INTRODUCTION
Cosmogenic radiocarbon (14C) is produced by the nuclear reaction 14N(n,p)14C in the upper atmosphere and has a generally accepted half-life of 5730 yr. The cosmogenic isotope is incorporated into the geochemical carbon cycle, penetrates into the subsurface, and interacts with the biosphere in the soil where partial pressure of CO2 often becomes elevated (Brook et al. Reference Brook, Folkoff and Box1983). Dissolved CO2 in water dissociates and forms bicarbonate and carbonate ions, which are together considered as dissolved inorganic carbon (DIC). In an ideal case, the 14C activity of DIC in phreatic zone of groundwater is close to that of atmospheric air, and with the isolation of water mass from the atmosphere the radioactive decay dating clock starts. Decades of 14C dating application on DIC in groundwater revealed that 14C activities can be modified by numerous geochemical processes both in phreatic and confined conditions, in most cases admixture of 14C-free carbon resulting in age overestimations. Nevertheless, sophisticated correction methods for such effects continue to develop (e.g. Plummer and Sprinkle Reference Plummer and Sprinkle2001; Han and Plummer Reference Han and Plummer2016), and 14C has been extensively used for the determination of groundwater residence time in order to constrain the recharge rate, flow dynamics, and paleoclimates [Plummer and Glynn (Reference Plummer and Glynn2013) and references therein].
Sample collection methods for 14C analyses are listed in Table 1. Because large quantities of carbon (3 g) were required for 14C analyses by decay counting, DIC in groundwater has traditionally been acquired by separating DIC from a large volume of groundwater (≥50 L) via chemical treatments: the gas evolution method acidifies the sample using H2SO4 to liberate CO2, which is then absorbed by hyperalkaline solution (NaOH or NH4OH) and precipitated as SrCO3 or BaCO3 (Feltz and Hanshaw Reference Feltz and Hanshaw1963; Hanshaw et al. Reference Hanshaw, Back and Rubin1965). The method had not been commonly applied in the field due probably to the relatively complex operation, but it has been refined and widely used in laboratories for efficiently extracting DIC from environmental water samples (e.g. Gao et al. Reference Gao, Xu, Zhou, Pack, Griffin, Santos and Southon2014; Gospodinova et al. Reference Gospodinova, Mcnichol, Gagnon and Shah Walter2016). A simpler and widely used method (direct deposition) is to precipitate DIC in the form of carbonate (SrCO3 or BaCO3) by adding hyperalkaline solution and an appropriate salt (e.g. SrCl2: Gleason et al. Reference Gleason, Friedman and Hanshaw1969; Plummer and Sprinkle Reference Plummer and Sprinkle2001). As recently demonstrated (Aggarwal et al. Reference Aggarwal, Araguas-Araguas, Choudhry, van Duren and Froehlich2014), the hyperalkaline solution exposed to the modern atmosphere is prone to rapid uptake of atmospheric CO2.
Table 1 List of 14C sampling methods. The × symbols indicate “problematic” or “disadvantageous,” whereas the ⃝ symbols indicate “advantageous” or “not a concern.” “Inhibitors” and “filters” are countermeasures commonly taken against the potential problems.

a Gleason et al. (Reference Gleason, Friedman and Hanshaw1969); Plummer and Sprinkle (Reference Plummer and Sprinkle2001).
b Feltz and Hanshaw (Reference Feltz and Hanshaw1963); Hanshaw et al. (Reference Hanshaw, Back and Rubin1965).
c McNichol (Reference McNichol, Osborne, Gagnon, Fry and Jones1994).
d Garnett et al. (Reference Garnett, Billett, Gulliver and Dean2016).
e This work.
f The authors state as conservative limit.
g Highest pH actually measured.
Accelerator mass spectrometry (AMS) dramatically decreased the required sample size for the 14C analyses to 0.05–1 L and enabled transport of bulk water sample (bulk method). Naturally occurring bulk water, if stored untreated, risks occurrence of biological and chemical processes that modify 14C activity of DIC. As countermeasures, inhibitors of microbial activity are often added in the field, and water samples are occasionally filtered, during which exposure of the small-size samples to modern atmosphere or CO2 loss by decompression is possible. Furthermore, 14C contamination may occur during sample storage (Takahashi et al. Reference Takahashi, Minami, Aramaki, Handa and Nakamura2016). Aggarwal et al. (Reference Aggarwal, Araguas-Araguas, Choudhry, van Duren and Froehlich2014) also suspect that some AMS analyses may have been prone to such contamination based on the data from literatures where 14C measurements level out around a few % modern carbon (pMC) over hundreds of kilometers. As a consequence of modern atmospheric contamination, the 14C age of old groundwater would be substantially underestimated, and the hydrological system of the region would be severely misinterpreted. The degree of modern atmospheric contamination during sampling and sample processing depends primarily on the details of the operations performed, introducing unsystematic biases that cannot be interpreted in a generalized way. Aggarwal et al. (Reference Aggarwal, Araguas-Araguas, Choudhry, van Duren and Froehlich2014) suggested to collect (and seal) old groundwater samples in plastic containers submerged in an overflowing container. This will minimize the atmospheric contamination, but makes the procedure of adding chemicals more complex. Sample collection methods and sample storage containers for DIC-14C have recently been going through a phase of re-evaluation (e.g. Nakata et al. Reference Nakata, Hasegawa, Iwatsuki and Kato2016; Takahashi et al. Reference Takahashi, Minami, Aramaki, Handa and Nakamura2016).
The advent of atom trap trace analyses (ATTA) at Argonne National Laboratory enabled the analyses of radiokrypton isotopes that are ideal tracers of groundwater age (Chen et al. Reference Chen, Li, Bailey, O’Connor, Young and Lu1999; Du et al. Reference Du, Purtschert, Bailey, Lehmann, Lorenzo, Lu, Mueller, O’Connor, Sturchio and Young2003; Sturchio et al. Reference Sturchio, Du, Purtschert, Lehmann, Sultan, Patterson, Lu, Muller, Bigler, Bailey, O’Connor, Young, Lorenzo, Becker, El Alfy, El Kaliouby, Dawood and Abdallah2004). ATTA is currently the only method capable of determining the isotopic abundance of the long-lived isotope 81Kr (t1/2=229,000 yr). Krypton-81 is an excellent tracer of old groundwater age and therefore provides the means of evaluating the hydrological significance of 14C leveling off as mentioned above (i.e. technical problem versus homogeneous reservoir). A detectable 85Kr (t1/2=10.8 yr) activity concentration in deep wells clearly indicates young water mixing or atmospheric contamination during sampling. It is therefore ideal to use these three isotope tracers together to assess the age structure of low-14C groundwater.
At present, the quantity of Kr used for each analysis is about 10 μL-STP (Jiang et al. Reference Jiang, Bailey, Lu, Mueller, O’Connor, Cheng, Hu, Purtschert, Sturchio, Sun, Williams and Yang2012; Lu et al. Reference Lu, Schlosser, Smethie, Sturchio, Fischer, Kennedy, Purtschert, Severinghaus, Solomon, Tanhua and Yokochi2014), which can be extracted from 100–200 L of groundwater. Because of the relatively large sample size, krypton and other bulk gases dissolved in groundwater are extracted in the field and stored in compressed gas cylinders (e.g. Purtschert et al. Reference Purtschert, Yokochi and Sturchio2013; Yokochi Reference Yokochi2016). During this process, proven to be robust against modern atmospheric contamination, dissolved CO2 is also extracted. In favorable cases, a significant fraction of DIC resides as dissolved CO2 in groundwater. Because the DIC chemical and isotope equilibrium occurs with an e-folding time scale of 10–25 s (Zeebe and Wolf-Gladrow Reference Zeebe and Wolf-Gladrow2001), 14C isotopic abundance of CO2 extracted by field degassing is likely to represent DIC of the groundwater, with minor isotopic fractionations that can be corrected for. Water-rock interaction and subsurface geochemical processes prior to sampling still require geochemical correction scheme (Plummer and Glynn Reference Plummer and Glynn2013). This method collects 14C and radiokrypton samples simultaneously, which is ideal for evaluating the degree of atmospheric contamination during sampling. Moreover, carbon is collected in the gas phase, eliminating the possibility of fostering microbial activities and aqueous chemical reactions. Recently, Garnett et al. (Reference Garnett, Billett, Gulliver and Dean2016) presented a field CO2 extraction method using headspace (super headspace method) for 14C analyses, similarly highlighting the sample preservation capability.
We herein present case studies where groundwater ages of 81Kr, 85Kr, and 14C of CO2 from bulk gas samples are compared with those of DIC-14C. The samples for 14C in gaseous CO2 and radiokrypton isotope analyses were simultaneously collected via field degassing. Our goal is to demonstrate the feasibility and advantages of analyzing 14C in CO2 as well as of using it together with noble gas radionuclides from the same bulk gas extracted in the field.
HYDROGEOLOGICAL BACKGROUND
The first set of samples was collected from the Judea Group carbonate aquifer and the underlying Kurnub Group sandstone aquifer in the Negev desert, Israel, in May 2014. These aquifers are precious sources of fresh and brackish water, and have been studied extensively (e.g. Issar et al. Reference Issar, Bein and Michaeli1972; Adar et al. Reference Adar, Rosenthal, Issar and Batelaan1992; Yechieli et al. Reference Yechieli, Starinsky and Rosenthal1992; Rosenthal et al. Reference Rosenthal, Zilberbrand and Livshitz2007). The Upper Cretaceous Judea Group carbonate aquifer in the studied region was probably replenished in the southern flanks of the Judea mountains, central to northeastern Sinai, and over the Negev Highlands. The Lower Cretaceous to Jurassic Kurnub (Nubian) sandstone aquifer extends beneath the Arabian Peninsula and the Sahara with two major clear hydrological discontinuities along the Gulf of Suez and the Jordan-Arava Rift Valley. The main recharge of Sinai-Negev Kurnub aquifer occurs in central Sinai where there are large exposures of Nubian sandstone. Additional small recharge zones are located in the erosion cirques of the northern Negev and Sinai deserts. 14C data in the literature indicate the “fossil” nature of water in both the Judea and Kurnub aquifers (Issar et al. Reference Issar, Bein and Michaeli1972; Kronfeld et al. Reference Kronfeld, Rosenthal, Weinberger, Flexer and Berkowitz1993; Vengosh et al. Reference Vengosh, Hening, Ganor, Mayer, Weyhenmeyer, Bullen and Paytan2007; Burg et al. Reference Burg, Zilberbrand and Yechieli2013). In most cases, those 14C analyses were conducted using the conventional method where BaCl2 solution was added to the samples in the field, together with NaOH, to precipitate the carbonates as BaCO3. The arid climate causes multiple difficulties in applying 14C dating of groundwater using DIC; the faint biological activities result in low P CO2 of the soil and hence relatively low DIC concentration in groundwater, which makes samples sensitive to mixing, both via modern atmospheric air contamination during sampling, and via chemical reactions with minerals in the subsurface. After applying corrections for the effect of chemical exchanges between groundwater and minerals in both phreatic and confined aquifers, the authors concluded that the groundwater from deep aquifers of the region was predominantly recharged during the last glacial period, around 25 kyr (Issar et al. Reference Issar, Bein and Michaeli1972; Gat and Issar Reference Gat and Issar1974; Vengosh et al. Reference Vengosh, Hening, Ganor, Mayer, Weyhenmeyer, Bullen and Paytan2007). The current sampling campaign was primarily aimed for 81Kr dating, and water samples for 14C analyses in DIC were not collected during this campaign. Therefore, the 14C isotopic abundances of CO2 in these samples are compared with published DIC-14C values in the literature (Carmi Reference Carmi1987; Kronfeld et al. Reference Kronfeld, Rosenthal, Weinberger, Flexer and Berkowitz1993; Vengosh et al. Reference Vengosh, Hening, Ganor, Mayer, Weyhenmeyer, Bullen and Paytan2007; Burg et al. Reference Burg, Zilberbrand and Yechieli2013).
The second sampling campaign took place in June 2015 in southern Florida. The Floridan aquifer system is one of the most productive aquifers on Earth. It underlays the entire state of Florida and beyond, consisting of a sequence of hydraulically connected carbonate rocks with minor amounts of evaporites of Paleocene to Miocene age (Bush and Johnson Reference Bush and Johnson1988). The recharge occurs near the potentiometric mound at Polk City, and the flow is south and outward (east and west) from the ridge of the peninsula. Low 14C activities of DIC were reported even at locations close to the recharge zone, partly due to the geochemical reactions that dilute the original 14C signal. Plummer and Sprinkle (Reference Plummer and Sprinkle2001) applied thorough correction of possible effects and concluded that most wells have adjusted 14C ages of 20–30 kyr and thus recharged during the last glacial period. A cavernous zone, known as the Boulder Zone, occurs in the southern Florida at depth where the chemical composition of the water is comparable with seawater and relatively high but not modern 14C activity was reported (Meyer Reference Meyer1989; Morrissey et al. Reference Morrissey, Clark, Bennett, Richardson and Stute2010). In Florida, two samples near the recharge zone and two high-salinity samples from the Boulder Zone were collected.
METHODS
The field degassing device (Yokochi Reference Yokochi2016) is equipped with a commercial membrane contactor, Liqui-Cel Extra-Flow. These membrane contactors permeate gas and serve as physical barriers to separate liquid water from the gas phase (Probst et al. Reference Probst, Yokochi and Sturchio2006; Purtschert et al. Reference Purtschert, Yokochi and Sturchio2013). A conceptual diagram of the devices is shown in Figure 1, with a projected configuration for radiocarbon sampling (i.e. small sample size) using a two-valve sample container. As filtered water runs through the shell-side of the contactor (MC), dissolved gas is pumped out from the lumen-side and compressed into a sample container. The operation is relatively simple: After connecting the water inlet, outlet, and sample container, the operator activates the pump and extracted gas comes out from the pump outlet. In both campaigns, carbon dioxide and krypton were collected together with other bulk gases (5–10 LSTP) from groundwater using the field degassing device (see for detail Yokochi Reference Yokochi2016). Sample gas cylinders were shipped to the University of Chicago or University of Bern for Kr separation (Yokochi et al. Reference Yokochi, Heraty and Sturchio2008; Purtschert et al. Reference Purtschert, Yokochi and Sturchio2013; Yokochi Reference Yokochi2016), and gas splits of ∼50 mL STP were set aside for the separation of CO2. The abundances of radiokrypton isotopes were measured by ATTA at Argonne National Laboratory (Jiang et al. Reference Jiang, Bailey, Lu, Mueller, O’Connor, Cheng, Hu, Purtschert, Sturchio, Sun, Williams and Yang2012).

Figure 1 Conceptual diagram of a field gas extraction apparatuses using a Liqui-Cel membrane contactor (MC), with a projected configuration for 14C sampling using a double ended sample container (SC). The letters F, G, and P denote “filter,” “pressure gauge,” and “pump.” Arrows show water and gas flow directions. For portability, a 12 VDC battery-operated vacuum pump may be implemented as Device A in Yokochi (Reference Yokochi2016).
In the field, temperature, pH, and conductivities were measured. In Florida, DIC-14C sample bottles were placed and overflowed for several minutes inside a bucket filled with water from the sampled well, then capped underwater without adding the inhibitor of biological activity, following the recommendation by Aggarwal et al. (Reference Aggarwal, Araguas-Araguas, Choudhry, van Duren and Froehlich2014). The samples were kept in a refrigerator except during transport, shipped directly from the field site to the AMS facility at the University of Georgia, and were analyzed within 3 weeks. The gas split container was connected to a vacuum line, and CO2 gas samples were sealed in 6-mm Pyrex tubes after cryogenic separation. The analyses of 14C were performed at the University of Georgia AMS facility. There, H2S and H2O were removed catalytically and cryogenically, and CO2 was graphitized with Fe catalyst (Vogel et al. Reference Vogel, Southon, Nelson and Brown1984).
RESULTS AND DISCUSSIONS
Carbon-14, Krypton-81 and -85 abundances of gas samples collected via field gas extraction are listed in Table 2, together with 14C data of CO2, DIC from the literature for Israeli samples and for four DIC samples from Florida. Table 3 includes δ13C of DIC and CO2 gas, as well as input and output parameters of correction for isotope fractionations. Krypton-81 abundance ranged between 0.49±0.04 and 1.05±0.05 relative to the modern atmospheric concentration. Krypton-85 activities were below 1% of modern atmosphere except for two samples. It suggests that the contamination by modern atmospheric air is insignificant in most CO2 gas samples. The 14C abundances of CO2 gas of all but two samples were comparable or lower than that of DIC (Figure 2).
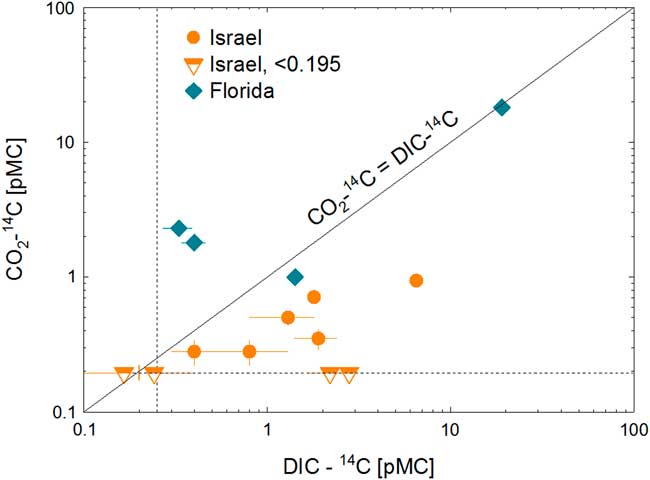
Figure 2 14C isotopic abundance [pMC] of dissolved inorganic carbon (DIC) is plotted against that of CO2 gas extracted in the field. DIC data of Israeli samples are from the literature. The dotted lines are the detection limit of 14C, and half-filled symbols represent data below detection limits. The solid line represents equal activity between DIC and CO 2 . All but two gaseous CO2 samples had lower 14 C activity than DIC samples from the same well.
Table 2 Radiokrypton and 14C isotopic abundances of gaseous CO2 and DIC. Numbers listed in the “UGA” columns are the analytical IDs of the AMS facility at University of Georgia (UGA). References are as follows: [1] Carmi Reference Carmi1987; [2] Kronfeld et al. Reference Kronfeld, Rosenthal, Weinberger, Flexer and Berkowitz1993; [3] Vengosh et al. Reference Vengosh, Hening, Ganor, Mayer, Weyhenmeyer, Bullen and Paytan2007; [4] Burg et al. Reference Burg, Zilberbrand and Yechieli2013; [5] Adar unpublished data. The 81Kr age of Paran 20 and Tamar 11 samples were corrected for mixing of young component using 85Kr abundance. The 14C data are corrected for the blank of 0.195 ±0.06 pMC, and data within 2σ of this value were reported as <0.195 pMC. The uncertainty of the blank was estimated based on the long-term reproducibility of ±30% reported for geologic graphite, coal, and calcite blanks in Cherkinsky et al. (Reference Cherkinsky, Ravi Prasad and Dvoracek2013), which reflects the reproducibilities of both the background and the measurement.

Table 3 The results of modeled carbon isotopic fractionation and relevant parameters.
![]() $${\rm F}_{{H}_{2}CO_{3}^{{\asterisk}} }}$$
, δ13CDIC,Model, and ∆13C are the fraction of H2CO3* among total DIC species, the δ13C of DIC modeled from the δ13C measured in CO2 gas, and the deviation of the modeled value from the actual DIC composition (∆13C=δ13CDIC–δ13CDIC,Model). This correction does not include any isotopic modifications caused by chemical reactions in the subsurface. *This sample did not contain sufficient CO2 or DIC for 14C analysis.
$${\rm F}_{{H}_{2}CO_{3}^{{\asterisk}} }}$$
, δ13CDIC,Model, and ∆13C are the fraction of H2CO3* among total DIC species, the δ13C of DIC modeled from the δ13C measured in CO2 gas, and the deviation of the modeled value from the actual DIC composition (∆13C=δ13CDIC–δ13CDIC,Model). This correction does not include any isotopic modifications caused by chemical reactions in the subsurface. *This sample did not contain sufficient CO2 or DIC for 14C analysis.

The field degassing method requires a sufficient amount of gaseous CO2 dissolved in the groundwater samples. Furthermore, the observed δ13C and 14C abundances need to be corrected for isotopic fractionation and mixing prior to geochemical interpretation. Therefore, general aspects of DIC chemical speciation and dissolved gas chemistry are first addressed in order to assess the accuracy and applicability of the method. Comparisons of different tracer ages and their implications follow.
DIC Speciation, Dissolved Gas Chemistry, and Atmospheric Contamination
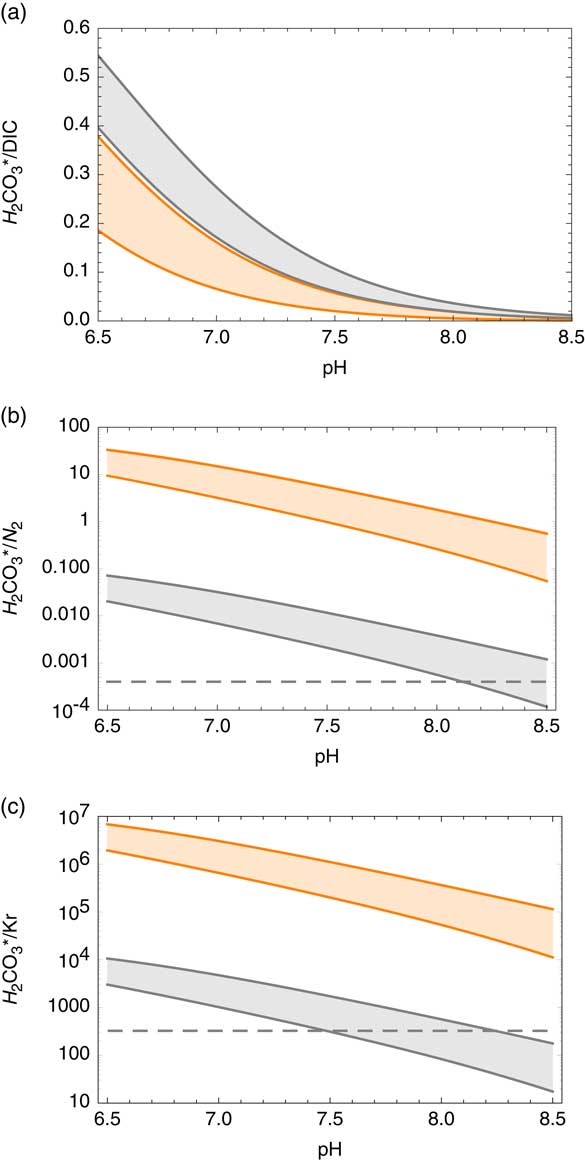
Carbon dioxide in soil gas dissolves in water, [CO2,aq ], at the unsaturated zone to reach solubility equilibrium following Henry’s law. The partial pressure of CO2 in soil gas can be significantly higher by >100 fold than that in the atmosphere due to subsurface biological activities (Brook et al. Reference Brook, Folkoff and Box1983). A fraction of [CO2,aq ] forms carbonic acid [H2CO3] that dissociates to bicarbonate [HCO−] and carbonate [CO2−] ions. These four chemical species all together are considered DIC and are used for the analyses of conventional 14C analyses in water. The former two species are expressed together as H2CO3*. The relative abundances of the carbon species in water are thermodynamically constrained (e.g. Millero et al. Reference Millero, Graham, Huang, Bustos-Serrano and Pierrot2006), depending primarily on pH and also on temperature (T) and salinity (S). Salinity was calculated based on the Cl concentration. In typical groundwater (6.5<pH<8.5, 0<T<50ºC and 0<S<45 ‰), the fraction of dissolved CO2 constitutes between 0.1% and 54% of total DIC (Figure 3a).

Figure 3 Carbon dioxide abundance relative to other species in groundwater as a function of pH: (a) fractional abundance of H2CO3* among dissolved inorganic carbon (DIC). Gray and orange zones represent 0–50ºC, respectively, for the salinity range of 0–45‰, calculated based on Millero et al. (Reference Millero, Graham, Huang, Bustos-Serrano and Pierrot2006). Salinity was calculated based on the Cl concentration. (b) Expected H2CO3*/N2 ratio of dissolved gas in groundwater assuming near neutral pH, zero salinity conditions for soil gas CO2-enrichment factors of 1 (orange zone) and 105 (gray zone) (Brook et al. Reference Brook, Folkoff and Box1983) at recharge temperatures range between 0 and 50ºC (see “Methods” section). The dashed line represents a desired minimum H2CO3* concentration of 0.04% among dissolved gases assuming N2-dominant chemistry. (c) Expected H2CO3*/Kr ratio of dissolved gas in groundwater using the same method described above for N2. The dashed line represents modern atmospheric CO2/Kr ratio. (Colors refer to online version.)
Ideally, AMS analyses of 14C require 2 cm3STP of CO2. Provided that 5–10 L STP of bulk gas is usually collected for the analyses of Kr radioisotopes, CO2 concentration of >0.04% in the extracted gas phase is required. Because N2 is the major constituent of dissolved atmospheric gas in groundwater, the CO2/N2 ratio serves as a good proxy for CO2 concentration in extracted gas. Carbon dioxide and molecular nitrogen solubility was calculated based on Ozima and Podosek (Reference Ozima and Podosek2002) and Weiss (Reference Weiss1974). For a range of possible recharge conditions (7.0<pH<7.5, 0<T<50◦C with low salinity) and P CO2 in soil gas (Brook et al. Reference Brook, Folkoff and Box1983), the DIC/N2 molar ratio varies between 0.12 and 35.5, significantly higher than the atmospheric CO2/N2 ratio (0.0005). Water–rock interactions may cause changes in pH and salinity, which determine the H2CO3*/DIC ratio. The anticipated H2CO3*/N2 ratio in groundwater for a range of subsurface conditions (6.5<pH<8.5, 0<T<50ºC and 0<S<45 ‰, assuming no significant gain or loss of C) is plotted in Figure 3b; In most cases, the gas sample will contain a sufficient concentration (>0.04%) of CO2 for 14C analyses. The gases extracted from groundwater often have >1% CO2.
Figure 3c depicts the H2CO3*/Kr ratio in groundwater for the same conditions. Most groundwater samples have higher H2CO3*/Kr ratios compared with atmospheric CO2/Kr ratio, often by orders of magnitude, due to the high CO2 solubility in water and elevated P CO2 in soil gas. This makes 85Kr a very sensitive indicator of atmospheric contamination for 14C in gaseous CO2 samples because the most liable source of atmospheric contamination is via gaseous atmospheric air leaks during the operation of the field gas extraction device. As shown in Figure 4, the 85Kr isotope signal is >10 fold more sensitive than 14C to a mixing of modern atmospheric component with old (zero-activity) groundwater. It is a remarkable advantage of the newly developed method that we can (semi-)quantitatively evaluate the potential impact of atmospheric contamination on 14C abundance during sample collection.
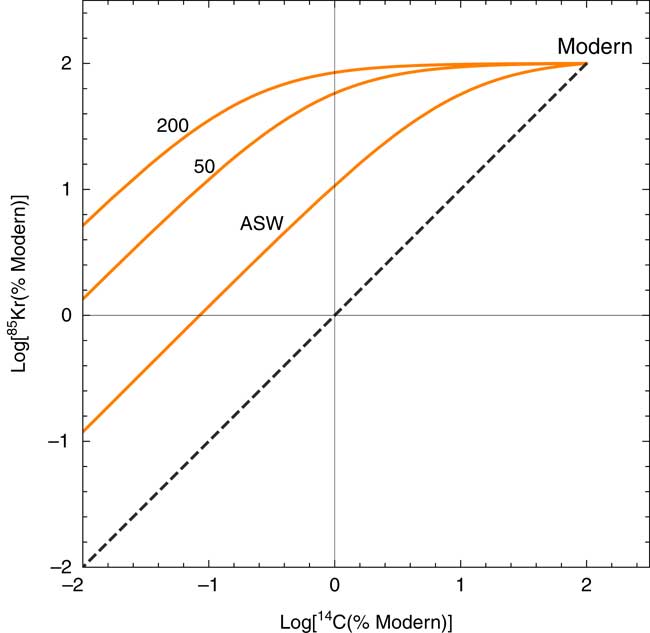
Figure 4 Modeled covariation of 14C-85Kr isotopic abundances in response to a mixing of modern atmosphere air into dissolved gas in old (14C-dead) groundwater with various DIC concentrations. The numbers correspond to the concentration of total DIC (mg/L) in groundwater under 25ºC at zero salinity. The line marked “ASW” represents mixing of modern atmospheric air with water in equilibrium with air. In all cases, the effect of modern air mixing is more significant for 85Kr. The dashed line is shown as a reference of mixing between modern and zero-activity components with identical C/Kr ratios.
One bulk gas sample, Shunit (Table 3), extracted from groundwater with a high pH of 9.7 had low CO2 concentration (71 ± 15 ppm), confirming a low H2CO3*/DIC ratio as expected. This sample did not contain sufficient CO2 or DIC for the analysis of 14C activity. The low CO2 concentration in the gas phase also implies that background CO2 contribution from the sample container and sampling device (e.g. degassing from the membrane contactor and leakage) is below this level. For samples with high pH (thus low CO2 concentrations), the procedural background needs to be more thoroughly tested for both modern and dead C contributions.
Two samples characterized with significant (>1% modern) 85Kr activities probably experienced either atmospheric air contamination during sampling or the admixture of young groundwater components, which would affect both 14C and 81Kr abundances: samples from Paran 20 and Tamar 11 wells had 85Kr activities of 5.5 and 1.2 dpm/cm3 Kr , respectively, corresponding to 7.3% and 1.6% modern atmospheric Kr contribution assuming 75 dpm/cm3 Kr 85Kr in atmospheric air (Winger et al. Reference Winger, Feichter, Kalinowski, Sartorius and Schlosser2005; Ahlswede et al. Reference Ahlswede, Hebel, Ross, Schoetter and Kalinowski2013). The contributing fraction of 81Kr is equal to that of 85Kr in the case of modern atmospheric contamination, or larger in case of the admixture of sub-modern young groundwater which contains lower 85Kr activities than modern atmosphere. Assuming modern 85Kr activity concentration for the young mixing component, 81Kr abundances of Tamar11 is corrected from 0.49 to 0.48. That of Paran 20 is corrected from 0.56 to 0.53. These values are upper limits, and they can be lower if the 85Kr carrier has a sub-modern 85Kr activity. Although a similar situation applies to 14C, the 14C activities of these two samples are still at baseline values, probably due to the high H2CO3*/Kr in the samples. Therefore, no correction was applied in these cases.
Carbon Isotope Fractionation and Mixing
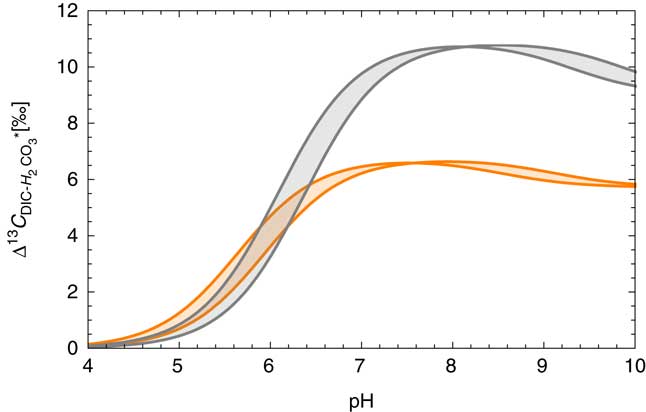
Carbon isotopic composition of DIC is often used for evaluating the degree of water–rock interaction and isotope exchange that disturb the 14C chronometer by the addition of dead carbon (Plummer and Glynn Reference Plummer and Glynn2013 and references therein). Equilibrium isotope fractionation factors α among different chemical species x and y are defined as [α] xy (T)=(13C/12C) x /13C/12C) y , also expressed as δ13C y =(δ13C x +1000)α xy –1000. Using the fractional abundance of each species discussed above, the equilibrium fractionation factor between DIC and H2CO3* over the range of T, S, and pH conditions can be estimated as shown in Figure 5. In typical groundwater (6.5<pH<8.5), it is expected that the δ13C value in H2CO3* is lower than δ13C value of total DIC by 6–10‰. If the field gas extraction device is only extracting the fraction of DIC that was in the state of H2CO∗ 3 prior to degassing, it is therefore expected that the δ13C composition of total DIC (TDIC) is heavier than the measured δ13C CO2 value by several ‰.

Figure 5 Carbon isotope fractionation between total DIC and H2CO3*. Equilibrium isotope fractionation factors are from Deines et al. (Reference Deines, Langmuir and Harmon1974), and the fractions of DIC species were calculated as in Figure 3a. The range of salinity is between 0 and 45‰ and the colors represent different temperatures, gray for 0ºC and orange for 50ºC. (Colors refer to online version.)
Assuming extraction of only H2CO∗
3 fraction (=
![]() $${\rm F}_{{H}_{2}CO_{3}^{{\asterisk}} }}$$
) and isotope equilibrium among DIC species, the 13C isotopic abundances of total DIC were estimated (δ13C
DIC,Model
) from the measured value of each CO2 sample collected by the field degassing method (δ13C
CO2). The modeled compositions (δ13C
DIC,Model
) are compared with the actual DIC data (δ13C
DIC
) to examine whether the DIC species are in chemical and isotopic equilibrium (Figure 6; Table 3). The
$${\rm F}_{{H}_{2}CO_{3}^{{\asterisk}} }}$$
) and isotope equilibrium among DIC species, the 13C isotopic abundances of total DIC were estimated (δ13C
DIC,Model
) from the measured value of each CO2 sample collected by the field degassing method (δ13C
CO2). The modeled compositions (δ13C
DIC,Model
) are compared with the actual DIC data (δ13C
DIC
) to examine whether the DIC species are in chemical and isotopic equilibrium (Figure 6; Table 3). The
![]() $${\rm F}_{{H}_{2}CO_{3}^{{\asterisk}} }}$$
ranged between 1.1% and 9.1%, resulting in ∆13C (=δ13C
DIC,Model
-δ13C
CO2) between 5.7 and 8.5‰ for the measured temperature range of 24.0–57.0ºC. The δ13C
DIC
of water samples agree, within a few ‰, with δ13C
DIC,Model
(solid symbols in Figure 6). The general agreement between δ13C
DIC
and δ13C
DIC,Model
indicates that the model assumptions well represent the actual degassing environment, and other effects that modifies carbon isotopic compositions such as diffusion and dehydration of bicarbonate ions appears minor (see Appendix for details).
$${\rm F}_{{H}_{2}CO_{3}^{{\asterisk}} }}$$
ranged between 1.1% and 9.1%, resulting in ∆13C (=δ13C
DIC,Model
-δ13C
CO2) between 5.7 and 8.5‰ for the measured temperature range of 24.0–57.0ºC. The δ13C
DIC
of water samples agree, within a few ‰, with δ13C
DIC,Model
(solid symbols in Figure 6). The general agreement between δ13C
DIC
and δ13C
DIC,Model
indicates that the model assumptions well represent the actual degassing environment, and other effects that modifies carbon isotopic compositions such as diffusion and dehydration of bicarbonate ions appears minor (see Appendix for details).
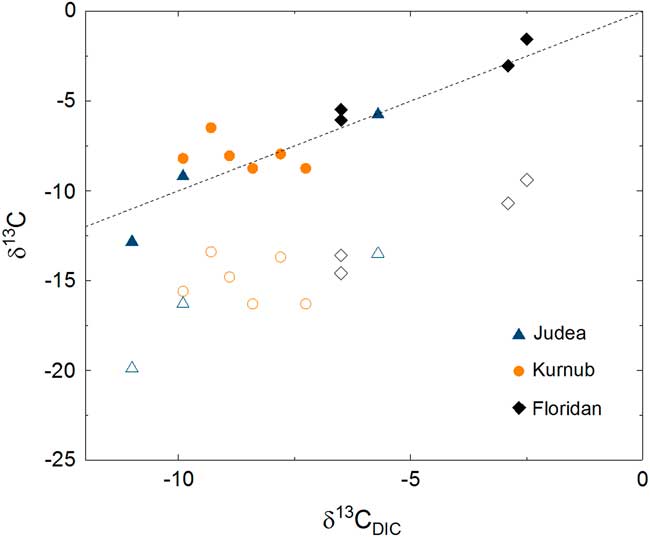
Figure 6 Carbon isotope compositions of CO2 gas (open symbols, δ13C CO2) and theoretically expected DIC (solid symbols, δ13C DIC,Model ) are plotted against measured δ13C DIC . The dashed line represents the case where measured and theoretical DIC values are identical, showing that they are in good agreement.
As mentioned above, the impact of atmospheric contamination is proven to be insignificant for most 14C data of CO2 samples based on the low 85Kr activities. Had there been no chemical processes that modify isotopic compositions inside the DIC sample container, 14C activity of DIC samples would be identical to that of CO2 or possibly higher if there was any atmospheric contamination. However, 14C activities for two of the DIC samples from Florida were lower than in the CO2 gas. In the gas phase acquired from the OKF105-Upper well, the concentration of CH4 and CO2 in the collected bulk gas were approximately 10% and 1%, respectively. Because no inhibitor of microbial activities was added in the DIC samples, it is possible that anaerobic oxidation of 14C-dead CH4 and possibly other organic carbon could have taken place. Inorganic dissolution of carbonate particles could lead to the same consequence, given that the water sample was not filtered. In either case, the data imply a possibility that there may have been a process that lowered 14C activity in water inside the DIC sample container. Although it is beyond the scope of this study, a large-scale, systematic study is desirable for ascertaining a reliable sampling method for DIC-14C. None of the above-mentioned processes can take place in an aluminum compressed gas cylinder when CO2 gas is collected and stored for 14C analyses.
Tracer Age Comparison and Implications
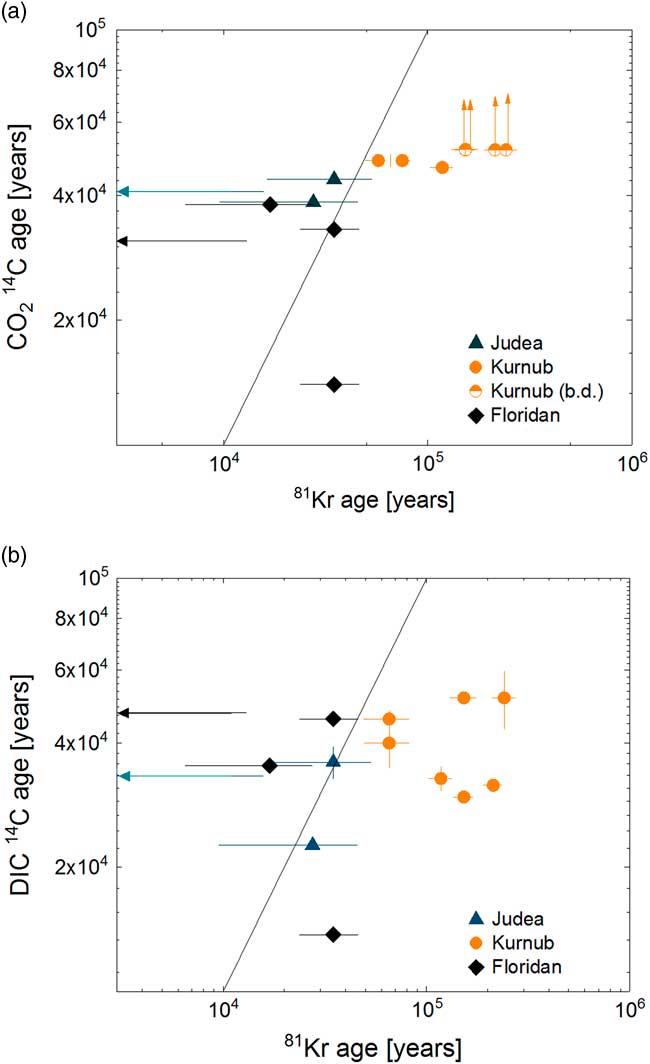
The groundwater ages calculated from 81Kr are listed in Table 2 and plotted in Figure 7 against apparent 14C ages (Table 2). For comparison with 81Kr age, 14C ages are calculated assuming simple decay with a half-life of 5730 yr, with an initial 14C activity of 100 pMC. No correction for dead C contribution was applied since it only affects in favor of our conclusion that previous DIC-14C ages may be underestimated by atmospheric contamination. Four CO2 gas samples from Israel had 14C abundances at background level (≤0.195 pMC), in agreement with 81Kr ages of >150 kyr (Figure 7a). The 14C abundances of DIC in these samples were somewhat higher, resulting in the apparent age range of 30–51 kyr (Figure 7 b). The difference in 14C abundances between DIC and gaseous CO2 is attributed to modern 14C contamination of DIC samples during the sampling and chemical preparation procedure in laboratories performed in previous studies. Krypton-81 age generally agrees better with 14C in CO2 gas from this study than 14C of DIC reported in the literature. Krypton-81 ages of three samples (Nitzana1, Qetora115, and LAB-PW2) agree with both CO2-14C and DIC-14C ages within relatively large uncertainty. Two samples collected near the recharge area of the Floridan aquifer and Revivim2 from Israel are characterized by younger 81Kr age than both CO2- and DIC-14C age. A plausible explanation for the apparently discordant ages is a contribution of dead C from the interaction with reservoir carbonate rocks (Plummer and Sprinkle Reference Plummer and Sprinkle2001; Vengosh et al. Reference Vengosh, Hening, Ganor, Mayer, Weyhenmeyer, Bullen and Paytan2007). Two samples, I75-MZ3 from Florida and Ein Yahav 6 from Israel, which showed measurable 14C and older 81Kr-ages can probably be explained by mixing of multiple water bodies with distinct ages.

Figure 7 Comparison of 81Kr age against (a) CO2-14C age and (b) DIC-14C age. For comparison with 81Kr age, 14C ages are calculated assuming simple decay with a half-life of 5730 yr, with an initial 14C activity of 100 pMC. 14C ages are uncorrected for the effects of chemical reactions in the subsurface. The solid line represents equal ages for the two tracers. The arrows represent cases where 81Kr ages were modern or where 14C activities were blank level.
The newly developed CO2-14C analyses of the groundwater with very long (>150 kyr) residence time proved that this method can provide 14C activities of groundwater devoid of modern atmospheric contamination. The sampling protocol can be conducted for the analyses of 14C using a relatively simple device at smaller scale (e.g. see Device A in Yokochi Reference Yokochi2016). It will significantly simplify the interpretation of 14C age of old groundwater by excluding any ambiguity on whether a measured low activity is due to contamination or the real presence of 14C-bearing groundwater components. Old samples give old age rather than a few pMC that corresponds to the Last Glacial Period, and therefore extends the reliable age range of 14C from 30 kyr to 50 kyr. Detectable 14C activity with a discordant 81Kr age serves as a proxy for mixing of water body with different ages.
The presented field gas extraction method can be used for collecting CO2-14C samples without radiokrypton analyses. Single-ended gas tight containers may be used with the configuration of Device A in Yokochi (Reference Yokochi2016), whereas the configuration for double ended sample containers proposed in Figure 1 allows simpler sample collection procedures. The sealed Pyrex tube containing CO2 sample gas is adaptable to an ampoule cracker type inlet of a gas source AMS (Ruff et al. Reference Ruff, Wacker, Gäggeler, Suter, Synal and Szidat2007, Reference Ruff, Fahrni, Gäggeler, Hajdas, Suter, Synal, Szidat and Wacker2010) as well as to the emerging optical radiocarbon detection method, saturated-absorption cavity ring-down (SCAR; Galli et al. Reference Galli, Bartalini, Cancio, De Natale, Mazzotti, Giusfredi, Fedi and Mandò2013), for which the graphitization of carbon is not necessary, and consequently lower blank and higher sample throughput may be achieved. There are ways of determining sample contamination or young groundwater contribution using alternative tracers to radiokrypton isotopes. Because relatively old groundwater is usually anoxic, modern atmospheric contamination during sampling may be quantified by measuring O2 concentration in the bulk gas phase collected (e.g. Yokochi et al. Reference Yokochi, Sturchio, Purtschert, Jiang, Lu, Mueller, Yang, Kennedy and Kharaka2013). Mixing with young groundwater component can be identified with other commonly used anthropogenic tracers such as tritium (-3He), CFCs, and SF6 (e.g. Corcho Alvarado et al. Reference Corcho Alvarado, Purtschert, Barbecot, Chabault, Rueedi, Schneider, Aeschbach-Hertig, Kipfer and Loosli2007). Compared to other membrane-based gas samplers in the literatures (Loose et al. Reference Loose, Stute, Alexander and Smethie2010; Garnett et al. Reference Garnett, Dinsmore and Billett2012; Matsumoto et al. Reference Matsumoto, Han, Jaklitsch and Aggarwal2013), this method does not wait for the gas–water solubility equilibrium to be attained. It enables rapid collection of large size samples, but chemical and isotope fractionations are anticipated. Whenever precise δ13C data is required, correction for such fractionations is essential. Alternatively, small-size sample for δ13C analyses may be collected separately.
The traditional DIC-14C method implied the recharge period around 30 kyr for groundwater from the Judea and Kurnub aquifers in Israel. The revised much older groundwater ages based on 81Kr brought new insights into the hydrology of the Negev Nubian aquifer. The 81Kr abundance of samples from the Floridan Aquifer confirmed the relatively young groundwater age anticipated from some of the corrected 14C abundances. Hydrological implications will be discussed in separate papers.
CONCLUSION
This study demonstrates that the field-degassing method used for the studies of noble gas radionuclides provides reliable samples for the analyses of 14C in the form of CO2 gas. Although dissolved CO2 gas is not usually the major species among DIC, sufficient quantities of gaseous CO2 can be acquired during most standard sampling procedures of noble gas radionuclides (Kr and Ar) because the analyses of rare noble gas radionuclides currently require comparably large amounts of gas to be extracted from the groundwater. The δ13C compositions of gaseous CO2 were significantly lighter than that of DIC samples but the degree of isotope fractionation is, in most cases, in good agreement with the theoretically expected value and therefore δ13C is still useful for evaluating exchange with 14C dead carbon sources. An obvious advantage of using field-degassed CO2 instead of DIC from a separate aliquot of water is that the gases are collected in a way that has proven to be highly immune against atmospheric contamination, and any potential atmospheric contamination occurring during sampling can be rigorously traced by the analyses of 85Kr. An additional advantage is that it is improbable that any biological activity would take place in compressed gas cylinders where the only liquid water inside the gas cylinders is condensate of water vapor that traveled through the hydrophobic membrane contactor. The field data presented here confirmed the reliability of the newly developed method and the significant role of radiokrypton isotopes as hydrological tracers.
ACKNOWLEDGMENTS
This work was supported by Ben Gurion University, Argonne National Laboratory, the University of Chicago Collaborative Water Research Initiative, and United States–Israel Binational Science Foundation (BSF Grant No. 2014351). The University of Chicago equipment was supported in part by NSF award #0923831, funded under the American Recovery and Reinvestment Act of 2009 (Public Law 111-5). J.C.Z., W.J., Z-T.L., P.M. and the Laboratory for Radiokrypton Dating at Argonne are supported by DOE, Office of Nuclear Physics, under contract DE-AC02-06CH11357. Reviews from three anonymous reviewers and advice from Albert Colman, Hiroshi Takahashi, Neil Sturchio, David Archer, and Associate Editor John Southon significantly improved the manuscript. Alex Cherkinsky at the UGA AMS facility is acknowledged for advice and discussions on sample preparation and data interpretations involving uncertainties and blanks. We thank Israel Water Authority and Mekorot LTD national water company for providing access to the observation boreholes and the production wells. We are grateful to Arik Kaplan for his support throughout the sampling campaign in Israel. We thank the South Florida Water Management District for the permission to access the wells, and Emily Richardson, Steven Krupa, and Brian Collins for their scientific inputs and help in the field. RY is grateful to Brian Lynch, James Eason, and PSD Computing for their support.
APPENDIX
Dissolved inorganic carbon (DIC) in water consists of dissolved CO2 [CO2]
aq
, carbonic acid [H2CO3], bicarbonate ion [HCO−] and carbonate ion [CO2−]. The former two species cannot be analytically distinguished and are thus treated together as [
![]() $${\rm H_{2} CO_{3}^{{\asterisk}}$$
]. The relative abundances of these species are thermodynamically constrained as a function of temperature and salinity as (for more detailed discussion, see Zeebe and Wolf-Gladrow Reference Zeebe and Wolf-Gladrow2001):
$${\rm H_{2} CO_{3}^{{\asterisk}}$$
]. The relative abundances of these species are thermodynamically constrained as a function of temperature and salinity as (for more detailed discussion, see Zeebe and Wolf-Gladrow Reference Zeebe and Wolf-Gladrow2001):
Total DIC concentration is defined as
The four equations can be solved for the four chemical species, and we obtain
When these reactions are primary controls of pH,
 $$pH{\equals}{\minus}Log_{{10}} \left[ {{{2K_{2} } \over {\sqrt {1{\minus}4{{K_{2} } \over {K_{1} }}{\plus}4{{K_{2} } \over {K_{0} \cdot K_{1} }}{{TDIC} \over {P_{{CO2}} }}{\minus}1} }}} \right]$$
$$pH{\equals}{\minus}Log_{{10}} \left[ {{{2K_{2} } \over {\sqrt {1{\minus}4{{K_{2} } \over {K_{1} }}{\plus}4{{K_{2} } \over {K_{0} \cdot K_{1} }}{{TDIC} \over {P_{{CO2}} }}{\minus}1} }}} \right]$$
The fraction of carbonic acid over TDIC is expressed as
As water flows through the membrane contactor in steady state,
![]() $${\rm H_{2} CO_{3}^{{\asterisk}}$$
is rapidly extracted via pumping so that TDIC decreases. The initial (prior to degassing) fraction
$${\rm H_{2} CO_{3}^{{\asterisk}}$$
is rapidly extracted via pumping so that TDIC decreases. The initial (prior to degassing) fraction
![]() $${\rm F}_{{H}_{2}CO_{3}^{{\asterisk}} }}$$
of TDIC is subject to this process, which constitute <10% for studied samples. Typical P
CO2 drop of about 90% is anticipated based on the membrane gas phase pressure around 0.1 bar during sample collection. As the CO2 gas is drawn off, the [
$${\rm F}_{{H}_{2}CO_{3}^{{\asterisk}} }}$$
of TDIC is subject to this process, which constitute <10% for studied samples. Typical P
CO2 drop of about 90% is anticipated based on the membrane gas phase pressure around 0.1 bar during sample collection. As the CO2 gas is drawn off, the [
![]() $${\rm H_{2} CO_{3}^{{\asterisk}}$$
] will decrease, driving the reaction
$${\rm H_{2} CO_{3}^{{\asterisk}}$$
] will decrease, driving the reaction
![]() $${HCO_{3}^{{\minus}}$$
+H+–>
$${HCO_{3}^{{\minus}}$$
+H+–>
![]() $${\rm H_{2} CO_{3}^{{\asterisk}}$$
. This raises the pH and decreases the P
CO2 /TDIC at equilibrium by about a factor of 10. The newly equilibrating (and still degassing) water has an equilibrium
$${\rm H_{2} CO_{3}^{{\asterisk}}$$
. This raises the pH and decreases the P
CO2 /TDIC at equilibrium by about a factor of 10. The newly equilibrating (and still degassing) water has an equilibrium
![]() $${\rm F}_{{H}_{2}CO_{3}^{{\asterisk}} }}$$
values lowered approximately by a factor of 10. The fraction of CO2 gas formed via dehydration of bicarbonate ion will constitute <10% of total CO2 gas extracted.
$${\rm F}_{{H}_{2}CO_{3}^{{\asterisk}} }}$$
values lowered approximately by a factor of 10. The fraction of CO2 gas formed via dehydration of bicarbonate ion will constitute <10% of total CO2 gas extracted.
The residence time of water in the membrane contactor is relatively short, on the order of 5–10 s. This is comparable to the relaxation time scale (5–20 s; Zeebe and Wolf-Gladrow Reference Zeebe and Wolf-Gladrow2001) of the H2CO3* equilibration in aqueous system. An incomplete dehydration of bicarbonate ion may occur, during which kinetic isotope fractionation is anticipated with a relatively large fractionation factor ∆13C ∼ –15‰ at 24ºC (Marlier and O’Leary Reference Marlier and O’Leary1984). Because the contribution of H2CO3* formed via this dehydration reaction is estimated to be minor (<10%), the effect on δ13C CO2 is also relatively small, <–1.5‰ compared to the δ13C CO2– δ13C DIC,Model of several ‰ (see subsection “Carbon Isotope Fractionation and Mixing”). The relatively good agreement between δ13C DIC and δ13C DIC,Model (Figure 6) confirms that the effect of this kinetic isotope fractionation is minor for the purpose of the presented method.
The apparatus effectively extracts inert gases with low diffusivity in water, which indicates that the degassing process is not diffusion-limited. We therefore do not consider isotopic fractionation by diffusion.