Significant outcomes
-
∙ Depressive symptoms, anxiety, and sleep disturbance were improved after l-theanine administration in patients with major depressive disorder (MDD).
-
∙ Cognitive functions were enhanced after l-theanine administration in patients with MDD.
-
∙ High tolerability of l-theanine was suggested.
Limitations
-
∙ This study was an open-label trial.
-
∙ The majority of the subjects was affected by relatively mild forms of MDD.
-
∙ The number of the subjects was small.
Introduction
MDD is a serious mental illness presenting with depressed mood, anxiety, sleep disturbance, various somatic symptoms, and cognitive impairments (Reference Kennedy1,Reference Rock, Roiser, Riedel and Blackwell2). Although many antidepressants acting on synaptic monoamine levels have been used as the first-line drug treatment for MDD, they are not effective in a substantial proportion of patients (Reference Rush, Trivedi and Wisniewski3). In addition, they can often elicit intolerable side effects in patients. This justifies the development of new treatment strategies in terms of efficacy and tolerability for patients suffering from MDD (Reference Hamon and Blier4). Several natural compounds (e.g. St. John’s wort, omega-3 fatty acids, and S-adenosyl-l-methionine) have been reported to be effective for MDD, whereas having fewer side effects and relatively low placebo-response rates than antidepressants (Reference Freeman, Mischoulon and Tedeschini5,Reference Nahas and Sheikh6).
l-theanine (l-γ-glutamylethylamide) is a non-proteinous amino acid, which is uniquely contained in green tea (Camellia sinensis). l-theanine crosses the blood–brain barrier, and is transported into the brain in a dose-dependent manner (Reference Yokogoshi, Kobayashi, Mochizuki and Terashima7). The chemical structure of l-theanine resembles that of l-glutamate and competes with it on glutamate receptors (Reference Kakuda, Nozawa, Sugimoto and Niino8). In experiments on rats, stress-induced suppression of recognition memory was rescued by l-theanine administration, which recovered the attenuation of in vitro hippocampal CA1 long-term potentiation (LTP) after stress exposure (Reference Tamano, Fukura, Suzuki, Sakamoto, Yokogoshi and Takeda9). Recognition memory was also enhanced in the l-theanine-administered rats, showing increased population spike amplitude in the induction of in vivo hippocampal dentate gyrus LTP (Reference Tamano, Fukura, Suzuki, Sakamoto, Yokogoshi and Takeda10). It has been shown that l-theanine has various psychotropic effects (Reference Lardner11). In experiments on mice, depression-like behaviour and cognitive dysfunction in a confront-housing condition, a model of chronic psychosocial stress, were suppressed in the l-theanine intake group (Reference Unno, Fujitani and Takamori12). We also reported the antidepressant-like effect of l-theanine together with the agonistic action on N-methyl-d-aspartate receptors (Reference Wakabayashi, Numakawa, Ninomiya, Chiba and Kunugi13). It has also been shown that anxiety-associated behaviours decrease (Reference Wise, Premaratne and Gamage14), and that chronic stress-induced cognitive impairments improve (Reference Tian, Sun and Gou15) in the l-theanine-administered mice.
In healthy humans, l-theanine was effective in reducing state anxiety scores, as well as in decreasing sympathetic nerve responses following acute stress task (Reference Kimura, Ozeki, Juneja and Ohira16). In electroencephalogram studies, l-theanine affected both resting state (Reference Nobre, Rao and Owen17) and attention task-related (Reference Gomez-Ramirez, Kelly, Montesi and Foxe18,Reference Gomez-Ramirez, Higgins and Rycroft19) α-wave activity which influences prefrontal cortical function, contributing to cognitive processes associated with attention and memory (Reference Knyazev20). Error rates in the attention task decreased in healthy participants (Reference Foxe, Morie, Laud, Rowson, de Bruin and Kelly21), and cognitive functions, including attention and memory, also improved in subjects exhibiting mild cognitive impairment (Reference Park, Jung and Lee22) in the l-theanine-administered group. Further, l-theanine showed augmentative effects on antipsychotic therapy in patients with schizophrenia and schizoaffective disorder, improving positive, activation, dysphoric mood, and anxiety symptoms (Reference Ritsner, Miodownik and Ratner23). We have also reported similar symptom-reducing effects of l-theanine in patients with schizophrenia (Reference Ota, Wakabayashi and Sato24).
Aims of the study
Although l-theanine has been shown to have various psychotropic effects, there has been no clinical study performed to show whether this compound affects symptoms and cognitive functions in patients with MDD. The aim of this study was to examine the psychotropic effects of l-theanine in patients with MDD.
Material and methods
Subjects
Subjects (mean age: 42.6±12.1 years, four males and 16 females) were 20 patients with the diagnosis of MDD according to the criteria in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (25). Each diagnosis was based on information obtained by the Mini-International Neuropsychiatric Interview (Reference Sheehan, Lecrubier and Sheehan26), and additional unstructured interviews, and medical records where available. We included some remitted patients with MDD as well as unremitted patients at baseline in order to elucidate the possible effects of l-theanine on mild forms of the illness. Participants were excluded if they had prior medical histories of central nervous system disease, severe head injury, substance abuse, or mental retardation. All patients were recruited from the outpatients of the National Center of Neurology and Psychiatry Hospital (n=6) or from the local community through prepared advertisements (n=14). After describing the study, written informed consent was obtained from every subject. Thereafter, patient anonymity has been preserved. Before starting the study, we registered the protocol at the university hospital medical information network – clinical trials registry and complied with it accordingly (UMIN000014653).
l-theanine administration
After the initial psychiatric assessments, l-theanine tablets (250 mg/day) (Suntheanine, Taiyo Kagaku Co. Ltd, Japan) were orally administered before sleep for 8 weeks. This dose was chosen because it was effective in our prior study (Reference Ota, Wakabayashi and Sato24). Psychiatric symptoms were assessed at baseline, 4 and 8 weeks after l-theanine administration. In total, 12 patients (60%) were treated with antidepressant medication at baseline and this medication was unchanged during our intervention period. Daily doses of antidepressants were converted to imipramine-equivalent doses using a published Japanese guideline (Reference Inada and Inagaki27). The mean imipramine-equivalent dose was calculated in the total subjects (n=20). Participants were instructed not to alter their usual intakes of green tea during the intervention period.
Clinical assessments
To evaluate depressive symptoms, anxiety, and sleep state, the 21-item version of the GRID Hamilton Depression Rating Scale (HAMD-21) (Reference Williams, Kobak and Bech28,Reference Hamilton29), State-Trait Anxiety Inventory (STAI) (Reference Spielberg30), and Pittsburgh Sleep Quality Index (PSQI) (Reference Buysse, Reynolds, Monk, Berman and Kupfer31) were used. Adverse events were assessed using the Udvalg for Klinicke Undersøgelser (UKU) side effect rating scale (Reference Lingjaerde, Ahlfors, Bech, Dencker and Elgen32). Cognitive functions were measured by the Stroop test (Reference Stroop33) and the Brief Assessment of Cognition in Schizophrenia (BACS) (Reference Keefe, Goldberg, Harvey, Gold, Poe and Coughenour34). The Stroop test was conducted on a computer using two types (control and experimental condition) of PowerPoint files composed of four (red, blue, yellow, and black) coloured words (Reference MacLeod and MacDonald35). After the participants practiced by the control condition in which colour and word were consistent, response latency (ms) and error rate (%) were evaluated in the experimental condition in which colour and word were inconsistent. Two versions (A and B) of BACS were used to exclude any learning effects.
Laboratory tests
Non-fasting blood samples were obtained before clinical assessments. Serum samples were prepared by centrifuging bloods for 10 min at 3,000 rotations/min. Plasma samples were prepared to measure haemoglobin A1c. The samples were evaluated at baseline, 4 and 8 weeks after l-theanine administration at SRL Co. Ltd, Japan to check general physical states and monitor side effects. Serum l-theanine blood concentrations were measured by a high-performance liquid chromatography system based on the AccQ·Tag method as described in the literature (Reference Gardener and Gillman36) using commercially available reagents and equipment (Waters Corp., Tokyo, Japan).
Statistics
Differences in clinical assessments and laboratory data between baseline and 8 weeks after l-theanine administration were analysed by the Wilcoxon’s signed-rank test. Effect sizes were calculated using r values. All statistical tests were two-tailed and p<0.05 indicated statistical significance. Statistical analyses were performed using the Statistical Package for the Social Sciences version 22.0 (IBM Corp., Tokyo, Japan).
Results
The clinical characteristics of the subjects are summarised in Table 1. All participants completed the entire 8-week study, suggesting high tolerability of l-theanine. Time courses of symptom scores are shown in Table 2. Six HAMD-21 subscale scores were calculated as described in Seretti et al. (Reference Seretti, Cusin, Lattuada, Di Bella, Catalano and Smeraldi37). There was a significant decrease in HAMD-21 total scores (p=0.007, r=−0.61), and a significant reduction in four subscale scores (core, psychic anxiety, somatic anxiety, and delusion) and a tendency of reduction in sleep subscale scores in the total patients (n=20). When the patients were divided into unremitted patients (HAMD-21>7; n=13) at baseline and the remaining remitted patients (n=7) according to Riedel et al. (Reference Riedel, Moller and Obermeier38), the former showed a significant decrease (p=0.004, r=−0.79), whereas the latter did not (p=0.35, r=−0.36) (Fig. 1). Among the unremitted patients at baseline, six (46.2%) were responders (50% or more reduction of HAMD-21 score) and six (46.2%) became remitters at 8 weeks. Among the remitted patients at baseline, there were only two (28.6%) responders. We did not detect any significant association of clinical variables (age, sex, height, weight, body mass index, education, onset of illness, single/recurrent episode, imipramine-equivalent dose, or smoking) with response to l-theanine (data not shown).

Fig. 1 Twenty-one-item version of the Hamilton Depression Rating Scale (HAMD-21) score change after l-theanine administration. There was a significant reduction of HAMD-21 scores in unremitted patients (n=13), whereas such a significant change was not observed in remitted patients (n=7, grey coloured). n.s., Not significant. **p<0.01.
Table 1 The clinical characteristics of the subjects
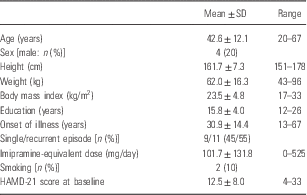
HAMD-21, 21-item version of the Hamilton Depression Rating Scale.
Table 2 Time courses of symptom scores after l-theanine administration

HAMD-21, 21-item version of the Hamilton Depression Rating Scale; PSQI, Pittsburgh Sleep Quality Index; STAI, State-Trait Anxiety Inventory; W, Wilcoxon’s signed-rank test statistic.
* HAMD-21>7 (n=13).
† HAMD-21≤7 (n=7).
*p<0.05, **p<0.01.
STAI consisted of anxiety-state and anxiety-trait indices. We found a tendency of reduction in anxiety-state (p=0.058, r=−0.43), and a significant reduction in anxiety-trait (p=0.012, r=−0.56) after l-theanine administration in the total patients. PSQI scores were significantly reduced after l-theanine administration in the patients who were unremitted at baseline (p=0.030, r=−0.60). We found no notable adverse events in the UKU side effect rating scale (4 weeks: 0.2±0.5, 8 weeks: 0.3±0.6). One patient reported slight sleepiness, two patients reported increased duration of sleep of up to 2 h longer than usual, and two patients reported slightly increased dream activity.
Time courses of cognitive function scores are shown in Table 3. In the Stroop test, response latency was significantly shortened after l-theanine administration (p=0.001, r=−0.72). There was also a significant decrease in error rate in the Stroop test (p=0.036, r=−0.47). Among seven indices of BACS, verbal memory score (p=0.005, r=0.63) and executive function score (p=0.016, r=0.54) were significantly enhanced after l-theanine administration.
Table 3 Time courses of cognitive function scores after l-theanine administration
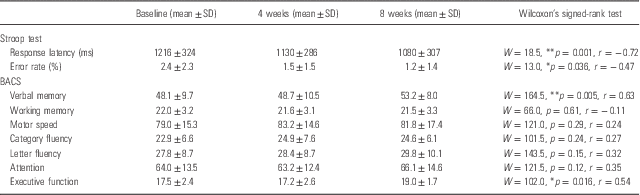
BACS, Brief Assessment of Cognition in Schizophrenia; W, Wilcoxon’s signed-rank test statistic.
*p<0.05, **p<0.01.
Changes in laboratory data are shown in Table 4. Except for a significant decrease of high-density lipoprotein (HDL) cholesterol (p=0.011, r=−0.57), general metrics such as liver function, renal function, and glucose metabolism were not significantly altered after l-theanine administration. As expected, l-theanine blood concentration significantly increased after administration (p=0.005, r=0.64).
Table 4 Changes in laboratory data after l-theanine administration

ALT, alanine transaminase; AST, aspartate transaminase; BUN, blood urea nitrogen; CRE, creatinin; GTP, glutamyltranspeptidase; Hb, haemoglobin; HDL-CHO, high-density lipoprotein cholesterol; LDL-CHO, low-density lipoprotein cholesterol; T-CHO, total cholesterol; TG, triglyceride; W, Wilcoxon’s signed-rank test statistic.
*p<0.05.
Discussion
We examined the possible effects of l-theanine in an open-label clinical trial. We found that psychiatric symptoms and cognitive functions of patients with MDD were improved after l-theanine administration. HAMD-21 scores, an objective index of depression, were reduced in unremitted patients at baseline; although the score-reducing effect was not observed any more in patients who were already remitted at baseline. Among the HAMD-21 subscales, core, psychic anxiety, somatic anxiety, and delusion scores were reduced. The scores in STAI and PSQI metrics were also reduced. Response latency and error rate decreased in the Stroop test and verbal memory and executive function increased in the BACS. Furthermore, there were no dropout subjects and no serious adverse events in either UKU side effect rating scale or laboratory data. To our knowledge, we showed, for the first time, reduced symptoms and enhanced cognitive functions in patients with MDD after l-theanine administration, suggesting that it possesses multiple favourable psychotropic effects.
Depressive symptoms were significantly reduced after l-theanine administration, which suggests that it has antidepressant effects. This is in line with previous reports in mice (Reference Unno, Fujitani and Takamori12,Reference Wakabayashi, Numakawa, Ninomiya, Chiba and Kunugi13). Considering the structural commonality of l-theanine to l-glutamate, the primary site of action is likely to be the glutamatergic pathway which is a therapeutic target for depression (Reference Skolnick, Popik and Trullas39,Reference Hashimoto40). Meta-analyses have reported that ketamine, a non-competitive glutamate receptor antagonist, is effective in treating depressive disorders (Reference Newport, Carpenter, McDonald, Potash, Tohen and Nemeroff41,Reference Caddy, Giaroli, White, Shergill and Tracy42). l-theanine has a lower affinity for glutamate receptors than does l-glutamate itself (Reference Kakuda, Nozawa, Sugimoto and Niino8). We showed that l-theanine could exert a partially agonistic effect on glutamate receptors (Reference Wakabayashi, Numakawa, Ninomiya, Chiba and Kunugi13) and regulate the concentration of glutamate and glutamine in the brain (Reference Ota, Wakabayashi and Sato24). Although detailed mechanisms are unknown, it is possible that, similar to a low dose of ketamine (Reference Miller, Moran and Hall43), l-theanine might normalise impaired glutamatergic transmission in MDD.
We found a tendency for reduction in anxiety-state scores and a significant reduction in anxiety-trait scores after l-theanine administration. This may suggest its anxiolytic effects in our patients with MDD, which is corroborated by previous studies in mice and humans (Reference Wise, Premaratne and Gamage14,Reference Kimura, Ozeki, Juneja and Ohira16,Reference Ritsner, Miodownik and Ratner23). This effect might be attributable again to the action of l-theanine on glutamate receptors, because modulators of the receptors have shown their anxiolytic effects (Reference Bergink, van Megen and Westenberg44). Increased level of central nervous system inhibitory neurotransmitters, such as glycine (Reference Yamada, Terashima, Okubo, Juneja and Yokogoshi45) and γ-aminobutyric acid (Reference Kimura and Murata46), following theanine administration may also contribute to the anxiolytic effects. Further, we found a significant improvement of sleep disturbance after l-theanine administration in our patients with MDD. In line with this, l-theanine has been suggested to help ameliorate sleep quality in patients with MDD as in attention deficit hyperactivity disorder (Reference Lyon, Kapoor and Juneja47) and schizophrenia (Reference Ota, Wakabayashi and Sato24).
We found enhanced cognitive functions after l-theanine administration in line with previous reports in rats, mice, and humans (Reference Tamano, Fukura, Suzuki, Sakamoto, Yokogoshi and Takeda9,Reference Tamano, Fukura, Suzuki, Sakamoto, Yokogoshi and Takeda10,Reference Unno, Fujitani and Takamori12,Reference Tian, Sun and Gou15,Reference Foxe, Morie, Laud, Rowson, de Bruin and Kelly21,Reference Park, Jung and Lee22). Although one recent double-blind randomised controlled trial reported that l-theanine had no beneficial effect on cognition in healthy coffee drinkers (Reference Dodd, Kennedy, Riby and Haskell-Ramsay48), the dose (50 mg) was substantially lower than ours (250 mg). Further, it was single, but not chronic l-theanine administration. In the Stroop test, response latency and error rates were significantly reduced in patients with MDD. The Stroop task is relevant to prefrontal cortical activity and other conflict-processing functional areas in the brain (Reference Roberts and Hall49). In the BACS, verbal memory and executive function scores of the patients significantly improved in this study. l-theanine may improve verbal memory and executive function by modulating the involved cortical areas through its putative α-wave activity related to attention and memory in healthy subjects in electroencephalogram studies (Reference Nobre, Rao and Owen17–Reference Gomez-Ramirez, Higgins and Rycroft19).
As discussed in Godsil et al. (Reference Godsil, Kiss, Spedding and Jay50), the hippocampus-prefrontal pathway might be related with the treatment response to l-theanine administration. In the hippocampus and prefrontal cortex, glutamatergic signalling is an essential regulator underlying synaptic plasticity. Disrupted function in this system can constitute the pathology of MDD and present a novel therapeutic target or research of antidepressant (Reference Marsden51). Considering that stress-related impairment of hippocampal synaptic plasticity was recovered (Reference Tamano, Fukura, Suzuki, Sakamoto, Yokogoshi and Takeda9), and that the augmentation of hippocampal synaptic plasticity was observed (Reference Tamano, Fukura, Suzuki, Sakamoto, Yokogoshi and Takeda10) in l-theanine-administered rats, the antidepressant and cognition-enhancing effects might also be related to the effect on synaptic plasticity.
In laboratory data, HDL cholesterol, alternatively called as ‘good’ cholesterol, was significantly reduced after l-theanine administration. This result is opposite to a report that the levels of HDL cholesterol increased in the theanine-supplemented group in hepatoma-bearing rats (Reference Zhang, Miura and Yagasaki52). Although the mechanisms underlying our result were elusive, there were no subjects who newly showed the value below the lower limit of reference range (40 mg/dl) at 8 weeks.
There are several limitations in our study. First, we cannot exclude the influence of the placebo effect on our results. Placebo-controlled clinical trials are required to confirm the therapeutic effect of l-theanine as an adjunct therapy in the treatment of MDD. Second, although a significant reduction in depressive symptoms were seen after l-theanine administration, the mean HAMD-21 score at baseline was 12.5 points, indicating that the patients presented relatively mild forms of MDD, which might have obscured the possible antidepressant effect.
In conclusion, reduced depressive symptoms, anxiety, sleep disturbance, and enhanced cognitive functions were found in patients with MDD after chronic (8-week) l-theanine (250 mg/day) administration. We also found high tolerability of l-theanine. These results suggest that l-theanine could be a useful natural compound in the treatment of MDD.
Acknowledgements
The authors thank Ikki Ishida, Moeko Hiraishi, Anna Nagashima, and Dr. Junko Matsuo for their assistance in the recruitment of subjects. Authors’ Contributions: S.H., M.O. and H.K. designed the study and H.K. supervised the results. C.W. was involved in supporting the intellectual content. T.N. helped recruitment of subjects from the outpatient clinic. T.O. provided important knowledge about l-theanine and critical comments to the study. H.O. carried out high-performance liquid chromatography analysis. S.H. evaluated clinical assessments, executed statistical analyses, and wrote the manuscript, which was revised and approved by all authors.
Financial Support
This study was supported by an Intramural Research Grant (M.O. and H.K., grant number 24–11, 27–1) for Neurological and Psychiatric Disorders of National Center of Neurology and Psychiatry, and an unrestricted research grant provided by the Taiyo Kagaku Co. Ltd.
Conflicts of Interest
H.O. and T.O. are employees of Taiyo Kagaku Co. Ltd., which provided l-theanine tablets used in the present study, and various food products.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the ethics committee of National Center of Neurology and Psychiatry and with the Helsinki Declaration of 1975, as revised in 2008.








