Acute tryptophan depletion induces transient symptoms in 50-60% of those with remitted depression treated with a serotonergic antidepressant (Reference Booij, Van der Does and RiedelBooij et al, 2003). Evidence suggests that it causes a return of symptoms that were present before treatment (Reference Delgado, Charney and PriceDelgado et al, 1990), but others argue that it mainly induces physical symptoms (Reference Lam and YathamLam & Yatham, 2003). Studies have almost exclusively relied on the Hamilton Rating Scale for Depression (HRSD), which includes many somatic items (Reference HamiltonHamilton, 1960). Hence, the behavioural specificity of acute tryptophan depletion is still uncertain. Furthermore, symptom provocation studies are now controversial. For instance, front-page articles in The Boston Globe (Reference Whitaker and KongWhitaker & Kong, 1998) accused researchers of abusing patients for financial profit or professional advancement. No lasting or serious side-effects have been reported for acute tryptophan depletion, suggesting that the procedure is safe. However, the views of participants have not been investigated. The aims of the present study were to investigate the behavioural specificity of acute tryptophan depletion and to collect data on participants' opinions on a depletion study.
METHOD
Participants
Eligible participants for the acute tryptophan depletion trial were out-patients of a mood disorders clinic. Inclusion criteria were: age between 18 and 65 years; ongoing treatment with a selective serotonin reuptake inhibitor (SSRI) or serotonin-noradrenaline reuptake inhibitor (SNRI) for at least 4 weeks; meeting DSM-IV criteria for depression in remission (American Psychiatric Association, 1994); and an HRSD (17-item version) score lower than 15. Exclusion criteria were: major physical illness; substance misuse within the past 3 months; psychosis (lifetime); lactation; and pregnancy. Diagnoses were clarified with the Structured Clinical Interview for DSM-IV (SCID; Reference First, Spitzer and GibbonFirst et al, 1995). After a complete oral and written description of the study, written informed consent was obtained. Twenty-three participants were enrolled in the trial, two of whom subsequently withdrew.
After the tryptophan challenge, all 23 participants, together with a further 18 (10 females) who had participated in another acute tryptophan depletion project, were asked to complete a questionnaire about their experiences during the study. Participants in the other project also had remitted depression and the design, procedure and inclusion criteria of that project were identical to the present study.
Design
The study had a randomised double-blind crossover design with two depletion sessions, separated by at least 4 days. The hospital pharmacist was responsible for the randomisation.
Amino acid mixtures
At each depletion session, patients received in randomised order either a 100 g or a 25 g amino acid mixture. The composition of the 100 g mixture (aimed at reducing tryptophan levels by 90%) was as follows: 5.5 g l-alanine, 3.2 g l-histidine, 13.5 g l-leucine, 12.2 g l-proline, 6.9 g l-tyrosine, 4.9 g l-arginine, 5.7 g l-phenylalanine, 6.9 g l-threonine, 6.9 g l-serine, 8.9 g l-valine, 2.7 g l-cysteine, 8.0 g l-isoleucine, 3.0 g l-methionine, 11.0 g l-lysine HCL and 3.2 g glycine. The 25 g mixture (aimed at 50% reduction) consisted of the same amino acids but in one-quarter the amount (Reference Krahn, Lu and KleeKrahn et al, 1996). Amino acids were mixed with cold water to a final volume of 300 ml. Liquid chocolate syrup was added, and the mixture was served chilled to limit the unpleasant taste of some of the amino acids. Participants were kept on a 24-h low-tryptophan diet (160 mg/day) prior to both sessions. During the depletion sessions, water, (de)caffeinated coffee, (herb) tea, orange juice and protein-poor (<0.05 g) biscuits were allowed in standard amounts. Patients had a low-tryptophan lunch 3 h after drinking the mixture.
Assessments
We used the Comprehensive Psychopathological Rating Scale (CPRS) to assess symptoms (Reference Goekoop, Hoeksema and Knoppert van der KleinGoekoop et al, 1992). The CPRS is a 68-item interview/observation scale with item scores ranging from 0 (absent) to 6 (very severe), including the Montgomery-Åsberg Depression Rating Scale (MADRS; Reference Montgomery and AsbergMontgomery & Åsberg, 1979) and the Brief Anxiety Scale (Reference Tyrer, Owen and CicchettiTyrer et al, 1984). Factor analytic research has revealed that the CPRS consists of six factors (Reference Goekoop, Hoeksema and Knoppert van der KleinGoekoop et al, 1992). Emotional dysregulation refers to a number of symptoms common to both anxiety and depression. Motivational inhibition refers to dysregulation of appetite, interest and motor inhibition, whereas motor disinhibition refers to ‘manic-like’ symptoms. The subscale behavioural disintegration refers to items such as agitation, slowness of movement and emotional indifference. Perceptual disintegration refers to psychotic symptoms. Autonomic dysregulation measures autonomic arousal/anxiety. For the present study, a number of questions were added to the CPRS interview, to allow scoring of the 17-item HRSD. Ratings were performed by a trained interviewer who was masked to the sequence of the mixtures and to the research question (i.e. the specificity question).
Self-report measures included the Beck Depression Inventory II (BDI-II; Reference Beck, Steer and BallBeck et al, 1996) and the Positive and Negative Affect Schedule (PANAS; Reference Watson, Clark and TellegenWatson et al, 1988). The BDI-II is more focused on cognitions than other depression scales, and has three subscales: affective, somatic, cognitive (Reference Van der DoesVan der Does, 2002). The PANAS is based on the tripartite model of anxiety and depression (Reference Watson, Clark and TellegenWatson et al, 1988). It measures negative affect (common to anxiety/distress and depression) and positive affect (low scores specific for depression). Participants also rated a list of 48 physical symptoms on a five-point scale.
The Self-referent Adjectives Encoding and Recall Task (SAERT; Reference Dobson and ShawDobson & Shaw, 1987) was used to assess self-schemata relevant to depression. The task consists of a random presentation of ten positive and ten negative adjectives and six neutral words, preceded by three neutral practice trials. Words were selected from a validated list of personality trait words (Reference AndersonAnderson, 1968) and matched on word frequency, word length and number of syllables. Each word was presented twice. At the first presentation, participants had to decide as quickly as possible whether or not the word was self-descriptive. Immediately thereafter, the same word was presented again, accompanied by a six-point scale ranging from -3 (not at all applicable) to +3 (very applicable). Response speed was emphasised for the initial (yes/no) ratings, not for the second presentation. Immediately after the second presentation, participants were asked to recall the adjectives presented.
Ethics questionnaire
The questionnaire contained 28 items concerning the quality of the informed consent procedure and participants' experiences during the study. Reasons for participation were also collected. The questionnaire was completed anonymously; a code could link the questionnaire data to the project in which the individual had participated. The psychometric properties of the questionnaire are unknown.
Blood plasma
We collected venous blood (10 ml) into tubes containing ethylenediamine tetra-acetic acid (EDTA) to determine the total plasma tryptophan concentration and the ratio of total tryptophan to large neutral amino acids. Immediately after sampling, plasma was centrifuged for 20 min at 2650 g and frozen at -65 8C prior to quantitative amino acid analysis by high-performance liquid chromatography (Reference Fekkes, van Dalen and EdelmanFekkes et al, 1995).
Procedure
After receiving oral and written information about the study, eligible participants were invited to a screening interview that included the SCID-IV, the HRSD and MADRS questionnaires and an interview with a dietician. During day 1 of each session, participants consumed the prepacked low-tryptophan meals. Participants arrived at the laboratory the next morning (8 or 9 a.m.) after an overnight fast. Symptoms and side-effects were assessed at baseline (arrival at the laboratory) (-1 h), +6.5 h and the next morning (+24 h). The SAERT was conducted at both sessions at +4.5 h. Blood samples were taken at -1 h, at +6 h and at +24 h. All participants were tested individually and were paid €115 for participation.
At the end of their participation, individuals were asked to complete the ethics questionnaire and to mail it in a prepaid envelope to an independent investigator. The address was a university in a different city, and it was emphasised that the data would remain confidential. To test whether the questions were well understood, we administered the questionnaire in a semi-structured interview format to the first four participants. The results did not differ from the other completed questionnaires. The study was approved by an independent medical ethics committee.
Statistical analysis
All variables were examined for accuracy of data entry, missing values and fit between their distributions and the assumptions of data analysis. To analyse the mood ratings, we used general linear models (GLM) for repeated measures with intervention (100 g amino acids v. 25 g amino acids) and time of assessment (pre- v. post-depletion v. the next day) as within-subject factors and gender as a between-subject factor. Contrasts tested for differences between specific time points.
Although the choice of the scales was based on prior research and theoretical considerations, a more stringent level of a may be needed to keep overall a under control. This could cause a power problem, although the sample size of our study is comparable with those of other acute tryptophan depletion studies. To correct for multiple comparisons and to retain reasonable power, we set a at 0.15 (Reference StevensStevens, 1996). Because there were 14 sub-scales, each variable was tested at the 0.15/14=0.01 level of significance. Contrast tests were not corrected because they were planned.
For each significant scale, effect size (Cohen's d) was calculated as follows (Reference Cortina and NouriCortina & Nouri, 2000; Reference Rosnow and RosenthalRosnow & Rosenthal, 2003):
where
![]() \batchmode \documentclass[fleqn,10pt,legalpaper]{article} \usepackage{amssymb} \usepackage{amsfonts} \usepackage{amsmath} \pagestyle{empty} \begin{document} \({\sigma}_{\mathrm{pooled}}={\surd}[({\sigma}_{1}^{2}+{\sigma}_{2}^{2}){/}2]\) \end{document}
; M
1 and M
2 are the means of each scale at baseline and at time t=+6.5 h; and σ2
1 and σ2
2 are the corresponding variances.
\batchmode \documentclass[fleqn,10pt,legalpaper]{article} \usepackage{amssymb} \usepackage{amsfonts} \usepackage{amsmath} \pagestyle{empty} \begin{document} \({\sigma}_{\mathrm{pooled}}={\surd}[({\sigma}_{1}^{2}+{\sigma}_{2}^{2}){/}2]\) \end{document}
; M
1 and M
2 are the means of each scale at baseline and at time t=+6.5 h; and σ2
1 and σ2
2 are the corresponding variances.
For the SAERT, intervention was the only within-subject factor. Clinical and demographic variables were analysed by univariate GLM and χ2-tests.
RESULTS
Demographic data
Of 23 individuals who satisfied the inclusion criteria, 21 completed the study. Two (both male) withdrew after the first session; the first owing to a severe headache on the depletion day (after 25 g amino acids), the second because of health problems after the first session (100 g amino acids) unlikely to be caused by acute tryptophan depletion. These two are not included in the analyses. One female participant vomited about 10 min after ingestion of the 100 g mixture, but she was able to complete both sessions. Because the reduction of plasma tryptophan levels with the 100 g mixture in this participant was only marginally higher than with the 25 g mixture (64% v. 55%), and much lower than the average reduction with the 100 g mixture (see below), this individual was also excluded.
Data screening
One participant missed one item on the PANAS positive scale; this item score was replaced by the mean of the remaining items for that individual on that scale. Using Mahalonobis distances and the standardised residuals criterion, we detected an outlier on the MADRS and CPRS sub-scales of emotional dysregulation and motivational inhibition (d 2 > 16.1; | z-residual | > 3) and HRSD (d 2=15.3; | z-residual | >3) and HRSD (d 2=15.3; | z-residual | >3). This patient had a response with the 25 g mixture and no response after 100 g. The clinical and demographic characteristics of the sample are presented in Table 1.
Table 1 Clinical and demographic characteristics of the participants (n=20)
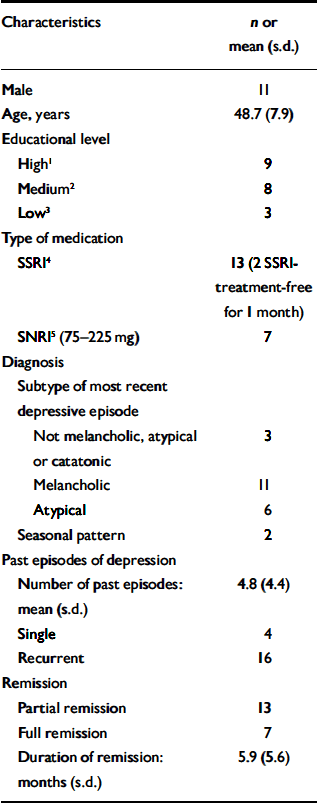
| Characteristics | n or mean (s.d.) |
|---|---|
| Male | 11 |
| Age, years | 48.7 (7.9) |
| Educational level | |
| High1 | 9 |
| Medium2 | 8 |
| Low3 | 3 |
| Type of medication | |
| SSRI4 | 13 (2 SSRI-treatment-free for 1 month) |
| SNRI5 (75-225 mg) | 7 |
| Diagnosis | |
| Subtype of most recent depressive episode | |
| Not melancholic, atypical or catatonic | 3 |
| Melancholic | 11 |
| Atypical | 6 |
| Seasonal pattern | 2 |
| Past episodes of depression | |
| Number of past episodes: | 4.8 (4.4) |
| mean (s.d.) | |
| Single | 4 |
| Recurrent | 16 |
| Remission | |
| Partial remission | 13 |
| Full remission | 7 |
| Duration of remission: | 5.9 (5.6) |
| months (s.d.) |
Biochemical effects
Full depletion significantly reduced total tryptophan and the tryptophan/large neutral amino acids ratio at +6 h by 86% (s.d.=5.5) and 93% (s.d.=4.2), respectively. During partial depletion, the average reductions were 47% (s.d.=14.3) for total tryptophan and 42% (s.d.=17.1) for the tryptophan/large neutral amino acids ratio.
Symptoms
The behavioural effects of both the 25 g and 100 g mixtures are presented in Table 2. Significant time × intervention effects were found for the HRSD (F=8.5, d.f.=2,34, P=0.001), the MADRS (F=10.6, d.f.=2,34, P < 0.001), and the CPRS sub-scales of emotional dysregulation (F=6.7, d.f.=2,34, P=0.003), motivational inhibition (F=7.8, d.f.=2,34, P=0.002) and disintegration (F=5.4, d.f.=2,36, P=0.009). The MADRS change score data revealed two clearly separable groups 7 participants had a mood change of at least six points (range 6-12) and the remaining 13 participants had no mood change (range -1 to +2). The HRSD gave a comparable division, but less clearly separable groups: 7 participants had a change of at least three points (range 3-6), the remaining participants had smaller or no changes (range -2 to +2). The agreement between the two scales was 90% (kappa 0.56, P=0.01). The interaction for the BDI-cognitive sub-scale approached significance (F=3.3, d.f.=2,36, P=0.05) and the BDI-total was significant (F=3.9, d.f.=2,36, P=0.03).
Table 2 Mood assessment scores according to intervention and time of assessment, t
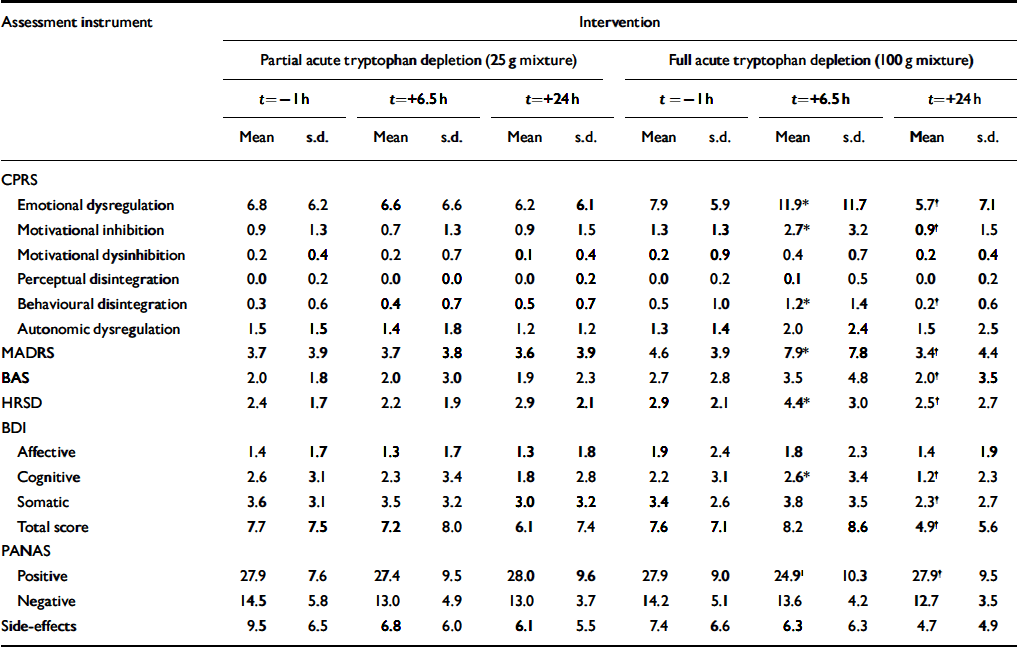
| Assessment instrument | Intervention | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Partial acute tryptophan depletion (25 g mixture) | Full acute tryptophan depletion (100 g mixture) | |||||||||||
| t=−1 h | t=+6.5 h | t=+24 h | t=−1 h | t=+6.5 h | t=+24 h | |||||||
| Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | |
| CPRS | ||||||||||||
| Emotional dysregulation | 6.8 | 6.2 | 6.6 | 6.6 | 6.2 | 6.1 | 7.9 | 5.9 | 11.9* | 11.7 | 5.7† | 7.1 |
| Motivational inhibition | 0.9 | 1.3 | 0.7 | 1.3 | 0.9 | 1.5 | 1.3 | 1.3 | 2.7* | 3.2 | 0.9† | 1.5 |
| Motivational dysinhibition | 0.2 | 0.4 | 0.2 | 0.7 | 0.1 | 0.4 | 0.2 | 0.9 | 0.4 | 0.7 | 0.2 | 0.4 |
| Perceptual disintegration | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | 0.2 | 0.1 | 0.5 | 0.0 | 0.2 |
| Behavioural disintegration | 0.3 | 0.6 | 0.4 | 0.7 | 0.5 | 0.7 | 0.5 | 1.0 | 1.2* | 1.4 | 0.2† | 0.6 |
| Autonomic dysregulation | 1.5 | 1.5 | 1.4 | 1.8 | 1.2 | 1.2 | 1.3 | 1.4 | 2.0 | 2.4 | 1.5 | 2.5 |
| MADRS | 3.7 | 3.9 | 3.7 | 3.8 | 3.6 | 3.9 | 4.6 | 3.9 | 7.9* | 7.8 | 3.4† | 4.4 |
| BAS | 2.0 | 1.8 | 2.0 | 3.0 | 1.9 | 2.3 | 2.7 | 2.8 | 3.5 | 4.8 | 2.0† | 3.5 |
| HRSD | 2.4 | 1.7 | 2.2 | 1.9 | 2.9 | 2.1 | 2.9 | 2.1 | 4.4* | 3.0 | 2.5† | 2.7 |
| BDI | ||||||||||||
| Affective | 1.4 | 1.7 | 1.3 | 1.7 | 1.3 | 1.8 | 1.9 | 2.4 | 1.8 | 2.3 | 1.4 | 1.9 |
| Cognitive | 2.6 | 3.1 | 2.3 | 3.4 | 1.8 | 2.8 | 2.2 | 3.1 | 2.6* | 3.4 | 1.2† | 2.3 |
| Somatic | 3.6 | 3.1 | 3.5 | 3.2 | 3.0 | 3.2 | 3.4 | 2.6 | 3.8 | 3.5 | 2.3† | 2.7 |
| Total score | 7.7 | 7.5 | 7.2 | 8.0 | 6.1 | 7.4 | 7.6 | 7.1 | 8.2 | 8.6 | 4.9† | 5.6 |
| PANAS | ||||||||||||
| Positive | 27.9 | 7.6 | 27.4 | 9.5 | 28.0 | 9.6 | 27.9 | 9.0 | 24.91 | 10.3 | 27.9† | 9.5 |
| Negative | 14.5 | 5.8 | 13.0 | 4.9 | 13.0 | 3.7 | 14.2 | 5.1 | 13.6 | 4.2 | 12.7 | 3.5 |
| Side-effects | 9.5 | 6.5 | 6.8 | 6.0 | 6.1 | 5.5 | 7.4 | 6.6 | 6.3 | 6.3 | 4.7 | 4.9 |
The total number of side-effects decreased in both sessions. Scores that were notably lower at t=+6.5 h compared with t= -1 h were ‘angry/irritable’, ‘sweating’ and ‘tension’. Scores on the items ‘nausea’ and ‘feel sick’ increased, with no differences between conditions.
Self-referent Adjectives Encoding and Recall Task (SAERT)
Prior to analysis, consistency of the answers across presentations was checked. Inconsistent answers (e.g. if a participant pressed the ‘yes’ button for the word ‘lazy’ and then rated it as ‘not applicable’ on second rating) were analysed separately. Outcome measures were highly skewed, except for percentage of words recalled. Reaction times were log10 transformed. Non-parametric tests were used for the other skewed variables of the SAERT because transformations were unsuccessful.
Participants were more likely to rate positive than negative traits as self-descriptive, and they needed less time for positive than for negative traits to decide whether they were self-descriptive. There was no main effect of acute tryptophan depletion. However, in responders (Δ MADRS ≥6) full depletion tended to decrease the consistency of positive trait ratings compared with partial depletion (mean % consistency 90% (s.d.=1.1) v. 96% (s.d.=0.8); Z= -71.89, P=0.06, two-tailed; Fisher exact P=0.04, one-tailed). This was not true for non-responders (mean % consistency 88% (s.d.=1.0) v. 92% (s.d.=1.0)). Participants who were inconsistent usually first pressed the ‘yes’ button (under time pressure) and then rated it as ‘not applicable’ (full depletion: 17 times; partial depletion: 18 times); the reverse happened only once under both conditions.
There were significant effects of intervention (F=4.7, d.f.=1,18, P=0.04) and intervention × mood response (F=5.7, d.f.=1,18, P=0.03) on memory for positive words. Full depletion decreased immediate recall of positive words in responders but not in non-responders (38% v. 2% relative to partial depletion). There were no other differences between responders and non-responders.
Computation of effect sizes
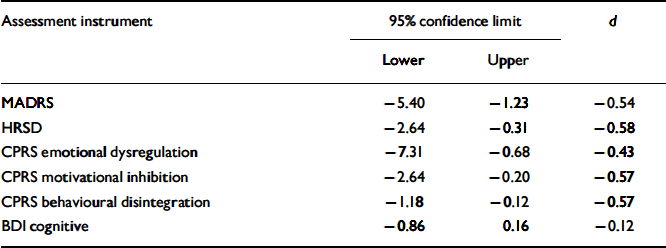
Cohen's d for the significant symptom scales are displayed in Table 3.
Table 3 Confidence intervals and effect sizes of the significant mood assessment scores. Values are calculated for the full depletion condition; t=+6.5 minus t=−1. Negative d means worsening of symptoms
| Assessment instrument | 95% confidence limit | d | |
|---|---|---|---|
| Lower | Upper | ||
| MADRS | −5.40 | −1.23 | −0.54 |
| HRSD | −2.64 | −0.31 | −0.58 |
| CPRS emotional dysregulation | −7.31 | −0.68 | −0.43 |
| CPRS motivational inhibition | −2.64 | −0.20 | −0.57 |
| CPRS behavioural disintegration | −1.18 | −0.12 | −0.57 |
| BDI cognitive | −0.86 | 0.16 | −0.12 |
Gender effects
At baseline, there were no significant demographic or clinical differences between males and females (Fig. 1). Morning (t= -1 h) MADRS scores were higher in females at both sessions (partial depletion F=4.3, d.f.=1,17, P=0.05; full depletion F=6.9, d.f.=1,17, P=0.02) and the intervention × time × gender interaction was significant (F=6.9, d.f.=2,34, P=0.003).
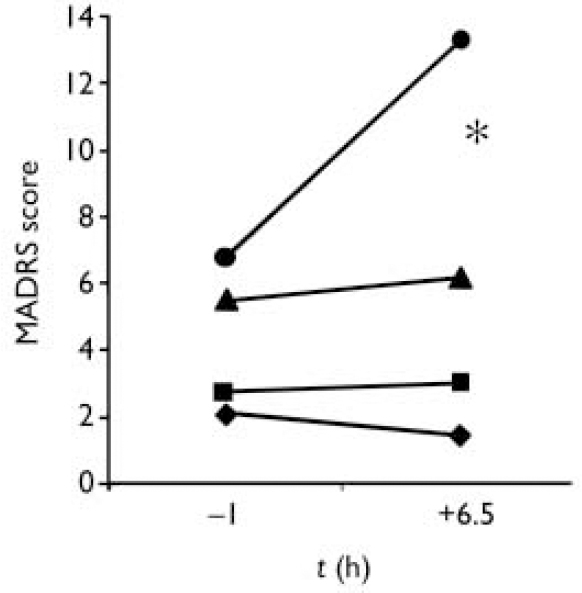
Fig.1 The effects of acute tryptophan depletion on the Montgomery-Åsberg Depression Rating Scale (MADRS) according to gender; -•- females, full depletion; -▪- males, full depletion; -▴- females, partial depletion; -♦- males, partial depletion. * F=5.1, d.f.=1,17, P=0.04.
The increase of MADRS scores between t= -1 h and t=+6.5 h after full depletion was larger in females (Fig. 1). All responders were women. Females also tended to have a larger increase of BDI-somatic scores than males in the full depletion condition at t=+6.5 h (P=0.07).
Order of administration
Type of intervention did not interact with order of administration; neither were there any higher-order interaction effects. On the SAERT, participants needed less time to decide whether a word was self-descriptive during the second session, irrespective of intervention or word valence (F=23.3, d.f.=1,18, P < 0.001).
Questionnaire about ethical aspects of the acute tryptophan depletion
Of the 41 questionnaires, 36 were returned (20 participants of the present study, 16 from the other depletion project). Two questionnaires (1 present study, 1 other project) were identified as completed by individuals who withdrew from the trials by the content of the answers to open-ended questions.
The main results are summarised in Table 4. All participants were quite satisfied with the informed consent procedure and reported that they had felt confident to decide whether to participate. More than half reported that participation in the study had changed their perception of their illness. Changes that were mentioned included that the study had helped them to gain more insight and facilitated acceptance of being vulnerable. Some participants mentioned that the transient return of symptoms had made them realise how much they had improved during treatment. Two participants interpreted their experiences as evidence for a biological cause of their depression, and felt less responsible. All changes were perceived as positive.
Table 4 Most relevant items of the ethics questionnaire
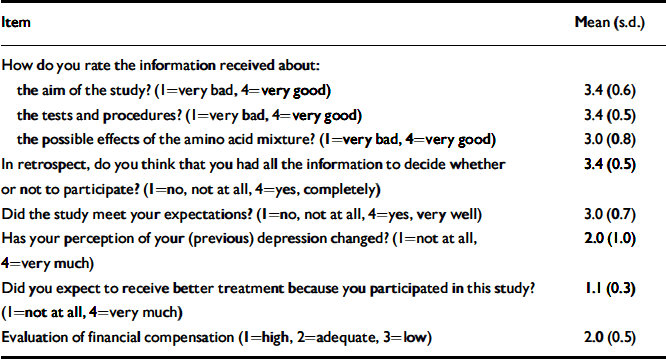
| Item | Mean (s.d.) |
|---|---|
| How do you rate the information received about: | |
| the aim of the study? (1=very bad, 4=very good) | 3.4 (0.6) |
| the tests and procedures? (1=very bad, 4=very good) | 3.4 (0.5) |
| the possible effects of the amino acid mixture? (1=very bad, 4=very good) | 3.0 (0.8) |
| In retrospect, do you think that you had all the information to decide whether or not to participate? (1=no, not at all, 4=yes, completely) | 3.4 (0.5) |
| Did the study meet your expectations? (1=no, not at all, 4=yes, very well) | 3.0 (0.7) |
| Has your perception of your (previous) depression changed? (1=not at all, 4=very much) | 2.0 (1.0) |
| Did you expect to receive better treatment because you participated in this study? (1=not at all, 4=very much) | 1.1 (0.3) |
| Evaluation of financial compensation (1=high, 2=adequate, 3=low) | 2.0 (0.5) |
The informed consent procedure had been very well understood. In response to a question about whether the participant expected to get better treatment after participation, 90% (n=32) answered ‘not at all’ and 11% (n=4) ‘slightly’. Regarding motivation to participate (open-ended question), 58% (n=21) wanted to contribute to the development of better treatments in the future. Others participated because of gratefulness towards the hospital or general interest in science. Two participants also mentioned financial reasons.
There were no significant differences between the two projects on any of the items investigated. The evaluation of the identified withdrawals was not different from the other participants. One who withdrew (a female mood responder) had felt unprepared for how big the effect would be, but she still reported that the study had been helpful, in the sense that it had convinced her that she is less responsible for her depression than she had thought.
DISCUSSION
Could the depletion procedure be explained well to participants?
Symptom provocation studies have become controversial, particularly in the USA. Questions have been raised concerning the risk/benefit ratio for participants, their capacity to decide whether to participate and the provision of adequate informed consent (Reference Miller and RosensteinMiller & Rosenstein, 1997; Reference MichelsMichels, 1999). This is the first acute tryptophan depletion study to systematically collect the views of participants. Individuals did not regret participating, and they had understood the informed consent procedure. Despite the provocation of symptoms and the fact that there was no direct benefit, some participants still experienced personal advantages. Two interpreted their experiences as evidence of a biological basis for their depression and felt less responsible for their illness. The influence of this on the future course of illness should be further investigated, as well as the generalisability to other symptom provocation studies. To justify future challenge studies, it may be useful to routinely investigate participants' subjective experiences in this type of research.
Is the acute tryptophan depletion response a specific model of depressive relapse?
Full acute tryptophan depletion significantly increased depressive symptoms as measured by the MADRS and HRSD. The MADRS increase was larger in females than in males. The menstrual cycle was not taken into account in the present study, but the finding is consistent with our pooled reanalysis of previous acute tryptophan depletion studies (Reference Booij, Van der Does and BenkelfatBooij et al, 2002).
Almost all acute tryptophan depletion studies in individuals with remitted depression have relied exclusively on the HRSD, and no previous study has measured a broad range of symptoms. However, some studies added self-report questionnaires such as the Profile of Mood States, or provided qualitative descriptions of the ‘relapse’ (Reference Smith, Fairburn and CowenSmith et al, 1997; Reference Lam and YathamLam & Yatham, 2003). Full but not partial depletion affected the CPRS sub-scales emotional dysregulation, motivational inhibition and behavioural disintegration. No significant effects were found on the sub-scales motivational disinhibition (elation, pressure of speech, ‘manic-like’ symptoms), perceptual disintegration (psychotic symptoms), autonomic dysregulation (autonomic arousal/anxiety), or on the Brief Anxiety Scale. Similarly, depletion did not change PANAS negative affect (common to anxiety and stress) but tended to decrease positive affect (indicative of depression). The lack of effect on anxiety is of interest, since different serotonergic projections and receptors may mediate both depression and anxiety (Reference Graeff, Guimaraes and DeAndradeGraeff et al, 1996).
It is noteworthy that the behavioural changes following acute tryptophan depletion were larger on observer-rating scales than on self-report questionnaires, the latter often resulting in trends or non-significant results. However, these changes were also consistent with a specific depressive response (increase of BDI-total and BDI-cognitive, decrease of positive affect). As noted previously (Reference Booij, Van der Does and RiedelBooij et al, 2003), people with depression may use standards other than those used by healthy controls to rate changes in mood over a short time interval. This is supported by the finding on the SAERT; responders more often rated positive traits as self-descriptive but rated these as ‘not applicable’ on reflection. The same effect may occur with self-rating scales, where individuals are typically instructed to choose the first answer that appears right.
Does acute tryptophan depletion change depression-related self-schemata?
Although acute tryptophan depletion significantly increased depression-related cognitions (indicated by BDI-cognitive), full depletion did not affect self-schemata. Acute tryptophan depletion negatively influenced the recall of positive traits, but only in responders. A number of studies have demonstrated that people with depression are impaired in the recall of positive information, and that they are more likely to store and recall information which is congruent with their mood state (Reference Burt, Zembar and NiedereheBurt et al, 1995). The present study suggests that this mood-congruent memory bias is mediated by low serotonin.
Are the statistically significant changes in mood also clinically significant?
Although statistically significant, the increases in HRSD and MADRS ratings were relatively low. However, effect sizes were around 0.50, which is clinically relevant (Reference CohenCohen, 1988).
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Acute tryptophan depletion specifically induces depressive symptoms in those with remitted depression.
-
▪ Mood-congruent memory bias in depression may be related to the serotonin system.
-
▪ Acute tryptophan depletion is a suitable model for investigating depression, both from scientific and ethical perspectives.
LIMITATIONS
-
▪ The psychometric properties of the ethics questionnaire are unknown.
-
▪ As participants were paid, this might have resulted in more favourable responses.
-
▪ Compared with other acute tryptophan depletion studies, there were few withdrawals; this may have influenced the results of the ethics questionnaire.
Acknowledgements
The authors thank Milad Kavehzadeh and Sanneke Van Vliet for assistance with the data collection. We also thank the dieticians and staff of the laboratory and hospital pharmacy of Parnassia and the Laboratory of Psychiatry of Erasmus Medical Center Rotterdam for technical assistance. This research was funded by grants from The Netherlands Organisation of Sciences-Medical Sciences (NWO-MW grant 904-57-132) and ‘Stichting tot Steun VCVGZ’ (a non-profit organisation supporting psychiatric research) to A.J.W.V.D.D.








eLetters
No eLetters have been published for this article.