Introduction
Recognition of the neuropsychological sequalae that emerge following treatment of childhood cancer has led to significant changes in treatment regimens and follow-up care for survivors. Technological advances in diagnosis and treatment over the past few decades have resulted in improved mortality and survival rates. Despite these improvements, childhood cancer survivors, particularly those treated for a brain tumor, are at risk for long-term neuropsychological sequelae, educational difficulties, psychological disorders, and chronic medical conditions (Armstrong, Reference Armstrong2010; Brinkman, Krasin, et al., Reference Brinkman, Krasin, Liu, Armstrong, Ojha, Sadighi, Gupta, Kimberg, Srivastavam, Merchant, Gajjar, Robison, Hudson and Krull2016; Gurney et al., Reference Gurney, Krull, Kadan-Lottick, Nicholson, Nathan, Zebrack, Tersak and Ness2009; Hudson et al., Reference Hudson, Mertens, Yasui, Hobbie, Chen, Gurney, Yeazel, Marina, Robison and Oeffinger2003; Zeltzer et al., Reference Zeltzer, Recklitis, Buchbinder, Zebrack, Casillas, Tsao, Lu and Krull2009). Given the enduring nature of these deficits, which impact independence, educational and employment attainment, and interpersonal relationships, research is needed to better understand means of ameliorating these neuropsychological sequelae.
Cranial radiation therapy (XRT), which utilizes photons to penetrate tissue and deliver radiation to the target (Yock & Tarbell, Reference Yock and Tarbell2004), is considered the standard of care for many pediatric central nervous system (CNS) tumors. Across time, XRT techniques (i.e., intensity-modulated RT, 3D conformal RT) have improved to provide greater conformality of the tumor. This has allowed for more precise radiation delivery to the tumor while simultaneously reducing irradiation of healthy tissue (Kun & Beltran, Reference Kun and Beltran2009). This increasingly focal approach has resulted in less cerebral tissue damage and improved intellectual outcomes compared to conventional XRT approaches (Moxon-Emre et al., Reference Moxon-Emre, Bouffet, Taylor, Laperriere, Scantlebury, Law, Spiegler, Malkin, Janzen and Mabbott2014, Reference Moxon-Emre, Bouffet, Taylor, Laperriere, Sharpe, Laughlin, Scantlebury, Law, Skocic, Richard and Mabbott2016). While there have been technical changes to XRT, all of these treatments utilize photons whereby energy is not only deposited to the tumor itself but also to the surrounding healthy brain tissue (Hoffman & Yock, Reference Hoffman and Yock2009). The impact of photons on the distal healthy brain tissues has been shown to contribute to neuropsychological late effects, particularly within the core domains of processing speed, working memory, and attention (King et al., Reference King, Ailion, Fox and Hufstetler2019; Palmer, Reference Palmer2008; Wolfe et al., Reference Wolfe, Madan-Swain and Kana2012). Deficits in these core domains have been shown to have a negative downstream impact on broader neuropsychological constructs such as IQ, academic achievement, and adaptive functioning (Brinkman, Li, et al., Reference Brinkman, Li, Vannatta, Marchak, Lai, Prasad, Di, Armstrong and Krull2016; Mabbott et al., Reference Mabbott, Spiegler, Greenberg, Rutka, Hyder and Bouffet2005; Semmel et al., Reference Semmel, Quadri and King2020). Within the domain of overall intellectual functioning, the emergence of late effects across time following XRT result in declines in IQ by 2–8 points per year (Ris et al., Reference Ris, Packer, Goldwein, Jones-Wallace and Boyett2001; Palmer et al., Reference Palmer, Goloubeva, Reddick, Glass, Gajjar, Kun, Merchant and Mulhern2001).
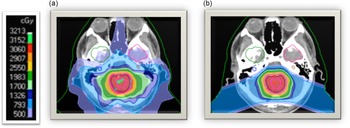
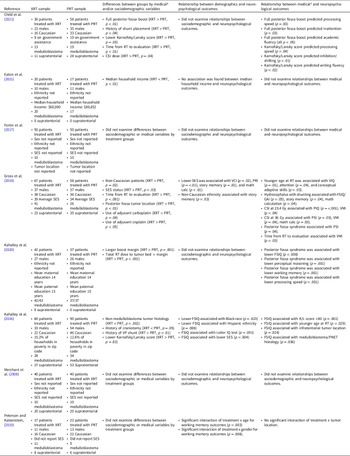
Proton beam radiation therapy (PRT) is a relatively newer cancer treatment that differs significantly from photons in terms of depth dose distribution. See Figure 1 for a dosimetric comparison of XRT and PRT. As protons enter the brain, they demonstrate an increasing energy deposition with penetration distance culminating in a peak (i.e., Bragg-peak). In front of the Bragg-peak, the energy dose level is modest as compared to photon beam; beyond the Bragg-peak the dose falls to nearly zero. Protons thereby reduce the radiation entrance and exit dose, thus providing exceptional conformality compared to XRT, thereby reducing radiation exposure to adjacent healthy brain tissue (Hoffman & Yock, Reference Hoffman and Yock2009; Kirsch & Tarbell, Reference Kirsch and Tarbell2004; Olsen et al., Reference Olsen, Bruland, Frykholm and Norderhaug2007). Given that PRT provides more precise tumor irradiation as compared to other radiation techniques, it is hypothesized to have many advantages over XRT including reduced adverse neuropsychological sequelae, better local tumor control, and diminished rates of normal tissue toxicity and secondary malignancies (Merchant et al., Reference Merchant, Hua, Shukla, Ying, Nill and Oelfke2008). In older brain tumor survivors, there was no significant change in intellectual, attention, executive, visuospatial and memory functions over time in adults treated with proton beam (Sherman et al., Reference Sherman, Colvin, Mancuso, Batchelor, Oh, Loeffler, Yeap and Shih2016; Shih et al., Reference Shih, Sherman, Nachtigall, Colvin, Fullerton, Daartz, Winrich, Batchelor, Oh, Curry, Loeffler and & Yeap2015).

Figure 1. Childhood medulloblastoma treated with (a) photon radiation therapy and (b) proton radiation therapy.
With increased awareness of PRT and its advantages relative to normative means (Greenberger et al., Reference Greenberger, Pulsifer, Ebb, MacDonald, Jones, Butler, Oberg, Tarbell and Yock2014; Jimenez et al., Reference Jimenez, Sethi, Depauw, Pulsifer, Adams, McBride, Ebb, Fullerton, Tarbell, Yock and MacDonald2013; Macdonald et al., Reference MacDonald, Sethi, Lavally, Yeap, Marcus, Caruso, Huang, Ebb, Tarbell and Yock2013; Pulsifer et al., Reference Pulsifer, Sethi, Kuhlthau, MacDonald, Tarbell and Yock2015), an important question remains as to whether PRT results in better neuropsychological outcomes as compared to XRT. Research has been fairly limited in comparing PRT and XRT given the number of inherent limitations. First, patients are not randomized to PRT or XRT, which impacts the ability to compare outcomes across treatments. In addition, PRT is costly, not consistently covered by insurance, and not widely available, which may pre-determine the patients that have access to this treatment technique and thereby reduce generalizability of findings. In spite of these limitations, in order to fully understand the potential neuropsychological benefits of PRT, it is essential to compare neuropsychological functioning across radiation therapy treatments. Thus, the aim of this study was to address these questions through a systematic literature review of neuropsychological outcomes in pediatric brain tumor patients and examine any plausible confounds that need to be considered.
Methods
This systemic review was performed following the guidance of the Preferred Reporting Items of Systemic Reviews and Meta-Analysis (PRISMA) statement (Liberati et al., Reference Liberati, Altman, Tetzlaff, Mulrow, Gøtzsche and Ioannidis2009) as well as proposed guidelines (Khan et al., Reference Khan, Kunz, Kleijnen and Antes2003). Similar to other systematic reviews (Ailion et al., Reference Ailion, Hortman and King2017; Wolfe et al., Reference Wolfe, Madan-Swain and Kana2012), a system was developed to provide an analysis of study strengths and weaknesses. For the purpose of this literature review, study quality was examined in thirteen categories. Please refer to Table 1 for the criteria for analyzing study strengths and limitations. These included but were not limited to study design, study methodology, homogeneity of sample, and sample size. As was used in previous systemic reviews (Semmel et al., Reference Semmel, Fox, Na, Kautiainen, Latzman and King2019), a small sample was defined as less than 15 patients, a moderate sample size was 15–49 patients, and a large sample size was 50 or more patients.
Table 1. Criteria for analyzing study strengths and limitations

The literature search was conducted within PubMed, PsychINFO, Embase, Web of Science, Scopus, and Cochrane databases using all possible combinations of the following search terms: “pediatric” or “childhood” + “brain tumor” or “neoplasm” + “proton” + “photon” + “XRT” or “RT” or “radiation” + “neurocognitive” or “cognitive” or “neuropsychology.” Inclusion criteria included published studies that compared neuropsychological outcomes in pediatric brain tumor patients, diagnosed at less than 19 years of age, and treated with proton or photon therapy. All brain pathologies, study selection criteria, and therapy protocols were eligible for inclusion. Only peer-reviewed studies on human populations written in English were considered. Poster abstracts and oral presentations were excluded from analyses.
Results
Study selection
Initial searches using the aforementioned combinations of search terms yielded 1101 peer-reviewed scientific articles. Of these, 501 were redundant; 600 remained after removing these duplicates. See Figure 2 for an overview of article screening and exclusion. In total, eight articles were analyzed in the present review. The strengths and limitations of these eight studies are provided in Table 2.

Figure 2. PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only.
Table 2. Studies included in systematic review

Main characteristics of included studies
Across all eight studies the total sample size of brain tumor patients was 648, with 322 patients treated with PRT and 326 treated with XRT. The majority of studies (n = 5) had large sample sizes (n > 49); the remaining three studies had medium sample size (Eaton et al., Reference Eaton, Fong, Ingerski, Pulsifer, Goyal, Zhang, Weyman, Ebb, MacDonald, Tarbell and Yock2021; Merchant et al., Reference Merchant, Hua, Shukla, Ying, Nill and Oelfke2008; Peterson & Katzenstein, Reference Peterson and Katzenstein2019). Most studies (n = 6) examined outcomes in heterogenous samples in terms of tumor histology and location. The most common tumor location was infratentorial (n = 402), with medulloblastoma tumor histology (n = 364).
In terms of study design, the majority of studies were retrospective (n = 6), with five studies using a longitudinal methodology. Six of the studies (n = 6) were empirical; two of the studies utilized theoretical models to conceptualize neuropsychological outcomes. Only one study (Gross et al., Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019) also included standardized rating scales to complement the neuropsychological performance-based measures. Of note, none of the studies examined relationship specificity by including a control task. Only one study adjusted for type 1 error (Child et al., Reference Child, Warren, Grosshans, Paulino, Okcu, Ris, Orobio, Cirino, Chintagumpala and Kahalley2021).
Theory-driven research provides a framework for examining specific neuropsychological constructs and proposes hypotheses related to these outcomes. Of the eight studies, four studies provided both a theoretical background as well as hypotheses (Child et al., Reference Child, Warren, Grosshans, Paulino, Okcu, Ris, Orobio, Cirino, Chintagumpala and Kahalley2021; Kahalley et al., Reference Kahalley, Ris, Grosshans, Okcu, Paulino, Chintagumpala, Guffey, Minard and Mahajan2016, Reference Kahalley, Peterson, Ris, Janzen, Okcu, Grosshans, Mahajan, Tsang, Laperriere, Whitehead, Dauser, Taylor, Conklin, Chintagumpala, Bouffet and Mabbott2020; Peterson & Katzenstein, Reference Peterson and Katzenstein2019). An additional study provided a hypothesis but no reference to why specific neuropsychological domains would be impacted by cancer treatment (Gross et al., Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019). The three remaining studies did not provide hypotheses related to neuropsychological outcomes.
Table 3 details the sociodemographic variables included in the eight studies examined as part of this literature review. The six empirical studies reported at least one sociodemographic variable. Sex was reported in all six of these studies, with no significant difference in treatment groups by sex. Four studies also included information on race/ethnicity (Child et al., Reference Child, Warren, Grosshans, Paulino, Okcu, Ris, Orobio, Cirino, Chintagumpala and Kahalley2021; Gross et al., Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019; Kahalley et al., Reference Kahalley, Ris, Grosshans, Okcu, Paulino, Chintagumpala, Guffey, Minard and Mahajan2016; Peterson & Katzenstein, Reference Peterson and Katzenstein2019). The majority of these studies had equal ethnic representation in the two treatment groups, though Gross et al. (Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019) had no Black patients in the PRT group. Socioeconomic status (SES) was not consistently considered in all studies. In the five studies that considered SES, it was measured in different ways. Child et al. (Reference Child, Warren, Grosshans, Paulino, Okcu, Ris, Orobio, Cirino, Chintagumpala and Kahalley2021) provided a frequency count of the number of families on government assistance. There was no difference between the two treatment groups. Gross et al. (Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019) examined SES continuously as well as categorically (low <$50,000 median household income by zip code, average $50,000–$90,000, and high >$90,000). SES was significantly higher in the PRT group. Eaton et al. (Reference Eaton, Fong, Ingerski, Pulsifer, Goyal, Zhang, Weyman, Ebb, MacDonald, Tarbell and Yock2021) also collected median household income by zip code. Patients in the PRT group had significantly higher median household incomes compared to those in the XRT group. Kahalley et al. (Reference Kahalley, Ris, Grosshans, Okcu, Paulino, Chintagumpala, Guffey, Minard and Mahajan2016) calculated SES by the percentage of households in poverty by the home zip code of the patient. There was no significant difference between the two treatment groups. In another study led by Kahalley et al. (Reference Kahalley, Peterson, Ris, Janzen, Okcu, Grosshans, Mahajan, Tsang, Laperriere, Whitehead, Dauser, Taylor, Conklin, Chintagumpala, Bouffet and Mabbott2020), maternal and paternal education were used in place of SES. There were no differences in parental education by treatment group.
Table 3. Sociodemographic and medical variables utilized in studies and relationships to neuropsychological outcomes

Abbreviations: CSI, craniospinal irradiation; FSIQ/Gai, full scale IQ/general ability index; Gy, Gray; PRT, proton radiotherapy; SES, socioeconomic status; VP, ventriculoperitoneal; XRT, photon radiotherapy.
a Medical variables other than treatment with XRT or PRT.
Table 3 also details the medical variables examined in the studies included in this literature review. Well-documented medical confounds that contribute to neuropsychological outcomes include younger age at cancer treatment (Hardy et al., Reference Hardy, Krull, Wefel and Janelsins2018) and history of posterior fossa syndrome (Cámara et al., Reference Cámara, Fournier, Cordero, Melero, Robles, Esteso, Lassaletta and Budke2020; Schreiber et al., Reference Schreiber, Palmer, Conklin, Mabbott, Swain, Bonner, Chapieski, Huang, Zhang and Gajjar2017). Of the eight studies, the six empirical studies provided information on the age of the child at diagnosis. There was no significant difference in age of diagnosis by treatment group in these studies. Two of the six studies considered age at diagnosis in analyses, which were found to predict neuropsychological outcomes (Gross et al., Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019; Peterson & Katzenstein, Reference Peterson and Katzenstein2019). Only two studies reported a history of posterior fossa syndrome. Both Kahalley et al. (Reference Kahalley, Peterson, Ris, Janzen, Okcu, Grosshans, Mahajan, Tsang, Laperriere, Whitehead, Dauser, Taylor, Conklin, Chintagumpala, Bouffet and Mabbott2020) and Gross et al. (Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019) found a history of posterior fossa syndrome to predict specific neuropsychological outcomes.
All studies examined overall intellectual functioning (FSIQ/GAI). Please refer to Table 4 for performance on individual intellectual indices by radiation therapy type. The majority of studies (n = 5) examined broader intelligence indices (e.g., VCI, VSI, FRI, WMI, PSI) (Child et al., Reference Child, Warren, Grosshans, Paulino, Okcu, Ris, Orobio, Cirino, Chintagumpala and Kahalley2021; Eaton et al., Reference Eaton, Fong, Ingerski, Pulsifer, Goyal, Zhang, Weyman, Ebb, MacDonald, Tarbell and Yock2021; Gross et al., Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019; Kahalley et al., Reference Kahalley, Peterson, Ris, Janzen, Okcu, Grosshans, Mahajan, Tsang, Laperriere, Whitehead, Dauser, Taylor, Conklin, Chintagumpala, Bouffet and Mabbott2020; Peterson & Katzenstein, Reference Peterson and Katzenstein2019). Three studies examined neuropsychological outcomes beyond IQ and intelligence indices, including academic achievement (Gross et al., Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019; Merchant et al., Reference Merchant, Hua, Shukla, Ying, Nill and Oelfke2008), attention, learning, and memory (Child et al., Reference Child, Warren, Grosshans, Paulino, Okcu, Ris, Orobio, Cirino, Chintagumpala and Kahalley2021; Gross et al., Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019), fine motor speed, executive functions, and academic fluency (Child et al., Reference Child, Warren, Grosshans, Paulino, Okcu, Ris, Orobio, Cirino, Chintagumpala and Kahalley2021), and visual-motor integration and adaptive functioning (Gross et al., Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019).
Table 4. Examining performance on IQ indices by radiation therapy type

Abbreviations: FSIQ, full scale IQ; PRI/PIQ, perceptual reasoning index/performance intelligence quotient; PRT, proton radiotherapy; PSI, processing speed index; VCI/VIQ, verbal comprehension index/verbal intelligence quotient; WMI, working memory index; XRT, photon radiotherapy.
PRT outcomes versus XRT outcomes
Cross-sectional studies
Three studies (Child et al., Reference Child, Warren, Grosshans, Paulino, Okcu, Ris, Orobio, Cirino, Chintagumpala and Kahalley2021; Eaton et al., Reference Eaton, Fong, Ingerski, Pulsifer, Goyal, Zhang, Weyman, Ebb, MacDonald, Tarbell and Yock2021; Gross et al., Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019) utilized a cross-sectional design to examine neuropsychological outcomes after radiation therapy. Of note, in two of these studies (Child et al., Reference Child, Warren, Grosshans, Paulino, Okcu, Ris, Orobio, Cirino, Chintagumpala and Kahalley2021; Gross et al., Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019) there was a significant difference in time from RT to evaluation by treatment type, with patients who received PRT being seen closer to time of treatment. This is notable as longer time since treatment is associated with worse neuropsychological outcomes (Hardy et al., Reference Hardy, Krull, Wefel and Janelsins2018). In their study, Child et al. (Reference Child, Warren, Grosshans, Paulino, Okcu, Ris, Orobio, Cirino, Chintagumpala and Kahalley2021) further disaggregated neuropsychological outcomes by radiation characteristics (focal or CSI); these findings will therefore be discussed in the respective section below.
Intellectual function
Following treatment, both Gross et al. (Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019) and Eaton et al. (Reference Eaton, Fong, Ingerski, Pulsifer, Goyal, Zhang, Weyman, Ebb, MacDonald, Tarbell and Yock2021) found that patients treated with PRT had higher (better) overall intellectual functioning (FSIQ/GAI; average range) whereas patients treated with XRT demonstrated low average overall intelligence. The difference in FSIQ by RT group was significant in both studies (p < .05), with a large effect size between the two groups (Eaton et al., Reference Eaton, Fong, Ingerski, Pulsifer, Goyal, Zhang, Weyman, Ebb, MacDonald, Tarbell and Yock2021; d = .81). Effect sizes could not be calculated from Gross et al. (Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019) as standard deviations were not reported.
Verbal reasoning
While both studies found that patients treated with PRT had average verbal reasoning skills (VIQ/VCI), there was a range in function in patients treated with XRT (low average in Gross et al., Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019, average in Eaton et al., Reference Eaton, Fong, Ingerski, Pulsifer, Goyal, Zhang, Weyman, Ebb, MacDonald, Tarbell and Yock2021). The difference in VCI by RT group was significant in both studies (p < .05) with a large effect size between the two groups (Eaton et al., Reference Eaton, Fong, Ingerski, Pulsifer, Goyal, Zhang, Weyman, Ebb, MacDonald, Tarbell and Yock2021; d = .92). Patients treated with XRT showed greater deficits than patients treated with PRT.
Perceptual reasoning
The studies by Gross et al. (Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019) and Eaton et al. (Reference Eaton, Fong, Ingerski, Pulsifer, Goyal, Zhang, Weyman, Ebb, MacDonald, Tarbell and Yock2021) found that perceptual/nonverbal reasoning (PIQ/PRI) was average in the PRT group and low average in the XRT group. Of note, this difference was only significant in Eaton et al. (Reference Eaton, Fong, Ingerski, Pulsifer, Goyal, Zhang, Weyman, Ebb, MacDonald, Tarbell and Yock2021), with a large effect size between the two groups (p = .01, d = .91), with patients treated with XRT demonstrating greater deficits than patients treated with PRT.
Working memory
Eaton et al. (Reference Eaton, Fong, Ingerski, Pulsifer, Goyal, Zhang, Weyman, Ebb, MacDonald, Tarbell and Yock2021) compared working memory outcomes (WMIs) by RT type in their cross-sectional study. They found that patients, regardless of treatment type, demonstrated average functioning at the time of their most recent neuropsychological evaluation. Gross et al. (Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019) did not utilize the WMI, but rather looked specifically at auditory working memory skills via the Wechsler Digit Span subtest. They found that patients treated with PRT demonstrated average abilities relative to low average performance in the XRT group. Of note, this difference was significant in the Gross et al. study (p = .03) but not Eaton et al. (Reference Eaton, Fong, Ingerski, Pulsifer, Goyal, Zhang, Weyman, Ebb, MacDonald, Tarbell and Yock2021) (p > .05).
Processing speed
The studies by Eaton et al. (Reference Eaton, Fong, Ingerski, Pulsifer, Goyal, Zhang, Weyman, Ebb, MacDonald, Tarbell and Yock2021) and Gross et al. (Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019) examined processing speed via the Wechsler PSI. In both studies, patients treated with PRT demonstrated low average performance. There was variability in outcomes in patients treated with XRT, with performance falling in the low average (Gross et al., Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019) to very low (Eaton et al., Reference Eaton, Fong, Ingerski, Pulsifer, Goyal, Zhang, Weyman, Ebb, MacDonald, Tarbell and Yock2021) range. Of note, this difference was significant in the Gross et al. (Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019) study (p = .03) but not Eaton et al. (Reference Eaton, Fong, Ingerski, Pulsifer, Goyal, Zhang, Weyman, Ebb, MacDonald, Tarbell and Yock2021) (p > .05).
Beyond IQ indices
Gross et al. (Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019) also examined neuropsychological outcomes beyond IQ indices in their cross-sectional study. They found that patients treated with PRT demonstrated average auditory attention span, story memory, word reading/decoding, math calculation, and parent-reported adaptive functioning. Patients treated with PRT also demonstrated low average visual-motor integration. In contrast, patients treated with XRT had a greater number of neuropsychological skills that fell below normative expectations. Specifically, these patients demonstrated low average auditory attention span, visual-motor integration, word reading/decoding, and math calculation. Parent reported adaptive functioning ranged from low average overall adaptive skills, conceptual, and social skills, to below average practical skills. The difference in neuropsychological outcomes by RT group was statistically significant.
Longitudinal studies
Five studies utilized a longitudinal study design, which allowed for the examination of change in neuropsychological functioning across time. Of note, of these studies, two exploratory (Kahalley et al., Reference Kahalley, Peterson, Ris, Janzen, Okcu, Grosshans, Mahajan, Tsang, Laperriere, Whitehead, Dauser, Taylor, Conklin, Chintagumpala, Bouffet and Mabbott2020; Peterson & Katzenstein, Reference Peterson and Katzenstein2019) and two theory driven (Fortin et al., Reference Fortin, Tsang, Ng, Laperriere and Hodgson2017; Merchant et al., Reference Merchant, Hua, Shukla, Ying, Nill and Oelfke2008) studies gathered baseline neuropsychological data prior to or while receiving radiation therapy. In the remaining study, Kahalley et al. (Reference Kahalley, Ris, Grosshans, Okcu, Paulino, Chintagumpala, Guffey, Minard and Mahajan2016) acknowledge that the absence of this baseline data made it difficult to determine the impact of treatment type alone on cognition; however, this is an inherent consequence of retrospective studies.
Baseline function
Of the four studies that considered baseline neuropsychological functioning, two of the studies provided this information. Kahalley et al. (Reference Kahalley, Peterson, Ris, Janzen, Okcu, Grosshans, Mahajan, Tsang, Laperriere, Whitehead, Dauser, Taylor, Conklin, Chintagumpala, Bouffet and Mabbott2020) found that at baseline (characterized as within 6 months of diagnosis and end of radiation therapy), patients regardless of radiation type demonstrated average overall intellectual functioning (FSIQ), verbal reasoning (VCI), and perceptual reasoning (PRI). Patients in the studies by Kahalley et al. (Reference Kahalley, Peterson, Ris, Janzen, Okcu, Grosshans, Mahajan, Tsang, Laperriere, Whitehead, Dauser, Taylor, Conklin, Chintagumpala, Bouffet and Mabbott2020) and Peterson and Katzenstein (Reference Peterson and Katzenstein2019) both demonstrated average working memory skills (WMI) as well. Processing speed (PSI) was low average for all patients at baseline regardless of RT group (Kahalley et al., Reference Kahalley, Peterson, Ris, Janzen, Okcu, Grosshans, Mahajan, Tsang, Laperriere, Whitehead, Dauser, Taylor, Conklin, Chintagumpala, Bouffet and Mabbott2020; Peterson & Katzenstein, Reference Peterson and Katzenstein2019). There was no significant difference in baseline intelligence scores by treatment group.
Intellectual function
Four of the five studies examined change in overall intellectual functioning (FSIQ) by treatment type. Of these, the theoretical models found that patients treated with PRT had shallower IQ declines as compared to patients treated with XRT 5 years following treatment (Fortin et al., Reference Fortin, Tsang, Ng, Laperriere and Hodgson2017; Merchant et al., Reference Merchant, Hua, Shukla, Ying, Nill and Oelfke2008), though patients regardless of RT type demonstrated declines in IQ across time. The difference in XRT and PRT IQ at 5 years was considered clinically significant (Merchant et al., Reference Merchant, Hua, Shukla, Ying, Nill and Oelfke2008).
Both empirical studies that examined FSIQ across time found that patients treated with PRT demonstrated stable IQ scores across time (Kahalley et al., 2016; Kahalley et al., Reference Kahalley, Peterson, Ris, Janzen, Okcu, Grosshans, Mahajan, Tsang, Laperriere, Whitehead, Dauser, Taylor, Conklin, Chintagumpala, Bouffet and Mabbott2020). In contrast, patients treated with XRT lost on average 0.9 (Kahalley et al., Reference Kahalley, Peterson, Ris, Janzen, Okcu, Grosshans, Mahajan, Tsang, Laperriere, Whitehead, Dauser, Taylor, Conklin, Chintagumpala, Bouffet and Mabbott2020) to 1.1 points (Kahalley et al., Reference Kahalley, Ris, Grosshans, Okcu, Paulino, Chintagumpala, Guffey, Minard and Mahajan2016) points per year. The difference between XRT and PRT IQ across time was not considered clinically significant (Kahalley et al., Reference Kahalley, Ris, Grosshans, Okcu, Paulino, Chintagumpala, Guffey, Minard and Mahajan2016; Kahalley et al., Reference Kahalley, Peterson, Ris, Janzen, Okcu, Grosshans, Mahajan, Tsang, Laperriere, Whitehead, Dauser, Taylor, Conklin, Chintagumpala, Bouffet and Mabbott2020).
Verbal reasoning
One study examined change in verbal reasoning (VCI). Kahalley et al. (Reference Kahalley, Peterson, Ris, Janzen, Okcu, Grosshans, Mahajan, Tsang, Laperriere, Whitehead, Dauser, Taylor, Conklin, Chintagumpala, Bouffet and Mabbott2020) found stable VCI outcomes in patients treated with PRT and XRT across time.
Perceptual reasoning
One study examined change in perceptual reasoning (PRI) by RT type. Kahalley et al. (Reference Kahalley, Peterson, Ris, Janzen, Okcu, Grosshans, Mahajan, Tsang, Laperriere, Whitehead, Dauser, Taylor, Conklin, Chintagumpala, Bouffet and Mabbott2020) found that mean PRI scores across time were significantly higher for patients treated with PRT as compared to those treated with XRT. Change in PRI also differed significantly between RT groups. Specifically, patients treated with PRT showed a 1.0 point increase per year, which was marginally significant (p = .05), whereas patients treated with XRT showed a 0.8 point decline per year, which was not statistically significant.
Working memory
Two studies examined change in working memory (WMI) across time. In the study by Peterson and Katzenstein (Reference Peterson and Katzenstein2019), all patients regardless of treatment type demonstrated a significant decline in working memory across time. In contrast, Kahalley et al. (Reference Kahalley, Peterson, Ris, Janzen, Okcu, Grosshans, Mahajan, Tsang, Laperriere, Whitehead, Dauser, Taylor, Conklin, Chintagumpala, Bouffet and Mabbott2020) found that WMI outcomes were stable across time in patients treated with PRT whereas patients treated with XRT declined by 2.2 points per year. While the mean WMI scores did not differ between RT groups, the change in WMI over time (slope) was significantly different between groups.
Processing speed
Two studies examined changes in graphomotor processing speed as measured by the PSI (Kahalley et al., Reference Kahalley, Peterson, Ris, Janzen, Okcu, Grosshans, Mahajan, Tsang, Laperriere, Whitehead, Dauser, Taylor, Conklin, Chintagumpala, Bouffet and Mabbott2020; Peterson & Katzenstein, Reference Peterson and Katzenstein2019). Both studies found that processing speed declined regardless of treatment type. In the study by Kahalley et al. (Reference Kahalley, Peterson, Ris, Janzen, Okcu, Grosshans, Mahajan, Tsang, Laperriere, Whitehead, Dauser, Taylor, Conklin, Chintagumpala, Bouffet and Mabbott2020) this equated to 0.9 IQ points per year regardless of RT group.
Neuropsychological outcomes by radiation characteristics
As noted above, the study by Child et al. (Reference Child, Warren, Grosshans, Paulino, Okcu, Ris, Orobio, Cirino, Chintagumpala and Kahalley2021) surveyed PRT and XRT outcomes more thoroughly by examining neuropsychological function in patients treated with focal or craniospinal irradiation (CSI), respectively.
Focal radiation
Before adjusting for clinical variables that differed between focal groups, Child et al. (2021) found that both focal RT groups fell significantly below the normative mean on measures of processing speed (PSI, academic fluency, fine motor speeded dexterity). In the focal PRT group, effect sizes ranged from medium (reading fluency d = .44) to large (verbal switching (d = .96). In the focal XRT group, effects sizes were large (d > .68). On all other neuropsychological measures examined, the PRT focal group did not differ significantly from the population mean. After adjusting for covariates, only inhibition/switching was significantly different between focal PRT and focal XRT (p = .04).
CSI radiation
The XRT CSI group performed significantly below the population mean on all neuropsychological measures apart from attention and impulse inhibition (d > 0.8). The PRT CSI group performed within the population mean on all neuropsychological measures with the exception of FSIQ, PSI, fine motor, graphomotor switching, verbal learning, and academic fluency. After adjusting for covariates, task vigilance (p = .02) and impulse inhibition (p = .05) were the only measures to differ significantly when comparing CSI PRT to CSI XRT.
Predictors of neuropsychological function
Some studies examined medical and demographic variables that were associated with neuropsychological outcomes. Significant medical variables that predicted worse neuropsychological outcomes included history of full posterior fossa boost (Child et al., Reference Child, Warren, Grosshans, Paulino, Okcu, Ris, Orobio, Cirino, Chintagumpala and Kahalley2021), lower performance on the Karnofsky/Lansky Scale (Child et al., Reference Child, Warren, Grosshans, Paulino, Okcu, Ris, Orobio, Cirino, Chintagumpala and Kahalley2021; Kahalley et al., Reference Kahalley, Ris, Grosshans, Okcu, Paulino, Chintagumpala, Guffey, Minard and Mahajan2016), higher cranial radiation dose (Fortin et al., Reference Fortin, Tsang, Ng, Laperriere and Hodgson2017; Gross et al., Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019; Merchant et al., Reference Merchant, Hua, Shukla, Ying, Nill and Oelfke2008), medulloblastoma tumor pathology (Fortin et al., Reference Fortin, Tsang, Ng, Laperriere and Hodgson2017), younger age at time of cancer treatment (Gross et al., Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019; Peterson & Katzenstein, Reference Peterson and Katzenstein2019), hydrocephalus requiring shunting (Gross et al., Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019), and history of posterior fossa syndrome (Gross et al., Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019; Kahalley et al., Reference Kahalley, Peterson, Ris, Janzen, Okcu, Grosshans, Mahajan, Tsang, Laperriere, Whitehead, Dauser, Taylor, Conklin, Chintagumpala, Bouffet and Mabbott2020).
Significant demographic variables that predicted worse neuropsychological outcomes included lower SES (Gross et al., Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019), non-White race/ethnicity (Kahalley et al., Reference Kahalley, Ris, Grosshans, Okcu, Paulino, Chintagumpala, Guffey, Minard and Mahajan2016), administration of the Leiter IQ test (Kahalley et al., Reference Kahalley, Ris, Grosshans, Okcu, Paulino, Chintagumpala, Guffey, Minard and Mahajan2016), and female sex (Peterson & Katzenstein, Reference Peterson and Katzenstein2019).
Discussion
This paper is the first systemic review to compare neuropsychological outcomes following PRT and XRT. Given the increasing awareness and use of PRT, research is needed to objectively quantify the neuropsychological advantages of PRT over XRT. In examining neuropsychological outcomes between the RT modalities, cross sectional studies showed that patients treated with PRT demonstrated better neuropsychological functioning as compared to those who received XRT across the domains of overall intellectual functioning, and verbal and perceptual reasoning. Fewer studies examined outcomes beyond intelligence. Thus, while results are promising for better visual-motor integration, attention, academic achievement, and parent-reported adaptive skills in patients treated with PRT, studies are limited in number. Performance on processing speed and working memory measures was inconclusive, with some studies showing sparing of these domains while others documented weaknesses in these domains that did not differ by treatment type. More research is needed to determine the effect of PRT on working memory and processing speed given that these skills are especially vulnerable in the pediatric brain tumor population (King et al., Reference King, Ailion, Fox and Hufstetler2019).
Longitudinal studies added to the well-documented findings that XRT results in lower neuropsychological functioning across time. In contrast, PRT was associated with stable neuropsychological skills across time, with the exception of working memory and processing speed which showed variable outcomes on both cross-sectional and longitudinal studies. Differences in cognitive outcomes may be due to underlying neuropathological pathways. XRT results in neuroinflammation by way of damage to glial cells, including microglia, astrocytes, and oligodendrocytes (Lumniczky et al., Reference Lumniczky, Szatmári and Sáfrány2017; Kalm et al., Reference Kalm, Lannering, Björk-Eriksson and Blomgren2009; Xue et al., Reference Xue, Dong, Huang, Qu, Wu and Dong2014). Activated microglia and astrocytes secrete inflammatory cytokines and produce reactive oxygen species, propagating an ongoing cascade of chronic neuroinflammation, which can persist for years after CRT (Burns et al., Reference Burns, Awad, Li and Grant2016; Jenrow et al., Reference Jenrow, Brown, Lapanowski, Naei, Kolozsvary and Kim2013; Kalm et al., Reference Kalm, Lannering, Björk-Eriksson and Blomgren2009; Panagiotakos et al., Reference Panagiotakos, Alshamy, Chan, Abrams, Greenberg, Saxena, Bradbury, Edgar, Gutin and Tabar2007; Xue et al., Reference Xue, Dong, Huang, Qu, Wu and Dong2014). Additionally, oligodendrocytes are highly sensitive to CRT due to their high metabolic demand and mitochondrial content, leaving them more vulnerable to oxidative stress and subsequent neuroinflammation (Burns et al., Reference Burns, Awad, Li and Grant2016). This neuroinflammation results in demyelination and neurogenesis (Boyd et al., Reference Boyd, Byrne, Middleton, Banati and Liu2021; Moxon-Emre et al., Reference Moxon-Emre, Bouffet, Taylor, Laperriere, Sharpe, Laughlin, Scantlebury, Law, Skocic, Richard and Mabbott2016). Because XRT comes into contact with normal brain parenchyma, neuroinflammation and oxidative stress can occur in healthy tissue. Given the more conformal radiation dose inherent to PRT, there is presumed to be less neuroinflammation and oxidate stress.
While the above noted studies may provide preliminary evidence of the advantage of PRT to XRT, patients were not comparable across medical and sociodemographic factors, which may have contributed to differences in neuropsychological outcomes. In terms of medical variables, patients treated with XRT more often had tumors in the posterior fossa (Gross et al., Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019), received cisplatin (Gross et al., Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019), received a full posterior fossa boost (Child et al., Reference Child, Warren, Grosshans, Paulino, Okcu, Ris, Orobio, Cirino, Chintagumpala and Kahalley2021), had higher CSI dose (Child et al., Reference Child, Warren, Grosshans, Paulino, Okcu, Ris, Orobio, Cirino, Chintagumpala and Kahalley2021), required a ventriculoperitoneal (VP) shunt (Kahalley et al., Reference Kahalley, Ris, Grosshans, Okcu, Paulino, Chintagumpala, Guffey, Minard and Mahajan2016), required a craniotomy (Kahalley et al., Reference Kahalley, Ris, Grosshans, Okcu, Paulino, Chintagumpala, Guffey, Minard and Mahajan2016), had greater boost margins (Kahalley et al., Reference Kahalley, Peterson, Ris, Janzen, Okcu, Grosshans, Mahajan, Tsang, Laperriere, Whitehead, Dauser, Taylor, Conklin, Chintagumpala, Bouffet and Mabbott2020), had a larger boost to the tumor bed (Child et al., Reference Child, Warren, Grosshans, Paulino, Okcu, Ris, Orobio, Cirino, Chintagumpala and Kahalley2021; Kahalley et al., Reference Kahalley, Peterson, Ris, Janzen, Okcu, Grosshans, Mahajan, Tsang, Laperriere, Whitehead, Dauser, Taylor, Conklin, Chintagumpala, Bouffet and Mabbott2020), and had a longer time between treatment and neuropsychological assessment (Child et al., Reference Child, Warren, Grosshans, Paulino, Okcu, Ris, Orobio, Cirino, Chintagumpala and Kahalley2021; Gross et al., Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019). These variables are important to highlight given that all of these medical factors in and of themselves are associated with greater neuropsychological sequelae.
In terms of sociodemographic factors, patients treated with XRT were of lower socioeconomic based on median household income of patient’s zipcode (Eaton et al., Reference Eaton, Fong, Ingerski, Pulsifer, Goyal, Zhang, Weyman, Ebb, MacDonald, Tarbell and Yock2021; Gross et al., Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019), and included a higher proportion of non-White patients (Gross et al., Reference Gross, Powell, Zelko, Hartsell, Goldman, Fangusaro, Lulla, Pillay Smiley, Han-Chih Chang and Gondi2019). Sociodemographic discrepancies between the RT groups is concerning given the importance of environmental enrichment and “cognitive reserve” on neuropsychological outcomes. In studies examining neurodevelopmental outcomes in very preterm neonates, higher maternal education and higher SES attenuated the association between brain injury and outcomes (Benavente-Fernandez et al., Reference Benavente-Fernández, Synnes, Grunau, Chau, Ramraj, Glass, DalitCayam-Rand, Siddiqi and Miller2019). Thus, it is not possible to unequivocally determine the treatment-related neuropsychological benefits of PRT relative to XRT. Moreover, these findings lend themselves to the discussion that PRT is perhaps encouraging disparities in access to healthcare services. Patients with higher SES and potentially increased “healthcare literacy” and access to resources are receiving what appears to be the more precise treatment, thus: (1) contributing to healthcare disparities; or (2) not capturing the possible negative consequences in outcomes in more vulnerable groups with less resources.
Interestingly, the majority of studies looked at one to two non-medical/sociodemographic factors, most frequently race/ethnicity, with some studies also incorporating SES or insurance type. There has been increasing interest in appreciating the relationship between family environmental factors and child neuropsychological function across medical populations, most notably childhood traumatic brain injury (Durish et al., Reference Durish, Yeates, Stancin, Taylor, Walz and Wade2018; Taylor et al., Reference Taylor, Yeates, Wade, Drotar, Stancin and Minich2002; Wade et al., Reference Wade, Cassedy, Walz, Taylor, Stancin and Yeates2011; Yeates et al., 2010). Pediatric oncology researchers inconsistently consider the role of family environmental factors such as two parent households, parental education and occupation, and familial supports, despite the albeit limited research suggesting a relationship between family function and neuropsychological outcomes (Ach et al., Reference Ach, Gerhardt, Barrera, Kupst, Meyer, Patenaude and Vannatta2013; Kullgren et al., Reference Kullgren, Morris, Morris and Krawiecki2003; Laliberté Durish et al., Reference Laliberté Durish, Moxon-Emre, Bouffet, Bartels and Mabbott2021; Quast et al., Reference Quast, Phillips, Li, Kazak, Barakat and Hocking2018). In addition, researchers should consider community and systemic factors that impact the child’s environment as well, such as via the Area Deprivation Index (ADI), which considers social determinants of health within the child’s community including education quality, income/employment, neighborhood housing, and household characteristics. Studies that have utilized the ADI have shown lower scores to be associated with worse health outcomes including morbidity and mortality (Hunt et al., Reference Hunt, Vogt, Jonaitis, Buckingham, Koscik, Zuelsdorff, Clark, Yu, Okonkwo, Johnson, Asthana, Bendlin and Kind2021).
While six studies utilized a longitudinal study design, none considered practice effects. Indeed, repeated neuropsychological evaluations are common in clinical practice in order to inform intervention efficacy and make determinations about improvements or declines in functioning. The fact that patients declined across time on specific repeated measures is concerning given that practice effects have been shown to provide diagnostic information (Duff et al., Reference Duff, Callister, Dennett and Tometich2012). Multiple studies have shown diminished practice effects in patients with mild neuropsychological impairment and dementia as compared to healthy adults who demonstrated the expected practice effects (Calero & Navarro, Reference Calero and Navarro2004; Duff et al., Reference Duff, Callister, Dennett and Tometich2012; Fernandez-Ballesteros et al., Reference Fernández-Ballesteros, Zamarrón and Tàrraga2005; Suchy et al., Reference Suchy, Kraybill and Franchow2011). This is important to consider within the context of childhood cancer, as survivors are at increased risk for accelerated aging, which is associated with early onset of chronic health conditions, physical frailty, and neuropsychological impairment typically associated with older adults (Schuitema et al., Reference Schuitema, Alexander, Hudson, Krull and Edelstein2021). The absence of practice effects in these studies may be suggestive of very early signs of accelerated aging and may be an important variable to monitor in these survivors.
Type of radiation therapy (focal or CSI) appears to influence neuropsychological outcomes. Patients who received focal PRT performed comparably to population means on most neuropsychological measures aside from speeded tasks (graphomotor processing speed, fine motor speed, academic fluency), which were relative weaknesses for both treatment groups. However, the difference between speeded outcomes by focal treatment groups was not statistically significant, suggesting that there is no pervasive benefit of focal PRT over focal XRT. Patients treated with CSI, regardless of PRT or XRT, performed well below normative means on multiple measures, indicating that CSI in and of itself is associated with neuropsychological deficits, though the XRT CSI group was the most impaired.
Limitations
There are several limitations that warrant consideration when drawing conclusions from these eight studies. First, only one study (Peterson & Katzenstein, Reference Peterson and Katzenstein2019) provided the neuropsychological data at baseline (prior to or during radiation treatment). Without baseline data, it is difficult to draw conclusions about neuropsychological outcomes across time given that premorbid functioning serves as a predictor of outcomes following an injury (i.e., “cognitive reserve”). While longitudinal studies are inherently stronger studies given that patients are compared to themselves as opposed to normative means, the clinical nature of these studies resulted in a large time frame between start of radiation treatment and first evaluation, as well as time between initial and follow-up evaluations. Additional differences in outcome by treatment type may be due to a number of additional factors. In order to increase sample sizes, studies utilized more heterogenous tumor histology. Utilizing more heterogenous pathologies limits the specificity of these findings. Moreover, treatment history above and beyond radiation type differed by medical and sociodemographic variables, which are all known risk factors for neuropsychological impairment in pediatric oncology populations. Lastly, inherent to research studies utilizing retrospective clinical data, neuropsychological outcomes were examined by a variety of neuropsychological measures or broad indices.
Future directions
As a field, we need to ensure that we are not contributing to systemic racism, poverty, and healthcare inequities, which impact long term neuropsychological outcomes. Therefore, to truly understand neuropsychological outcomes differences between patients treated with XRT and PRT, studies need a comparable representation of SES and race/ethnicity, parental education, material hardships, and school quality. Additionally, studies should gather baseline measures of neuropsychological functioning prior to radiation therapy in order to examine change in function over time as premorbid function is predictive of long-term neuropsychological outcomes in pediatric oncology patients (Hoskinson et al., Reference Hoskinson, Wolfe, Yeates, Mahone, Cecil and Ris2018; Raghubar et al., Reference Raghubar, Rothhaar, Yeates, Mahone, Grosshans, Scheurer and Ris2020). Moreover, studies should utilize neuropsychological measures with strong psychometric properties and consider reliable change indices when examining plausible change over time.
In order to bolster claims regarding the efficacy of PRT over XRT, future studies should utilize neuroimaging metrics such as magnetic resonance imaging and diffusion tensor imaging to correspond with neuropsychological outcomes. To date, no studies have directly compared imaging metrics between the two treatment groups despite the awareness that cranial radiation results in diffuse and multifocal white matter abnormalities, cerebral atrophy, and white matter volume loss, likely secondary to alterations in brain microstructure, damage to oligodendrocytes, and glial apoptosis (Ailion et al., Reference Ailion, King, Roberts, Tang, Turner, Conway and Crosson2020; Ailion et al., Reference Ailion, Roberts, Crosson and King2019). Given that cranial radiation therapy directly impacts white matter microstructure, it is critical to consider the anatomical and physiological impacts of radiation type. In addition to neuroimaging measures, complementary measures of neuroinflammation that are less time intensive than neuroimaging should also be considered in future studies examining white matter integrity following radiation therapy. While this has not been examined in pediatric neuro-oncology samples to date, studies in other medical conditions with white matter injury such as traumatic brain injury and neonatal hypoxic ischemic injury have shown elevated serum biomarkers of glial injury (Dadas et al., Reference Dadas, Washington, Diaz-Arrastia and Janigro2018). Capturing these measures at the time of other medically necessary blood panel monitoring may help reduce overall burden on the patients as science better understands the benefits of different and plausibly complementary biomarkers. Furthermore, future studies should consider homogeneity of samples to provide greater precision in characterizing individual contributors to outcomes including tumor location (e.g., cerebellum; Clark et al., Reference Clark, Semmel, Aleksonis, Steinberg and King2021), tumor genetics (e.g., Northcott et al., Reference Northcott, Robinson, Kratz, Mabbott, Pomeroy, Clifford, Rutkowski, Ellison, Malkin, Taylor and Pfister2019) and host genetics (Kautianen et al., Reference Kautiainen, Aleksonis and King2022).
Conclusions
This systemic review illuminates the sociodemographic inequities in access to PRT, which may contribute to neuropsychological outcomes. As has been articulated in previous studies (Ofuya et al., Reference Ofuya, McParland, Murray, Brown, Sebag-Montefiore and Hall2019), patients are not randomized to PRT, thus outcomes may reflect greater health literacy and community resources. More information is needed to objectively determine whether neuropsychological outcomes—including those beyond IQ indices—following PRT are better as a result of improved conformality and not sociodemographic differences between treatment groups.
Acknowledgements
We thank the informationists at the Johns Hopkins University Welch Medical Library for assistance with designing and conducting the systematic review.
Funding statement
None.
Conflicts of interest
None.