finland. The number of women was larger in the cancer population than that of men, as 53% of our cancer population was female. Among men, the most common SMI was substance use disorder, whereas among women mood disorders and psychoses were more common (Table 1). Patients with a history of substance use disorder were younger than other cancer patients, and together with patients with a history of psychosis more often had a metastasised cancer at presentation among both men and women. Comorbidities were more common among men, but there were few differences between patient groups in Charlson's comorbidity indices.
Table 1 Basic background characteristics of the 1990–2013 population with a first cancer in Finland by gender and history of severe mental illness (SMI)
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Substance misuse |
Psychosis | Mood disorder |
No SMI | Substance misuse |
Psychosis | Mood disorder |
No SMI | |
| n | 13 165 | 5 325 | 4 367 | 273 599 | 3 058 | 7 988 | 7 793 | 313 140 |
| % | 4.4 | 1.8 | 1.5 | 92.3 | 0.9 | 2.4 | 2.4 | 94.3 |
| Age, mean | 64.8 | 64.4 | 67.3 | 67.6 | 60.0 | 65.8 | 67.6 | 64.7 |
| Breast cancer, % | – | – | – | – | 25 | 29 | 24 | 25 |
| Prostate cancer, % | 21 | 16 | 25 | 26 | – | – | – | – |
| Colorectal cancer, % | 7 | 7 | 7 | 8 | 5 | 6 | 6 | 7 |
| Lung cancer, % | 21 | 23 | 13 | 11 | 11 | 6 | 5 | 3 |
| Other, % | 51 | 54 | 55 | 56 | 59 | 58 | 64 | 65 |
| Metastasised at presentation, % | 42 | 42 | 34 | 33 | 39 | 39 | 33 | 32 |
| Charlson's index, mean | 0.19 | 0.19 | 0.21 | 0.21 | 0.13 | 0.15 | 0.16 | 0.15 |
Among men the top-10 cancers were cancer of the prostate (ICD-10 code C61), skin (C44), lung (C34), colon and rectum (C18, C20), cancers of the haematopoietic and reticuloendothelial system (C42), bladder (C67), stomach (C16), pancreas (C25), kidney (C64) and brain (C71). Among women, the top-10 cancers were cancer of the breast (C50), skin (C44), colon and rectum (C18, C20), cervix uteri (C53), corpus uteri (C54), ovaries (C56), lung (C34), cancers of the haematopoietic and reticuloendothelial system (C42), pancreas (C25) and stomach (C16).
The Kaplan–Meier curves in Fig. 1 indicate that cancer-specific mortality was higher among men with a history of psychosis and substance use disorder, whereas there were only small differences between those with a mood disorder history and those without a history of SMI. Among women a stepwise gradient was found from those without SMI through mood disorders and substance use disorders to those with psychosis. Additionally, among both men and women with psychosis and substance use disorders excess mortality was found already, during the first years after cancer diagnosis.
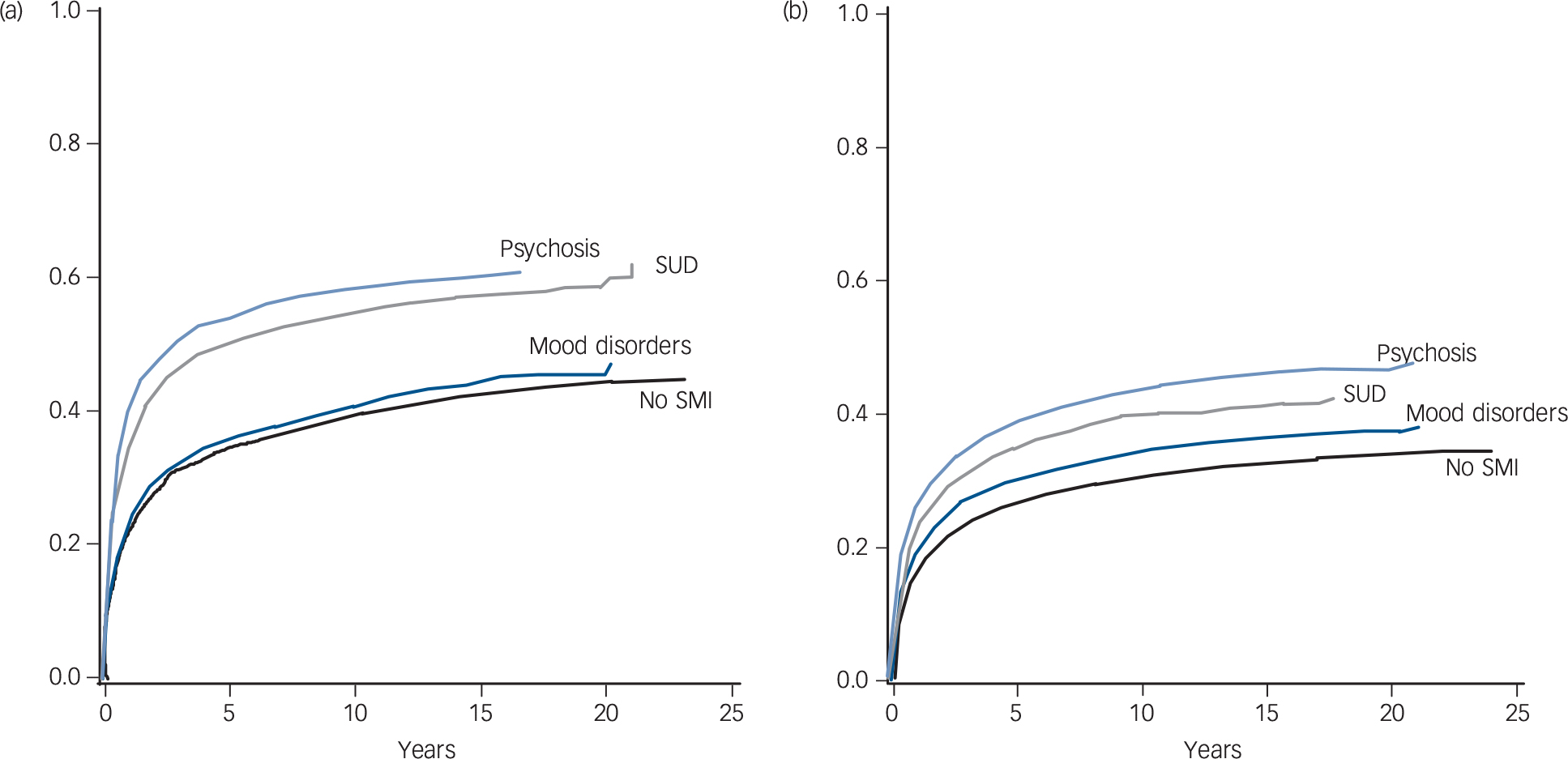
Fig. 1 Kaplan–Meier curves for the distribution of cancer-specific mortality (cumulative distribution function) among Finnish (a) men and (b) women diagnosed with their first cancer in 1990–2013.
SMI, severe mental illness; SUD, substance use disorder.
Table 2 presents the risk of cancer-specific mortality among the four patient groups and the mortality risk when taking into account stepwise age, cancer type and year of cancer diagnosis, cancer stage at presentation, cancer treatment and comorbidities. When taking into account only age, year of cancer diagnosis and cancer type, all groups with a history of SMI had higher mortality risk compared with those without a history of SMI. The excess mortality risk was especially high among those with history of psychosis both among men and among women. Although all the examined factors were important predictors of mortality overall, the only factor that had an effect on differences between patient groups was cancer treatment. Taking into account cancer treatment (model III) decreased the mortality risk among those with a history of psychosis and substance use problems. Findings regarding all-cause mortality were similar (online Table DS1).
Table 2 The effect of a history of severe mental illness on risk of cancer-specific mortality among Finnish men and women in 1990–2013a

| Model I | Model II | Model III | Model IV | |
|---|---|---|---|---|
| Men | ||||
| Substance misuse, HR (95% CI) | 1.38 (1.34–1.41) | 1.38 (1.35–1.42) | 1.31 (1.28–1.35) | 1.31 (1.28–1.35) |
| Psychosis, HR (95% CI) | 1.59 (1.53–1.65) | 1.59 (1.53–1.65) | 1.41 (1.36–1.47) | 1.42 (1.36–1.47) |
| Mood disorder, HR (95% CI) | 1.09 (1.04–1.15) | 1.11 (1.06–1.17) | 1.08 (1.03–1.14) | 1.08 (1.03–1.14) |
| Generalised R 2 | 0.3927 | 0.4662 | 0.5130 | 0.5131 |
| Women | ||||
| Substance misuse, HR (95% CI) | 1.44 (1.36–1.53) | 1.43 (1.34–1.51) | 1.37 (1.28–1.45) | 1.36 (1.28–1.45) |
| Psychosis, HR (95% CI) | 1.64 (1.58–1.70) | 1.61 (1.55–1.66) | 1.47 (1.42–1.53) | 1.47 (1.42–1.53) |
| Mood disorder, HR (95% CI) | 1.11 (1.07–1.16) | 1.12 (1.08–1.17) | 1.10 (1.05–1.14) | 1.10 (1.05–1.14) |
| Generalised R 2 | 0.3720 | 0.4452 | 0.4906 | 0.4906 |
a. People without a history of mental illness used as a reference group. Model I: controlling for age, year and cancer type; model II: model I + stage at presentation; model III: model II + cancer treatment; model IV: model III + comorbidities (Charlson's comorbidity index).
When examining the average annual decrease in cancer-specific mortality, we found that the decrease was 3.1% (95% CI 3.0–3.2) among men and 3.2% (3.1–3.3) among women without a history of severe mental disorders. Among men with a history of psychosis or substance use disorder the decrease was significantly slower; 1.7% (1.1–2.3) and 2.5% (2.1–2.8), respectively. Among women a similar difference was found only among those with history of psychosis; among them the average annual decrease was 2.2% (1.6–2.7).
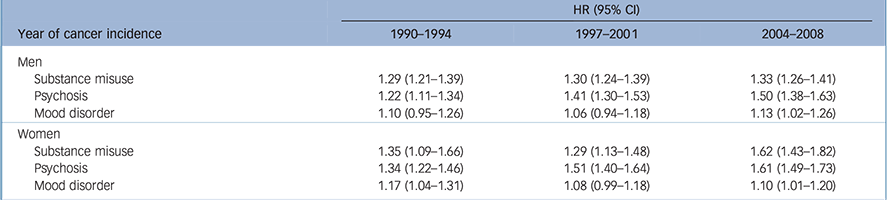
Table 3 presents the 5-year cancer-specific mortality risk of incident cancer patients in three time periods. The interaction between period and SMI type was statistically significant among women (P = 0.0091); among patients with a history of psychosis the relative excess mortality risk (compared with patients with no history of SMI) increased more than among other SMI groups. Among men, the pattern was similar, but the interaction was not statistically significant (P = 0.0649). Cancer-specific mortality risk was higher among patients with a history of psychosis or substance use disorders compared with those without a history of mental disorders in each three periods among men and women. The risk was not consistent among patients with a history of mood disorder, but the risk was significantly higher in 2004–2008 compared with patients without a history of mental disorders.
Table 3 Hazard ratios (HR) and their 95% confidence intervals for 5-year cancer-specific mortality in incident cancer patients with a history of severe mental illness in three time periods in 1990–2013 a
| HR (95% CI) | |||
|---|---|---|---|
| Year of cancer incidence | 1990–1994 | 1997–2001 | 2004–2008 |
| Men | |||
| Substance misuse | 1.29 (1.21–1.39) | 1.30 (1.24–1.39) | 1.33 (1.26–1.41) |
| Psychosis | 1.22 (1.11–1.34) | 1.41 (1.30–1.53) | 1.50 (1.38–1.63) |
| Mood disorder | 1.10 (0.95–1.26) | 1.06 (0.94–1.18) | 1.13 (1.02–1.26) |
| Women | |||
| Substance misuse | 1.35 (1.09–1.66) | 1.29 (1.13–1.48) | 1.62 (1.43–1.82) |
| Psychosis | 1.34 (1.22–1.46) | 1.51 (1.40–1.64) | 1.61 (1.49–1.73) |
| Mood disorder | 1.17 (1.04–1.31) | 1.08 (0.99–1.18) | 1.10 (1.01–1.20) |
a. People without a history of mental illness in each time period used as the reference group, adjusted for age, cancer type, stage at presentation, cancer treatment and Charlson's comorbidity index.
In the group having their first cancer episode in 1997–2001, the mortality risk increased among those with a history of psychosis, both among men (P = 0.0234) and among women (P = 0.0411), compared with the 1990–1994 period. The mortality risk further increased in this patient group among those with their first cancer in 2004–2008 compared with the 1990–1994 period (among men P = 0.0012; among women P = 0.0022). Whereas the hazard ratio also increased among women with a history of substance use problems, the change was not statistically significant.
Discussion
Main findings and comparison with findings from other studies
In this study we examined the effect of a history of SMI on mortality among a total population of people with their first cancer episode in 1990–2013. Our study adds to the literature by examining all cancers and history of psychosis, substance use disorders and mood disorders simultaneously using nationwide register data. In line with earlier research, we found excess mortality especially among individuals with a history of psychotic and substance use disorders. Reference Lawrence, Holman, Jablensky, Threlfall and Fuller1–Reference Batty, Whitley, Gale, Osborn, Tynelius and Rasmussen5 Cancer was more often detected at a metastasised stage in those with a history of psychotic and substance use disorders than in individuals with a mood disorder or no SMI history. However, although stage at presentation was an important predictor of mortality, it had little effect on differences in mortality. In general, earlier studies have reported that patients with mental disorders present at a later stage, receive less treatment in terms of surgery and chemotherapy, and, taking into account stage at presentation, have poorer survival. Reference Lawrence, Holman, Jablensky, Threlfall and Fuller1–Reference Batty, Whitley, Gale, Osborn, Tynelius and Rasmussen5,Reference Chang, Hou, Su, Chen, Ho and Lee8,Reference Boyd, Benarroch-Gampel, Sheffield, Han, Kuo and Riall9 In our study, taking into account differences in treatment did decrease the differences but did not abolish them, and taking into account comorbidity did not have an effect on the differences.
Consistent with many previous studies, psychotic and substance use disorders were associated with larger excess mortality than mood disorders, Reference Kisely, Crowe and Lawrence2,Reference Chang, Hayes, Broadbent, Hotopf, Davies and M⊘ller4 although in some studies mortality risk has been similar in schizophrenia and depressive disorders Reference Batty, Whitley, Gale, Osborn, Tynelius and Rasmussen5 or even higher in mood disorders. Reference Lawrence, Holman, Jablensky, Threlfall and Fuller1,Reference Pinquart and Duberstein20 However, it should be pointed out that because of the hierarchical diagnostic classification in our study the mood disorder group did not include patients with comorbid substance use disorders. In previous studies, comorbid substance use disorders have contributed to excess mortality from cancer in patients with mood disorders. Reference Crump, Sundquist, Winkleby and Sundquist21 Lung cancer was more common in men with a history of psychotic and substance use disorders and in women with substance use disorder compared with the other groups, which is consistent with the high rates of smoking in these patient groups. Reference Guydish, Passalacqua, Pagano, Martinez, Le and Chun22,Reference Partti, Vasankari, Kanervisto, Perälä, Saarni and Jousilahti23 However, cancer type was adjusted for in our analyses. Although comorbidity with various chronic conditions is more common in people with SMI than in the general population Reference Crump, Winkleby, Sundquist and Sundquist24,Reference Crump, Sundquist, Winkleby and Sundquist25 our results showed no difference in Charlson's comorbidity index between cancer patients with or without SMI, and comorbidity did not explain excess cancer mortality in the SMI groups.
An alarming finding was that the cancer-specific mortality gap between patients with a history of psychosis who had cancer and other cancer patients increased over time. This is consistent with findings on a worsening mortality gap in people with schizophrenia in general. Reference Saha, Chant and McGrath26 The increasing mortality gap among people with the most severe forms of mental illness and cancer compared with people without a history of mental illness is likely to partly reflect differences in quality of cancer treatment.
Poorer quality of cancer treatment in patients with schizophrenia is a consistent finding in previous literature. Reference Kisely, Crowe and Lawrence2 Barriers in cancer care among people with SMI have not been studied in Finland but a recent international review identified patient-level, provider-level and systems-level factors contributing to this disparity in cancer treatment in patients with schizophrenia. Reference Irwin, Henderson, Knight and Pirl27 Stigma toward patients with schizophrenia may affect treatment decisions and create barriers to optimal care. Reference Irwin, Henderson, Knight and Pirl27 There is also evidence that patients with schizophrenia experience barriers to clinical trials including challenges with trust and high medical comorbidity. They may also have more difficulties in navigating the healthcare system. Social factors including social isolation and lack of social support are also important factors likely to contribute to diagnostic delay and treatment differences. Reference Irwin, Henderson, Knight and Pirl27 Patients with schizophrenia may also have cognitive deficits and problems in communication, have difficulties agreeing with more intensive treatment regimens and may not be able to complete these treatments. It is therefore important to allow more time for their appointments, to engage family members or caregivers in the treatment and to arrange home nursing services or other assistance during cancer treatment. Collaboration between mental healthcare and oncological care is crucial for the optimal cancer care for these patients, Reference Irwin, Henderson, Knight and Pirl27 but in practice it is often limited or lacking.
The quality of cancer care in patients with substance use disorders has received little attention. Substance use disorders are associated with comorbidities that affect cancer treatment decisions and options Reference Pinter, Trauner, Peck-Radosavljevic and Sieghart28 but the possible role of stigma has received little attention. The possible role of treatment adherence in the poorer prognosis of cancer in patients with substance use and mood disorders should be studied further. As in schizophrenia, collaboration between mental health or substance use services and oncological care is needed to ensure optimal cancer care for these patients.
Administrative databases contain only limited information on the diagnosis and treatment process. Previous studies point to multiple problems: less frequent participation in cancer screening, delays in the diagnosis of cancer, difficulties in access to different treatments but also increased risk of side-effects because of interactions with psychotropic medications, to name a few. Reference Irwin, Henderson, Knight and Pirl27,Reference Howard, Barley, Davies, Rigg, Lempp and Rose29 Further studies including detailed clinical information are needed to address these issues comprehensively.
Methodological considerations
The strength of our study was the use of nationwide, unselective representative data concerning the total population of Finnish residents with their first cancer diagnosis in 1990–2013 collected from the Finnish Cancer Registry and Hospital Discharge Register covering the total population and based on clinical diagnoses. Notification of cancer to the Cancer Registry is mandatory in Finland and a good comparability and validity has been documented in the Cancer Registries of all the Nordic countries in a survey comparing the practices of the registries. 30 Close to 100% coverage of incident cases has been reported in the Finnish Cancer Registry and the accuracy of the records has been found to be high. Reference Korhonen, Malila, Pukkala, Teppo, Albanes and Virtamo31 We were also able to include patients with a history of substance use disorders from the Hospital Discharge Register. The accuracy and coverage of the register has, in general, been reported to be good. Reference Sund32 The Finnish Causes of Death statistics are considered valid and reliable by international standard, thus enabling us to study cancer-specific mortality. Reference Lahti33 Furthermore, the rate of confirmation of the diagnosis by autopsy in Finland is high compared with many other countries (in 2014, 23% for all deaths and 53% of those under 65 years of age). 34
The benefit of using a register-based total population of incident cancer patients leads to having no missing data or selection bias in the analysis as would be the case in, for example, hospital populations. In our study people with less severe mental disorders who have not been admitted to hospital are excluded from the SMI population. Although most patients with psychotic disorder are admitted to hospital at some point in their illness, Reference Suvisaari, Perälä, Saarni, Juvonen, Tuulio-Henriksson and Lönnqvist35 hospital-treated substance use disorder and mood disorders capture only the most severe forms of these disorders. In our study, we were able to define a group of individuals with SMI who had a definite history of contact with healthcare and a diagnosis confirmed by a psychiatrist. A weakness of administrative registers is that they do not contain information about health behaviours, disease severity or data on the treatment process. Another weakness in our study is that we could not analyse bipolar disorder and depressive disorders separately because of the small numbers of patients with bipolar disorder. We could, however, examine the Kaplan–Meier survival curves of these patient groups and found no differences in them.
Policy implications
People with SMI have increased mortality from cancer compared with other cancer patients, and this seems mainly to be because of the poorer quality of cancer treatment. Different models of integrated medical and psychiatric care and involving professionals responsible for the treatment of SMI in the cancer treatment plan at the time of diagnosis helps to improve this situation. However, further studies should collect more in-depth information on the whole cancer treatment process in order to identify the best opportunities to improve cancer treatment in people with SMI.
Funding
This study was partly funded by the Cancer Society in Finland, but the Society had no involvement in its design, data collection, findings or decision to publish.







eLetters
No eLetters have been published for this article.