Introduction
Sepsis is a syndrome of physiological, pathological and biochemical abnormalities induced by infection [Reference Singer1]. Septic shock causes circulatory and metabolic abnormalities, leading to increased mortality in hospitalised patients, especially in intensive care unit (ICU) patients [Reference Mayr, Sachin and Angus2, Reference Gaieski3]. Studies showed that once sepsis advanced to septic shock, the mortality rate rose from 25% to 52%, despite adoption of therapeutic strategies according to international sepsis guidelines [Reference Rhodes4, Reference Leligdowicz5]. Given the poor prognosis of septic shock in critical illness, researchers have found multiple risk factors predicting the prognosis of these patients, with the aim of early intervention to reduce mortality [Reference Jones, Trzeciak and Kline6, Reference Gilani, Razavi and Azad7]. Nevertheless, the mortality caused by sepsis remains high.
Neutrophils play crucial roles in the innate cellular immune system. Previous studies suggested that early higher neutrophil counts correlated with increased sepsis severity [Reference Shen8, Reference Park9], and neutrophil percentage was predictive of bloodstream infection [Reference Pan10]. Albumin is a medium-sized molecule that is the most abundant protein in human plasma. For a variety of physiological mechanisms, albumin is indispensable. It has a variety of functions, including serving as a major buffer, extracellular antioxidant, immunomodulator, antidote and transporter in plasma [Reference Artigas11, Reference Ha and Bhagavan12]. Increased capillary leakage of albumin is one of the features of SIRS [Reference Gradel13]. This means that lower albumin levels correlate with severe systemic inflammation and organ failure [Reference Norberg14]. Moreover, several studies demonstrated that low albumin levels correlated with adverse clinical outcomes [Reference Artigas11, Reference Arnau-Barres15].
Recently, the neutrophil-albumin ratio has been identified as a prognostic predictor in patients with rectal cancer and palliative pancreatic cancer [Reference Tingle16, Reference Tawfik17]. Nevertheless, to our knowledge, no previous study has focused on the neutrophil percentage-to-albumin ratio (NPAR). In this study, we hypothesised that NPAR is a novel marker of inflammation associated with all-cause mortality in patients with severe sepsis or septic shock.
Methods
Data source
Similar to our previous studies, we followed the methods of Wang et al., 2019 [Reference Wang18, Reference Wang19]. The study was based on a publicly accessible clinical database called the Multiparameter Intelligent Monitoring in Intensive Care III version 1.4 (MIMIC-III v1.4). It includes approximately 40 000 critical care patients at the Beth Israel Deaconess Medical Center (Boston, USA) from 2001 to 2012 [Reference Johnson20]. The demographics, vital signs, laboratory tests, medications, nursing progress notes and other clinical variables were recorded in this database. The project was approved by the institutional review boards of the Massachusetts Institute of Technology (MIT) and Beth Israel Deaconess Medical Center (BIDMC). To apply for access to the database, we passed the Protecting Human Research Participants exam and obtained a certificate (No. 6182750). Health data of all patients in this database were de-identified; therefore, informed consent was waived.
Population selection criteria
Inclusion criteria included: (1) adult patients (⩾18 years) diagnosed with severe sepsis or septic shock; (2) hospitalisation in the ICU at first admission for more than 2 days. Exclusion criteria were as follows: (1) no neutrophil percentage and albumin measured during ICU stay and (2) more than 5% of individual data missing. Severe sepsis was defined as systemic inflammatory response syndrome caused by infection combined with acute organ dysfunction (SOFA scoring system). Septic shock was defined as the presence of infection and systemic inflammatory response syndrome as defined in severe sepsis as well as the presence of arterial hypotension with a systolic blood pressure ⩽90 mmHg or a mean arterial blood pressure ⩽70 mmHg for at least 2 h or administration of a vasopressor (dopamine ⩾5 μg/kg/min; norepinephrine, epinephrine, phenylephrine, or vasopressin in any dosage) to maintain systolic blood pressure ⩾90 mmHg or mean arterial blood pressure ⩾70 mmHg despite adequate fluid loading [Reference Dellinger21].
Data extraction
Structured Query Language (SQL) with the PostgreSQL tool (version 9.6) was used to extract the data from MIMIC-III. Extracted data included demographics, vital signs, comorbidities, laboratory parameters and others upon admission. We extracted comorbidities, including congestive heart failure (CHF), coronary artery disease (CAD), atrial fibrillation (AFIB), stroke, renal disease, liver disease, pneumonia, malignancy, respiratory failure, chronic obstructive pulmonary disease (COPD) and acute respiratory distress syndrome (ARDS). The laboratory parameters included neutrophil percentage, albumin, bicarbonate, anion gap, creatinine, bilirubin, chloride, glucose, haematocrit, haemoglobin, platelet, sodium, potassium, blood urea nitrogen (BUN), white blood cell (WBC), lactate, prothrombin time (PT), international normalised ratio (INR) and activated partial thromboplastin time (APTT). Sequential organ failure assessment (SOFA) scores [Reference Allard22] and simplified acute physiology scores II (SAPSII) [Reference Le, Lemeshow and Saulnier23] were calculated for each patient. Age, gender, ethnicity, systolic blood pressure (SBP), diastolic blood pressure (DBP), mean blood pressure (MBP), respiratory rate, temperature, heart rate, SPO2, renal replacement therapy, vasopressor use and length of stay in the ICU were extracted. All baseline data were extracted within 24 h after ICU admission; 30-, 90- and 365-day all-cause mortality were the endpoints.
Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) or medians and interquartile range, and were tested by One-Way ANOVA (normal distribution) and Kruskal–Wallis H (skewed distribution). Categorical data were summarised as number or percentage and were compared using the chi-squared test. The association between NPAR levels and 30-, 90- and 365-day all-cause mortality was evaluated using Cox proportional hazards models. The results of the multivariate analysis were presented as hazard ratios (HRs) with 95% confidence intervals (CIs).
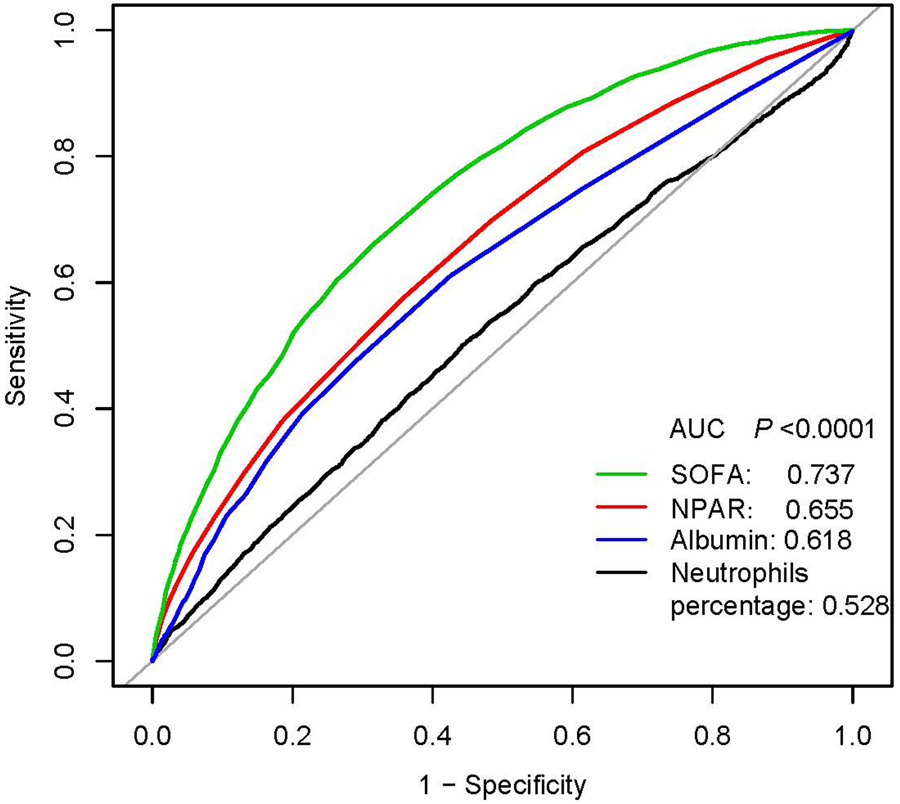
Two multivariate models were used to evaluate the prognostic values of NPAR for each endpoint. In model I, covariates were only adjusted for age, ethnicity and gender. In model II, we further adjusted for age, gender, ethnicity, SBP, DBP, temperature, SPO2, anion gap, bicarbonate, chloride, haemoglobin, lactate, platelet, APTT, PT, BUN, WBC, vasopressor use, atrial fibrillation, liver disease, respiratory failure, SOFA and SAPSII. We selected these confounders based on a change in effect estimate of more than 10%. The receiver operating curve (ROC) test was performed to measure the sensitivity and specificity of NPAR and other variables (SOFA score, albumin and neutrophils percentage) and calculated the area under the curve (AUC) to ascertain the quality of NPAR as a predictor of 365-day all-cause mortality.
Subgroup analysis of the associations between NPAR and 90-day all-cause mortality was performed to examine whether the effect of the NPAR differed across various subgroups. All statistical analyses were performed using EmpowerStats version 2.17.8 (http://www.empowerstats.com/cn/, X&Y solutions, Inc., Boston, MA) and R software version 3.42; P < 0.05 was considered statistically significant.
Results
Subject characteristics
A total of 2166 patients were eligible for this analysis. The demographic characteristics of participants stratified by NPAR tertiles are summarised in Table 1. Of these patients, there were 1204 (55.6%) men and 1595 (73.6%) white. According to NPAR levels, patients were divided into three groups (tertile 1: NPAR <24.4; tertile 2: NPAR ⩾24.4, <31.4; tertile 3: NPAR ⩾31.4), and the number of patients in each group was 722. Patients in the high tertile of NPAR (NPAR ⩾31.4) were more likely to use vasopressor, to report a history of malignancy, had lower SBP, DBP, MBP, haematocrit, haemoglobin and had higher values of chloride, BUN, WBC and mortality.
Table 1. Characteristics of the study patients according to NPARs
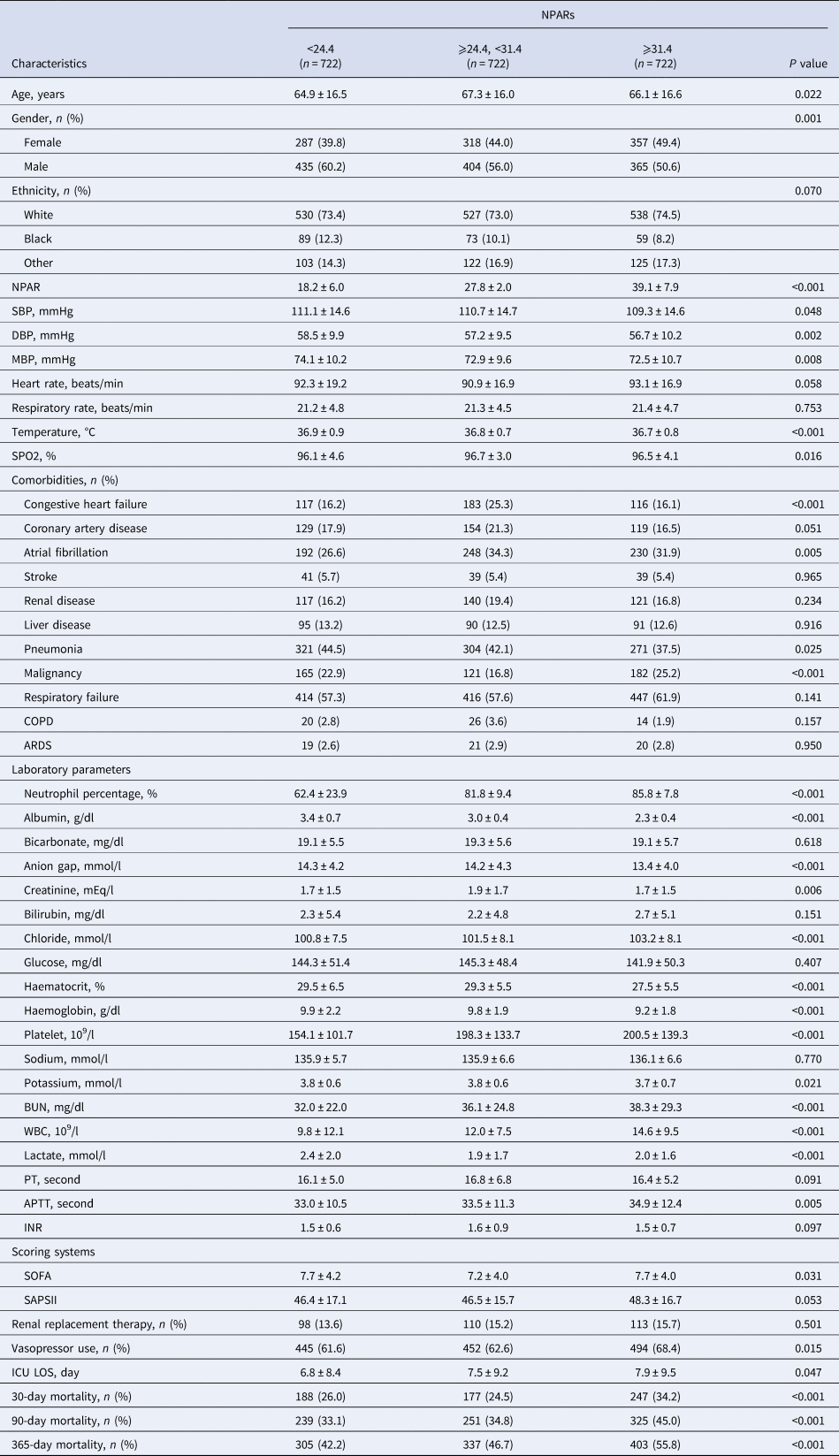
NPAR, neutrophil percentage-to-albumin ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; COPD, chronic obstructive pulmonary disease; ARDS, acute respiratory distress syndrome; BUN, blood urea nitrogen; WBC, white blood cell; PT, prothrombin time; APTT, activated partial thromboplastin time; INR, international normalised ratio; SOFA, Sequential Organ Failure Assessment; SAPSII, Simplified Acute Physiology Score II; ICU, intensive care unit; LOS: length of stay.
The statistical methods used for comparisons were the One-Way Anova (normal distribution), Kruskal–Wallis H (skewed distribution) test and chi-square tests (categorical variables).
NPAR as a predictor of the clinical endpoints
In multivariate analysis, we stratified NPAR levels by tertiles and quartiles, to assess whether NPAR was associated with 30-, 90- and 365-day all-cause mortality (Table 2). In model I, after adjustments for age, ethnicity and gender, higher NPAR was associated with increased risk of all-cause mortality. In model II, after adjusting for age, gender, ethnicity, SBP, DBP, temperature, SPO2, anion gap, bicarbonate, chloride, haemoglobin, lactate, platelet, APTT, PT, BUN, WBC, vasopressor use, AFIB, liver disease, respiratory failure, SOFA and SAPSII, higher NPAR was still significantly associated with 30-, 90- and 365-day all-cause mortality compared with the low NPAR levels (tertile 3 vs. tertile 1: HR, 95% CI: 1.29, 1.04–1.61; 1.41, 1.16–1.72; 1.44, 1.21–1.71). A similar trend was observed in NPAR levels stratified by quartiles; high-NPAR levels were also independently associated with these clinical endpoints (quartile 4 vs. quartile 1: HR, 95% CI: 1.37, 1.07–1.77; 1.43, 1.15–1.79; 1.41, 1.16–1.73). The generated ROC curves were shown in Figure 1. The AUCs for NPAR, albumin, neutrophils percentage and SOFA scores were 0.655, 0.618, 0.528 and 0.737, respectively. The findings indicated that NPAR was a better predictor of 365-day all-cause mortality than either albumin or neutrophil percentage alone (P < 0.0001). Moreover, we compared NPAR with neutrophil: lymphocyte (NLR) and lymphocyte in the supplementary materials, and the AUCs for NPAR, NLR and lymphocyte were 0.655, 0.646 and 0.576, respectively.

Fig. 1. ROC curves for the prediction of 365-day all-cause mortality in critically ill patients with severe sepsis or septic shock. The AUCs for NPAR, albumin, neutrophils percentage and SOFA scores were 0.655, 0.618, 0.528 and 0.737, respectively.
Table 2. HRs (95% CIs) for all-cause mortality across groups of NPARs
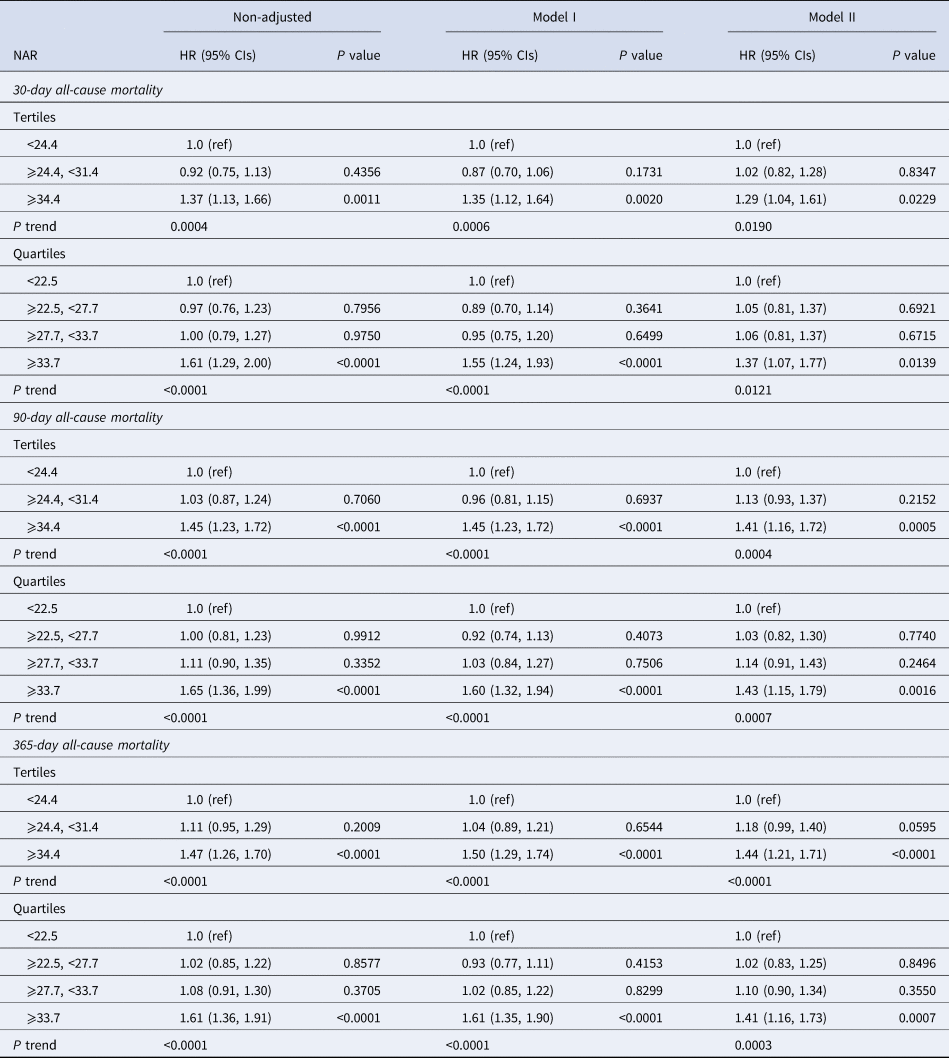
HR, hazard ratio; CI, confidence interval.
Models were derived from Cox proportional hazards regression models.
Non-adjusted model adjust for: none.
Adjust I model adjust for: age, ethnicity and gender.
Adjust II model adjust for: age, gender, ethnicity, systolic blood pressure, diastolic blood pressure, temperature, SPO2, anion gap, bicarbonate, chloride, haemoglobin, lactate, platelet, APTT, PT, BUN, WBC, vasopressor use, atrial fibrillation, liver disease, respiratory failure, SOFA, SAPSII.
Subgroup analyses
Subgroup analysis of the associations between NPAR and 90-day all-cause mortality was performed (Table 3), and there were no interactions in most strata (P = 0.0697–0.8841). Patients with a sodium ⩾136 mmol/l had a significantly higher risk of 90-day mortality with a NPAR ⩾31.4 (HR 1.89, 95% CI 1.49–2.40, P = 0.0354). Similarly, patients with a chloride ⩾102 mmol/l, WBC ⩾10.3 × 109/l, haematocrit ⩾28.7% and haemoglobin ⩾9.5 g/dl showed an increased risk with a NPAR ⩾31.4 (HR, 95% CI: 1.72, 1.35–2.18; 1.74, 1.34–2.25; 1.81, 1.40–2.35; 1.76, 1.36–2.27, respectively).
Table 3. Subgroup analysis of the associations between the NPARs and 90-day all-cause mortality
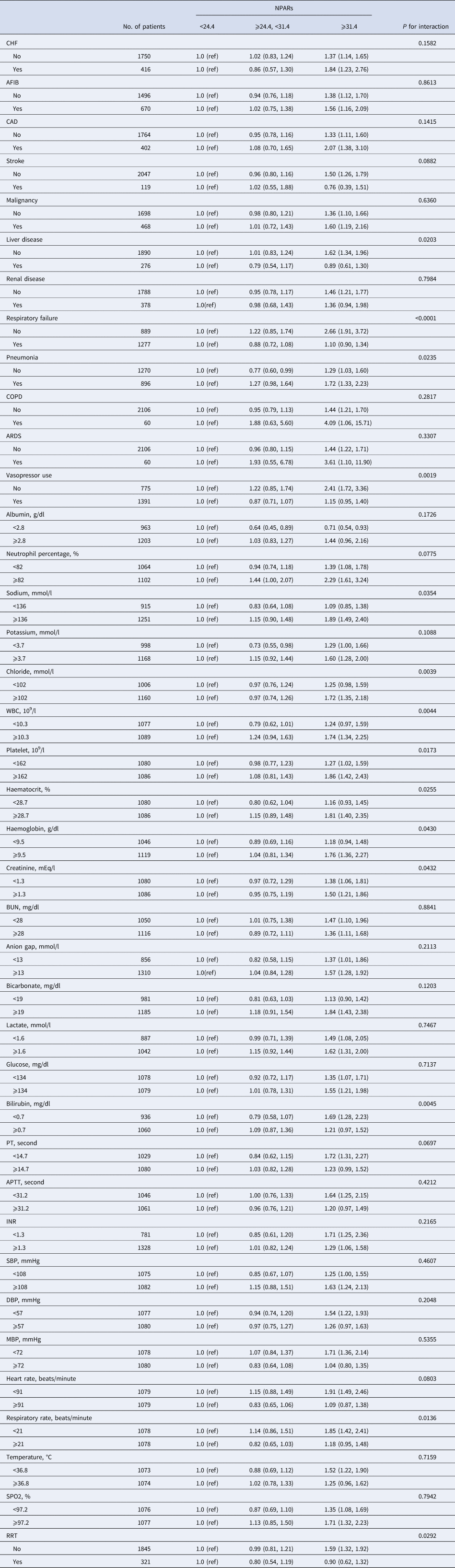
CHF, congestive heart failure; AFIB, atrial fibrillation; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; ARDS, acute respiratory distress syndrome; WBC, white blood cell; BUN, blood urea nitrogen; PT, prothrombin time; APTT, activated partial thromboplastin time; INR, international normalised ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; RRT, renal replacement therapy.
The modification and interaction of subgroup were inspected by the likelihood ration test.
Discussion
Our main findings can be summarised as follows. First, higher NPAR was associated with increased risk of 30-, 90- and 365-day all-cause mortality in critically ill patients with severe sepsis or septic shock after adjustments for age, ethnicity and gender. Furthermore, after adjustments for more potential confounders, higher NPAR remained significantly associated with all-cause mortality. To our knowledge, this was the first study to investigate the prognostic value of NPAR in critically ill patients with severe sepsis or septic shock; we found that higher NPAR was a novel predictor of poorer prognosis, and it was a better predictor than either albumin or neutrophil percentage alone.
Clinically, we found a phenomenon in which the high neutrophil percentage and the low albumin levels are associated with poor outcomes in patients with severe sepsis or septic shock. Previous studies focused on the neutrophil-to-albumin ratio, mainly in significantly predicting prognosis of palliative pancreatic cancer treatment and rectal cancer [Reference Tingle16, Reference Tawfik17]. Neutrophil percentage can be used as a practical marker to assess inflammation, and the serum neutrophil percentage and inflammatory cytokines are increased in infected patients [Reference Sun, Jiang and Shao24, Reference Lau25]. Moreover, previous studies have described the relationship between hypoproteinemia and mortality in stroke, myocardial infarction and hip fracture [Reference Plakht, Gilutz and Shiyovich26–Reference Pioli28]. These findings suggested that lower albumin values correlated with poorer prognosis of the disease. On the other hand, lower albumin values correlated with higher the values of NPAR. In our study, by comparing changes in NPAR values of patients with severe sepsis or septic shock, we found that increasing values of NPAR predicted poor sepsis prognosis. Russell et al., [Reference Russell29] showed that peripheral blood leucocyte ratios are useful biomarkers for infection. In critical illness due to sepsis, there is a signal and prognosis associated with NLR, and longitudinal measurements of these biomarkers during infection could be informative. Our findings also indicated that NPAR and NLR had similar predictive abilities for poor outcomes.
Neutrophils are part of the differential of WBC counts that are typically sensitive to bacterial and fungal infections [Reference Zarkesh30]. Walling et al., [Reference Walling and Manian31] demonstrated that neutrophil percentages above 80% provided a good distinction between positive and negative blood cultures among sepsis patients. However, the role of neutrophils in predicting bloodstream infection remained questionable, because stress, medication, trauma and abnormal bone marrow formation could cause these changes [Reference Pagano32, Reference Murray33]. Therefore, looking for a simple and reliable clinical predictor of mortality in sepsis is significant. Albumin levels reflect nutritional status and organ function, and the underlying inflammatory state give rise to a decrease of albumin production in liver by increasing inflammatory factors, the primary cause of hypoalbuminemia that occurs early in sepsis [Reference Churpek34, Reference Akirov35]. Therefore, based on our findings, NPAR, a new biomarker composed of neutrophil percentage and albumin that closely related to the inflammatory response, can significantly predict the prognosis of sepsis.
Our study had some limitations. First, the study was a single-centre retrospective design, and was therefore subject to selection bias. Second, we extracted NPAR in patients only upon admission to the ICU and did not assess changes during the ICU stay. Third, this database does not use the latest sepsis definitions (sepsis 3.0), and severe sepsis no longer forms part of the sepsis 3.0 definitions, this may affect the conclusion. Fourth, missing the aetiology of sepsis and specific cause of death in the MIMIC database failed to make the study more detailed and comprehensive. Fifth, although we have done our best to use a multivariate model to control bias, there remain numerous other known and unknown factors. Furthermore, the database contains a few inaccurate data elements. Therefore, multi-centre prospective studies are needed to confirm these findings.
Conclusions
Our findings demonstrated that higher NPAR was associated with increased risk of all-cause mortality in critically ill patients with severe sepsis or septic shock. Nevertheless, the conclusions need to be confirmed in large prospective multicentre studies.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268820000771.
Data
The clinical data used to support the findings of this study were supplied by Monitoring in Intensive Care Database III version 1.4 (MIMIC-III v.1.4). Although the database is publicly and freely available, researchers must complete the National Institutes of Health's web-based course known as Protecting Human Research Participants to apply for permission to access the database.
Financial support
This research was supported by the Zhejiang Provincial Natural Science Foundation of China (grants No. LY19H150002 and LY13H150007) and the Scientific Research Foundation of Wenzhou (grant No. Y20150038)
Conflict of interest
The authors declare that they have no competing interests.
Ethical standards
The MIMIC-III database has received ethical approval from the institutional review boards (IRBs) at Beth Israel Deaconess Medical Center and Massachusetts Institute of Technology. Because the database does not contain protected health information, a waiver of the requirement for informed consent was included in the IRB approval.
Disclosure
The funders of the project were not involved in study design, collection, data analysis, writing of the report and publication.
Consent for publication
Not applicable.