Coronavirus disease 2019 (COVID-19) is a highly contagious disease, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Reference Huang, Wang and Li1 The SARS-CoV-2 virus is transmitted through respiratory droplets Reference Meng, Hua and Bian2 and close contact with infected individuals. Reference Morawska and Cao3 Aerosolized particles generated by medical procedures such as transsphenoidal endoscopic pituitary surgery could be another transmission route. Reference Patel, Fernandez-Miranda and Hwang4 Healthcare providers taking care of COVID-19 patients without appropriate personal protective equipment (PPE) are at high risk for infection. A shortage of surgical masks has resulted from an abrupt rise in global demand. Reference Wu, Huang, Zhang, He and Ming5 While surgical masks filter infectious particles spreading via droplets, filtering facepiece respirators (FFRs) filter >95% of airborne particles. These masks are designed for single use. Reference Desai and Mehrotra6 Reuse of these disposable masks has been implemented during the COVID-19 pandemic, Reference Feng, Shen, Xia, Song, Fan and Cowling7 although the appropriate method of decontamination remains unclear. Concerns include sterility, filtration efficiency, and structural integrity. Reference Heimbuch, Wallace and Kinney8,9 In this systematic review, we assessed the evidence of various decontamination methods of surgical masks and FFRs, including N95 and P100.
Methods
Eligibility criteria
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Reference Moher, Liberati, Tetzlaff and Altman10 . We applied the following inclusion criteria: trials studying the performance of decontamination and reuse of surgical masks and/or FFRs, any study designs, any device, any methods, and any models of FFRs. We also applied the following exclusion criteria: studies published in a language other than English, nonexperimental studies, and studies without original data. The outcome measures were disinfection of bacteria and virus, post-decontamination filtration efficiency, and physical structure degradation.
Information sources and search strategy
Electronic systematic searches were conducted. The last search was performed on April 11, 2020. Literature searches were performed using Ovid MEDLINE and Ovid EMBASE. We also scanned references of the included studies to identify any missing published or unpublished trials. We used the following search strategy: (“exp Respiratory Protective Devices/ or Filtering Facepiece Respirators.mp.” or “exp Respiratory Protective Devices/ or N95.mp.” or “face mask.mp.” or “exp Masks/ or surgical mask.mp.” or “medical masks.mp.”) and (“decontamination.mp. or exp Decontamination/” or “exp Recycling/ or reuse.mp.” or “reusability.mp.”).
Study selection and data collection
Two review authors (V.P. and T.C.) independently performed trial selection by title and abstract screening based on predetermined eligibility criteria. The full-text articles of the selected studies were reviewed for the final study selection. Two authors (K.Se. and K.T.) extracted data from the included studies. Disagreements were resolved by the fifth author (K.Sn.).
Results
We identified 196 studies: 190 studies from electronic searches, and 6 studies from manual searches. During the title and abstract screening, 173 studies were irrelevant and excluded. After full-text screening, 8 studies were excluded. Finally, 15 studies were included in the qualitative synthesis (Fig. 1). Reference Heimbuch, Wallace and Kinney8,Reference Viscusi, Bergman, Eimer and Shaffer11–Reference Fisher, Williams and Shaffer24
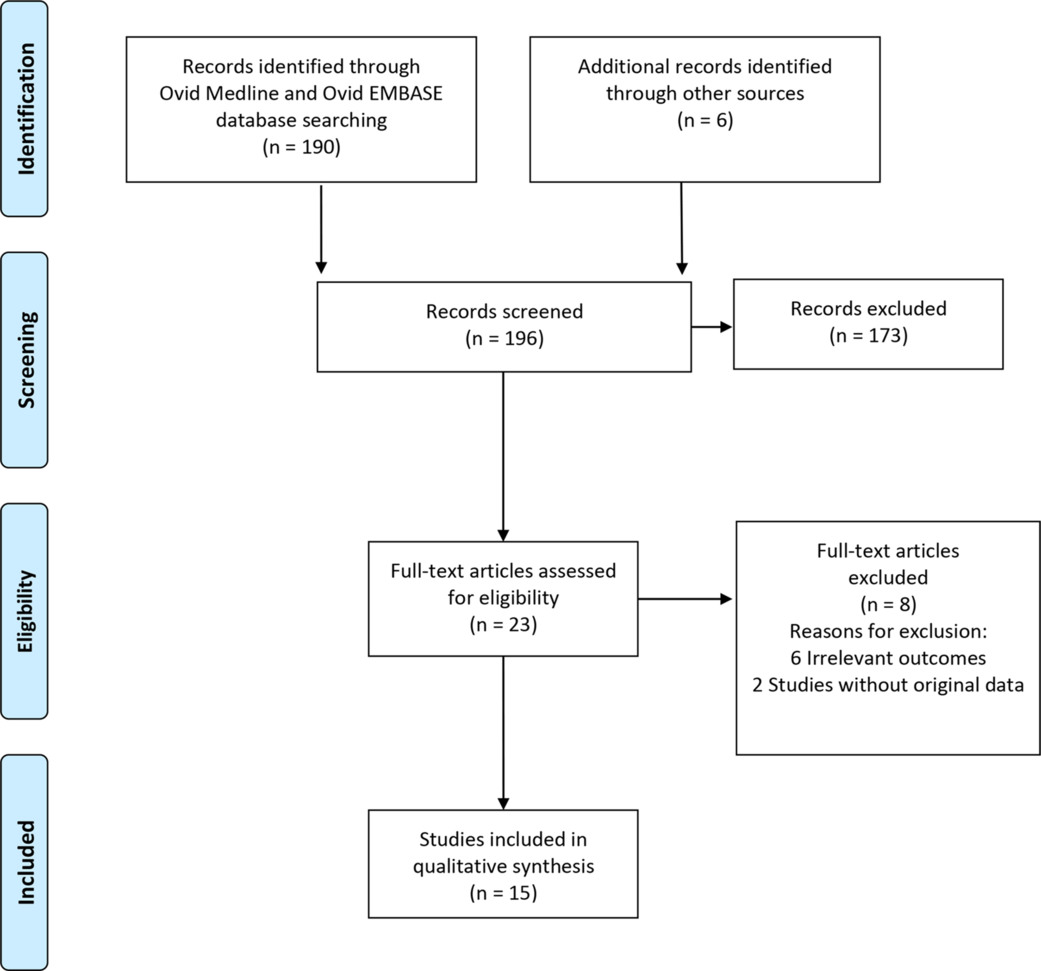
Fig. 1. Flow diagram of study selection for the systematic review.
Included studies
None of the 15 included studies assessed surgical masks. All studies assessed FFRs, including N95 and P100. All included studies were nonhuman subject research. Of the 15 studies, 4 studies (27%) assessed disinfection of bacteria, Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,Reference Lin, Tang, Hung, Hua and Lai17,21,Reference Schwartz, Stiegel and Greeson22 7 studies (47%) assessed disinfection of virus, Reference Heimbuch, Wallace and Kinney8,Reference Viscusi, Bergman, Eimer and Shaffer11,Reference Bergman, Viscusi, Zhuang, Palmiero, Powell and Shaffer13–Reference Lore, Heimbuch, Brown, Wander and Hinrichs15,Reference Mills, Harnish, Lawrence, Sandoval-Powers and Heimbuch18,Reference Fisher, Williams and Shaffer24 9 studies (60%) assessed postdecontamination filtration efficiency, Reference Viscusi, Bergman, Eimer and Shaffer11,Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,Reference Lore, Heimbuch, Brown, Wander and Hinrichs15,Reference Lindsley, Martin and Thewlis16,Reference Viscusi, King and Shaffer19–Reference Schwartz, Stiegel and Greeson22,Reference Fisher, Williams and Shaffer24 and 12 studies (80%) assessed physical structure degradation. Reference Heimbuch, Wallace and Kinney8,Reference Viscusi, Bergman, Eimer and Shaffer11–Reference Viscusi, Bergman and Novak14,Reference Lindsley, Martin and Thewlis16,Reference Viscusi, King and Shaffer19–Reference Fisher, Williams and Shaffer24
We identified 14 decontamination methods. Data regarding ultraviolet germicidal irradiation (UVGI) (9 studies) Reference Heimbuch, Wallace and Kinney8,Reference Viscusi, Bergman, Eimer and Shaffer11–Reference Mills, Harnish, Lawrence, Sandoval-Powers and Heimbuch18 are described in Supplementary Table 1 (online). Data regarding moist heat (5 studies) Reference Heimbuch, Wallace and Kinney8,Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12–Reference Lore, Heimbuch, Brown, Wander and Hinrichs15 are described in Supplementary Table 2 (online). Data regarding microwave-generated steam (MGS) (4 studies) Reference Heimbuch, Wallace and Kinney8,Reference Bergman, Viscusi, Zhuang, Palmiero, Powell and Shaffer13–Reference Lore, Heimbuch, Brown, Wander and Hinrichs15 and hydrogen peroxide vapor (HPV) (4 studies) Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,21–Reference Kenney, Chan and Kortright23 are described in Supplementary Table 3 (online). Data regarding microwave steam bags (1 study), Reference Fisher, Williams and Shaffer24 bleach (5 studies), Reference Viscusi, Bergman, Eimer and Shaffer11,Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,Reference Lin, Tang, Hung, Hua and Lai17,Reference Viscusi, King and Shaffer19,Reference Lin, Chen, Huang, Kuo, Lai and Lin20 steam treatment, (3studies), Reference Lin, Tang, Hung, Hua and Lai17,Reference Viscusi, King and Shaffer19,Reference Lin, Chen, Huang, Kuo, Lai and Lin20 dry heat (3 studies), Reference Lin, Tang, Hung, Hua and Lai17,Reference Viscusi, King and Shaffer19,Reference Lin, Chen, Huang, Kuo, Lai and Lin20 ethanol or isopropyl alcohol (3 studies) Reference Lin, Tang, Hung, Hua and Lai17,Reference Viscusi, King and Shaffer19,Reference Lin, Chen, Huang, Kuo, Lai and Lin20 , ethylene oxide (EtO) (3 studies), Reference Viscusi, Bergman, Eimer and Shaffer11,Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,Reference Viscusi, King and Shaffer19 hydrogen peroxide gas plasma (HPGP) (2 studies), Reference Viscusi, Bergman, Eimer and Shaffer11,Reference Viscusi, King and Shaffer19 liquid hydrogen peroxide (LHP) (2 studies), Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,Reference Viscusi, King and Shaffer19 microwave irradiation (1 study), Reference Viscusi, King and Shaffer19 and soap and water (1 study) Reference Viscusi, King and Shaffer19 are described in Supplementary Table 4 (online).
Ultraviolet germicidal irradiation
Overall, 9 studies assessed the performance of UVGI decontamination method. Reference Heimbuch, Wallace and Kinney8,Reference Viscusi, Bergman, Eimer and Shaffer11–Reference Mills, Harnish, Lawrence, Sandoval-Powers and Heimbuch18 All studies evaluated ultraviolet light-C (UV-C) with a primary wavelength of 254 nm. However, a great variety of equipment and delivery techniques were employed: a laminar-flow cabinet with UV-C light, Reference Viscusi, Bergman, Eimer and Shaffer11,Reference Bergman, Viscusi, Zhuang, Palmiero, Powell and Shaffer13–Reference Lore, Heimbuch, Brown, Wander and Hinrichs15 a UV-C lamp, Reference Heimbuch, Wallace and Kinney8,Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,Reference Lin, Tang, Hung, Hua and Lai17 and a chamber with a UV-C bulb. Reference Lindsley, Martin and Thewlis16,Reference Mills, Harnish, Lawrence, Sandoval-Powers and Heimbuch18 Diversity across studies included the intensity of UV-C (W/cm2), the dose of UV-C (J/cm2), the distance between the source of UV-C and the FFRs, the exposure surface of the FFRs, the total exposure time, and the number of cycles. The exposure time per cycle varied from 1 minute, Reference Lindsley, Martin and Thewlis16–Reference Mills, Harnish, Lawrence, Sandoval-Powers and Heimbuch18 15 minutes, Reference Heimbuch, Wallace and Kinney8,Reference Bergman, Viscusi, Zhuang, Palmiero, Powell and Shaffer13,Reference Lore, Heimbuch, Brown, Wander and Hinrichs15 20 minutes, Reference Lin, Tang, Hung, Hua and Lai17 30 minutes Reference Viscusi, Bergman, Eimer and Shaffer11,Reference Viscusi, Bergman and Novak14 to 45 minutes. Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12 The number of cycles varied from 1 Reference Heimbuch, Wallace and Kinney8,Reference Viscusi, Bergman, Eimer and Shaffer11,Reference Viscusi, Bergman and Novak14–Reference Mills, Harnish, Lawrence, Sandoval-Powers and Heimbuch18 to 3 cycles. Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,Reference Bergman, Viscusi, Zhuang, Palmiero, Powell and Shaffer13
UVGI was effective for influenza virus inactivation, including H5N1 (2 studies) Reference Viscusi, Bergman, Eimer and Shaffer11,Reference Lore, Heimbuch, Brown, Wander and Hinrichs15 and H1N1 (3 studies), Reference Heimbuch, Wallace and Kinney8,Reference Bergman, Viscusi, Zhuang, Palmiero, Powell and Shaffer13,Reference Mills, Harnish, Lawrence, Sandoval-Powers and Heimbuch18 and Bacillus subtilis spore inactivation. Reference Lin, Tang, Hung, Hua and Lai17 Post-decontamination filtration efficiency was unchanged in 4 studies. Reference Viscusi, Bergman, Eimer and Shaffer11,Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,Reference Lore, Heimbuch, Brown, Wander and Hinrichs15,Reference Lindsley, Martin and Thewlis16 The physical structure was unchanged in 3 studies, Reference Viscusi, Bergman, Eimer and Shaffer11–Reference Bergman, Viscusi, Zhuang, Palmiero, Powell and Shaffer13 but physical strength partially lost with high doses of UV-C at 120 J/cm2 and 950 J/cm2 and the head strap strength lost at 590 J/cm2. Reference Lindsley, Martin and Thewlis16 The optimal UV-C dose should be <2 J/cm2. Laminar flow cabinet was suggested for 3M 1860, 3M 1870, Kimberly Clark PFR 95-270. Reference Viscusi, Bergman, Eimer and Shaffer11,Reference Bergman, Viscusi, Zhuang, Palmiero, Powell and Shaffer13–Reference Lore, Heimbuch, Brown, Wander and Hinrichs15 UV-C chamber was suggested for 3M 1860, 3M 9210, Gerson 1730, Kimberly Clark 46727. Reference Lindsley, Martin and Thewlis16,Reference Mills, Harnish, Lawrence, Sandoval-Powers and Heimbuch18 UV-C lamp was effective, but the FFRs model was unspecified. Reference Heimbuch, Wallace and Kinney8,Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,Reference Lin, Tang, Hung, Hua and Lai17
Moist heat
In 5 studies, the performance of moist heat decontamination method was assessed, Reference Heimbuch, Wallace and Kinney8,Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12–Reference Lore, Heimbuch, Brown, Wander and Hinrichs15 and 2 types of equipment were used. In 3 studies, N95 FFRs were decontaminated with a laboratory incubator for a 30-minute incubation at 60°C. The FFRs were air dried after each incubation: overnight after the first incubation and for 30 minutes after the second and the third incubations. Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12–Reference Viscusi, Bergman and Novak14 In 2 studies, a 6-L sealable container filled with 1 L tap water was warmed in a 65°C oven for a minimum of 3 hours. Then the FFRs were placed on a rack to isolate the FFRs from the liquid, and the containers were sealed and returned to the oven. Reference Heimbuch, Wallace and Kinney8,Reference Lore, Heimbuch, Brown, Wander and Hinrichs15 The exposure time per cycle ranged from 15 minutes Reference Bergman, Viscusi, Zhuang, Palmiero, Powell and Shaffer13 to 20 minutes Reference Lore, Heimbuch, Brown, Wander and Hinrichs15 to 30 minutes. Reference Heimbuch, Wallace and Kinney8,Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,Reference Viscusi, Bergman and Novak14 The number of cycles ranged from 1 Reference Heimbuch, Wallace and Kinney8,Reference Viscusi, Bergman and Novak14,Reference Lore, Heimbuch, Brown, Wander and Hinrichs15 to 3. Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,Reference Bergman, Viscusi, Zhuang, Palmiero, Powell and Shaffer13
Moist heat was effective for the H1N1 Reference Heimbuch, Wallace and Kinney8 and H5N1 Reference Lore, Heimbuch, Brown, Wander and Hinrichs15 influenza virus inactivation when using a prewarmed sealable container. Viral inactivation was not achieved by a laboratory incubator. Bacterial disinfection was not assessed. The postdecontamination filtration efficiency was >97.5%. Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,Reference Lore, Heimbuch, Brown, Wander and Hinrichs15 The physical structure was reported unchanged by 1 study Reference Heimbuch, Wallace and Kinney8 but degradation was reported in some models by 3 studies. Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12–Reference Viscusi, Bergman and Novak14 A 6-L prewarmed sealable container filled with 1 L tap water was suggested for models 3M 1860 and 3M 1870. Reference Heimbuch, Wallace and Kinney8,Reference Lore, Heimbuch, Brown, Wander and Hinrichs15
Microwave-generated steam
In 4 studies, the performance of MGS decontamination was assessed. Reference Heimbuch, Wallace and Kinney8,Reference Bergman, Viscusi, Zhuang, Palmiero, Powell and Shaffer13–Reference Lore, Heimbuch, Brown, Wander and Hinrichs15 FFRs were placed outer-side down on top of 2 side-by-side pipette tip boxes with 50 mL room-temperature tap water, in a 1,100W, 1,250W (2,450 MHz) microwave oven with a revolving glass carousel. The exposure time was 2 minutes at the maximum power setting. Then the FFRs were dried overnight on a laboratory benchtop. In 3 studies, the FFRs were decontaminated with 1 cycle, Reference Heimbuch, Wallace and Kinney8,Reference Viscusi, Bergman and Novak14,Reference Lore, Heimbuch, Brown, Wander and Hinrichs15 and in 1 study FFRs were decontaminated with 3 cycles. Reference Bergman, Viscusi, Zhuang, Palmiero, Powell and Shaffer13 MGS inactivated >4-log reduction of the viable virus. Reference Heimbuch, Wallace and Kinney8,Reference Bergman, Viscusi, Zhuang, Palmiero, Powell and Shaffer13–Reference Lore, Heimbuch, Brown, Wander and Hinrichs15 Bacterial disinfection was not assessed. The post-decontamination filtration efficiency was unchanged. Reference Lore, Heimbuch, Brown, Wander and Hinrichs15 In 3 studies a slight separation of the inner-foam nose cushion was observed in some samples. Reference Heimbuch, Wallace and Kinney8,Reference Bergman, Viscusi, Zhuang, Palmiero, Powell and Shaffer13,Reference Viscusi, Bergman and Novak14 Although a minor physical structure degradation was reported, the FFRs had a 90%–100% fit-test passing rate. Reference Bergman, Viscusi, Zhuang, Palmiero, Powell and Shaffer13 A 1,250W (2450 MHz) microwave oven with a revolving glass carousel was suggested for 3M 1860. Reference Heimbuch, Wallace and Kinney8,Reference Lore, Heimbuch, Brown, Wander and Hinrichs15
Hydrogen peroxide vapor
In 4 studies, the performance of HPV decontamination was assessed for 3, 5, 30, and 50 cycles (225, 405, and 480, and 1,440 minutes per cycle). Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,21–Reference Kenney, Chan and Kortright23 An HPV generator utilizing 30% or 35% hydrogen peroxide solution was placed in a room. The FFRs were placed on stainless-steel wire racks. The HPV run consisted of the following 5 stages: conditioning, pre-gassing, gassing, gassing dwell, and aeration. The processing room attained the 480+ parts per million (ppm) level of HPV with gassing times of 25 and 40 minutes and gassing dwell times of 15, 20, and 25 minutes (ie, the sterilization process). During the aeration stage, fresh air was introduced into the room to increase the rate of catalytic conversion of hydrogen peroxide into water and oxygen. Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,21–Reference Kenney, Chan and Kortright23 In addition, 4 hours of aeration eliminated the toxicity of hydrogen peroxide. Reference Schwartz, Stiegel and Greeson22 Viral disinfection was not assessed. HPV was effective for Geobacillus stearothermophilus spore inactivation. Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,21,Reference Schwartz, Stiegel and Greeson22 Post-decontamination filtration efficiency 21,Reference Schwartz, Stiegel and Greeson22 and the physical structure were unchanged. Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,Reference Schwartz, Stiegel and Greeson22,Reference Kenney, Chan and Kortright23 Placement of the FFRs on stainless-steel wire racks in the room with an HPV generator was suggested for 3M 1860. 21,Reference Schwartz, Stiegel and Greeson22
Microwave steam bags
One study assessed the performance of microwave steam bag decontamination. Reference Fisher, Williams and Shaffer24 The FFRs were placed inside separate bags filled with 60 mL tap water. The bags were sealed, placed in a microwave oven, and irradiated on high power for 90 seconds. Bacteriophage MS2, a surrogate for a pathogenic virus, was thereby inactivated, but bacterial inactivation was not assessed. The postdecontamination filtration efficiency and the physical structure were unchanged. Reference Fisher, Williams and Shaffer24
Bleach
The performance of bleach decontamination was assessed in 5 studies. Reference Viscusi, Bergman, Eimer and Shaffer11,Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,Reference Lin, Tang, Hung, Hua and Lai17,Reference Viscusi, King and Shaffer19,Reference Lin, Chen, Huang, Kuo, Lai and Lin20 The FFRs were submerged in a 0.6% aqueous solution of sodium hypochlorite for 1–3 cycles. The exposure time ranged from 10 to 30 minutes. Reference Viscusi, Bergman, Eimer and Shaffer11,Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,Reference Lin, Tang, Hung, Hua and Lai17,Reference Viscusi, King and Shaffer19,Reference Lin, Chen, Huang, Kuo, Lai and Lin20 After treatment, they were hung on a laboratory pegboard and allowed to air dry overnight. Virus inactivation was not assessed. Bleach was effective for Bacillus subtillis spore inactivation. Reference Lin, Tang, Hung, Hua and Lai17 The postdecontamination filtration efficiency was unchanged in 3 studies, Reference Viscusi, Bergman, Eimer and Shaffer11,Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,Reference Viscusi, King and Shaffer19 but it decreased in 1 study. Reference Lin, Chen, Huang, Kuo, Lai and Lin20 The physical structure of N95 FFRs was degraded after the 30 minutes of decontamination. Reference Viscusi, Bergman, Eimer and Shaffer11,Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,Reference Viscusi, King and Shaffer19
Steam
The performance of steam decontamination was assessed in 3 studies. Reference Lin, Tang, Hung, Hua and Lai17,Reference Viscusi, King and Shaffer19,Reference Lin, Chen, Huang, Kuo, Lai and Lin20 Characteristics of these studies assessing steam are described in Supplementary Table 5 (online). The FFRs were sealed in an autoclave bag and treated in an autoclave at 121°C. The FFRs were air dried for 72 hours. Virus inactivation was not assessed. Steam was effective for Bacillus subtillis spore inactivation. Reference Lin, Tang, Hung, Hua and Lai17 The filtration efficiency decreased in 2 studies, Reference Viscusi, King and Shaffer19,Reference Lin, Chen, Huang, Kuo, Lai and Lin20 and the outer layer of the N95 FFRs was deformed, shrunken, and stiff. Reference Viscusi, King and Shaffer19,Reference Lin, Chen, Huang, Kuo, Lai and Lin20
Dry heat
The performance of dry heat decontamination was assessed in 3 studies Reference Lin, Tang, Hung, Hua and Lai17,Reference Viscusi, King and Shaffer19,Reference Lin, Chen, Huang, Kuo, Lai and Lin20 using 2 types of equipment. One study used a hot-air oven; respirators were placed in a metal pan on racks of a laboratory oven and were turned over midway through the exposure period (60 minutes) for 1 cycle at 80°C and 160°C. Reference Viscusi, King and Shaffer19 Two studies used an electric rice cooker at 149–164°C for 3 minutes for 1 cycle, Reference Lin, Tang, Hung, Hua and Lai17,Reference Lin, Chen, Huang, Kuo, Lai and Lin20 but virus inactivation was not assessed. Dry heat with an electric cooker was effective on the disinfection of Bacillus subtilis spores. Reference Lin, Tang, Hung, Hua and Lai17 The postdecontamination filtration efficiency was unchanged, but the FFRs were melted at 160°C after 22 minutes of decontamination. Reference Viscusi, King and Shaffer19
Ethanol or isopropyl alcohol
The performance of ethanol or isopropyl alcohol decontamination was assessed in 3 studies. Reference Lin, Tang, Hung, Hua and Lai17,Reference Viscusi, King and Shaffer19,Reference Lin, Chen, Huang, Kuo, Lai and Lin20 Ethanol with various concentrations and volumes was added to the center of the surface of the N95 FFRs. The FFRs were then dried in a petri dish placed in a biosafety cabinet for 10 minutes, followed by another 10 minutes of submersion in 100% isopropanol solution. Virus inactivation was not assessed. Ethanol was effective in the disinfection of Bacillus subtilis spores. Reference Lin, Tang, Hung, Hua and Lai17 The postdecontamination filtration efficiency decreased, Reference Viscusi, King and Shaffer19,Reference Lin, Chen, Huang, Kuo, Lai and Lin20 and the physical structure was unchanged. Reference Viscusi, King and Shaffer19,Reference Lin, Chen, Huang, Kuo, Lai and Lin20
Other methods
Other decontamination methods assessed neither viral nor bacterial inactivation. Post-decontamination filtration efficiency remained unchanged for EtO, Reference Viscusi, Bergman, Eimer and Shaffer11,Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,Reference Viscusi, King and Shaffer19 HPGP, Reference Viscusi, Bergman, Eimer and Shaffer11,Reference Viscusi, King and Shaffer19 LHP, Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,Reference Viscusi, King and Shaffer19 microwave irradiation decontamination. Reference Viscusi, King and Shaffer19 In contrast, soap and water decreased filtration efficiency. Reference Viscusi, King and Shaffer19 Physical degradation was shown in the ethylene oxide, HPGP, LHP, microwave irradiation methods Reference Viscusi, Bergman, Eimer and Shaffer11,Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,Reference Viscusi, King and Shaffer19 but the physical structure was unchanged for soap and water. Reference Viscusi, King and Shaffer19
Discussion
None of the existing published articles had data on the SARS-CoV-2 disinfection. However, both the influenza virus and the SARS-CoV-2 are in the same group of lipid bilayer enveloped viruses. Reference Ivanova, Myers, Milne, McClaren, Thomas and Brown25–Reference Peiris, Guan and Yuen27 Therefore, the data on the decontamination of the influenza virus could be applied to the COVID-19 setting. The studies assessed Bacillus subtilis Reference Lin, Tang, Hung, Hua and Lai17 and Geobacillus stearothermophilus Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,21,Reference Schwartz, Stiegel and Greeson22 disinfection. Spores of these bacteria are more challenging to disinfect than viruses; thus, the data can be applied to the COVID-19 pandemic. Reference Palenik28–Reference Lemieux, Sieber, Osborne and Woodard30 Bacteriophage MS2, a surrogate for a pathogenic virus, was also assessed. Fisher et al Reference Fisher, Williams and Shaffer24 reported that steam bags were 99.9% effective in inactivating MS2 on the FFRs. However, they commented that more research was required before the data could be applied.
We recommend 4 decontamination methods as options in response to a preponderance of benefit over harm shown by nonhuman subject research: UVGI, Reference Heimbuch, Wallace and Kinney8,Reference Viscusi, Bergman, Eimer and Shaffer11–Reference Mills, Harnish, Lawrence, Sandoval-Powers and Heimbuch18 moist heat, Reference Heimbuch, Wallace and Kinney8,Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12–Reference Lore, Heimbuch, Brown, Wander and Hinrichs15 MGS, Reference Heimbuch, Wallace and Kinney8,Reference Bergman, Viscusi, Zhuang, Palmiero, Powell and Shaffer13–Reference Lore, Heimbuch, Brown, Wander and Hinrichs15 and HPV, Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,21–Reference Kenney, Chan and Kortright23 UVGI, Reference Heimbuch, Wallace and Kinney8,Reference Viscusi, Bergman, Eimer and Shaffer11–Reference Mills, Harnish, Lawrence, Sandoval-Powers and Heimbuch18 moist heat, Reference Heimbuch, Wallace and Kinney8,Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12–Reference Lore, Heimbuch, Brown, Wander and Hinrichs15 MGS Reference Heimbuch, Wallace and Kinney8,Reference Bergman, Viscusi, Zhuang, Palmiero, Powell and Shaffer13–Reference Lore, Heimbuch, Brown, Wander and Hinrichs15 and HPV. Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,21–Reference Kenney, Chan and Kortright23 These methods were effective in disinfecting virus and bacteria and in maintaining the filtration efficiency and the physical structure of the FFRs (Fig. 2). We do not recommend other decontamination methods for 3 reasons. (1) Several methods did not assess the virus and bacteria disinfection: microwave steam bag, Reference Fisher, Williams and Shaffer24 EtO, Reference Viscusi, Bergman, Eimer and Shaffer11,Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,Reference Viscusi, King and Shaffer19 HPGP, Reference Viscusi, Bergman, Eimer and Shaffer11,Reference Viscusi, King and Shaffer19 LHP, Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,Reference Viscusi, King and Shaffer19 microwave irradiation, Reference Viscusi, King and Shaffer19 and soap and water. Reference Viscusi, King and Shaffer19 (2) Several methods decreased the filtration efficiency: soap and water, Reference Viscusi, King and Shaffer19 ethanol and isopropyl alcohol, Reference Viscusi, King and Shaffer19,Reference Lin, Chen, Huang, Kuo, Lai and Lin20 and microwave irradiation. Reference Viscusi, King and Shaffer19 And (3) several methods destroyed the physical structure of the masks: bleach, Reference Viscusi, Bergman, Eimer and Shaffer11,Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,Reference Viscusi, King and Shaffer19 HPGP, Reference Viscusi, Bergman, Eimer and Shaffer11,Reference Viscusi, King and Shaffer19 and microwave irradiation. Reference Viscusi, Bergman, Eimer and Shaffer11,Reference Viscusi, King and Shaffer19 A summary of the performance of the 14 decontamination methods is displayed in Supplementary Table 5 (online).
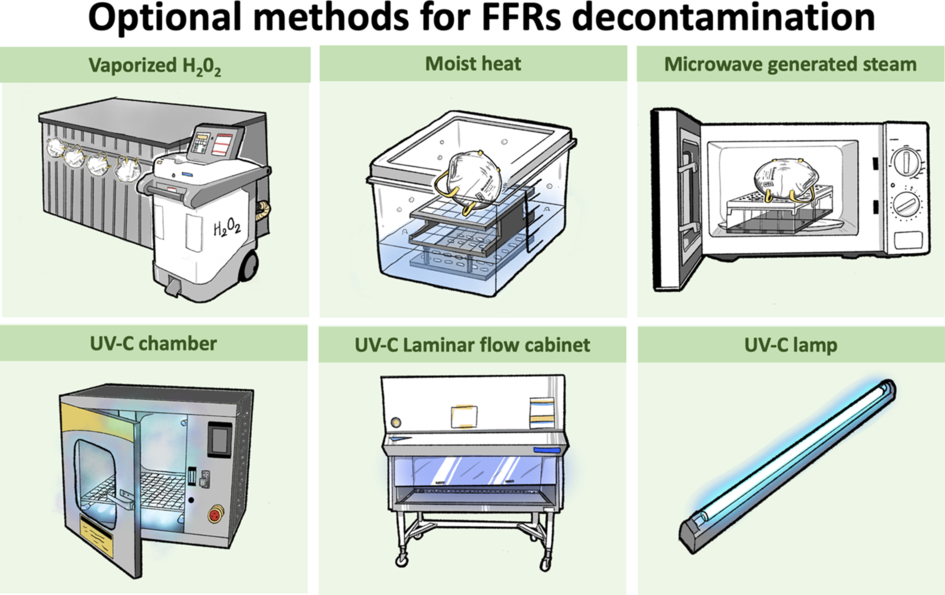
Fig. 2. Optional methods for FFRs decontamination.
The UV-C light decontaminates viruses by damaging the DNA and RNA of the virus. A study by Darnell et al Reference Darnell, Subbarao, Feinstone and Taylor31 showed that the UV-C light source (254 nm), which emitted 4.016 W/cm2 at a distance of 3 cm for 15 minutes, could inactivate severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) virus. For clinical applicability, the UV-C concentration, a distance between the UV-C light and the masks, and exposure time must be considered. The findings from this review showed that the UV-C light exposure at 1.6–2.2 W/cm2 for 1–3 cycles (15–30 minutes per cycle) could inactivate the H1N1 and H5N1 influenza viruses, maintain filtration efficiency, and restore the physical structure of the FFRs. Reference Heimbuch, Wallace and Kinney8,Reference Viscusi, Bergman, Eimer and Shaffer11–Reference Mills, Harnish, Lawrence, Sandoval-Powers and Heimbuch18 Heat inactivates viruses by modifying the protein structures of the virus that affects the attachment and replication within a host cell. Heat at 65°C inactivates most SARS-CoV-1 after 4 minutes. Reference Darnell, Subbarao, Feinstone and Taylor31 One cycle of moist heat exposure using a sealable container for 20 minutes inactivates the H5N1 virus Reference Lore, Heimbuch, Brown, Wander and Hinrichs15 and the H1N1 after 30 minutes. Reference Heimbuch, Wallace and Kinney8 Physical structure degradation may occur when the temperature is >60–70°C or when >1 cycle is used. Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12–Reference Viscusi, Bergman and Novak14 Mid-to-high relative humidity increases viral inactivation, although 100% humidity is not effective. Reference Casanova, Jeon, Rutala, Weber and Sobsey32,Reference Lin and Marr33 The SARS-CoV-1 infectivity is reduced by 60–75°C heat exposure in various liquid media. Reference Darnell, Subbarao, Feinstone and Taylor31 McDevitt et al Reference McDevitt, Rudnick, First and Spengler34 showed that H1N1 inactivation in a dried solution on stainless steel when either temperature or relative humidity was increased. Reference McDevitt, Rudnick, First and Spengler34 Therefore, MGS was recommended as an option. Although a minor physical structure degradation was reported, the FFRs passed a fit test. Reference Heimbuch, Wallace and Kinney8,Reference Bergman, Viscusi, Zhuang, Palmiero, Powell and Shaffer13,Reference Viscusi, Bergman and Novak14 The combination of hydrogen peroxide gas and the generation of hydroxyl and hydroperoxyl free radicals inactivates spores of the Geobacillus stearothermophilus bacteria. Reference Bergman, Viscusi, Heimbuch, Wander, Sambol and Shaffer12,21,Reference Schwartz, Stiegel and Greeson22 Compared to other decontamination methods, HPV can increase the number of cycles up to 20 cycles and still maintain filter efficiency and physical structure. 21 The Battelle Decontamination System, an HPV system for decontaminating N95 masks, received emergency use authorization from the FDA on March 28, 2020. 35
Our recommendations align with the Centers for Disease Control and Prevention (CDC) recommendations about the emergency reuse of UVGI, moist heat, and HPV. Furthermore, the CDC suggests that healthcare workers should have at least 5 pieces of N95 FFRs and that the used FFRS should be kept in a breathable paper bag and labeled at the end of each shift. The FFRS should be reused with a minimum of 5 days after the last use. 35 This recommendation is based on the study by van Doremalen et al Reference van Doremalen, Bushmaker, Morris, Holbrook, Gamble and Williamson36 showing that SARS-CoV-2 can survive for up to 72 hours on plastic, stainless steel, and cardboard surfaces. In contrast, Chin et al Reference Chin, Chu and Perera37 found that the SARS-CoV-2 could be detected on the outer layer of a surgical mask after 7 days. We believe that no good evidence supports the safety of the reuse of medical masks after keeping the used masks for 72–98 hours. 35
Although surgical masks are not indicated to protect general people from the transmission of respiratory pathogens, masks are overused by the public and surgical masks are scarce. The reuse of masks was not recommended in normal situations. The masks were not manufactured for multiple uses; they were not intended for extended wear and should not be worn for several hours at a time. Medical personnel should follow the recommended reuse techniques summarized in Supplementary Table 5 (online). Otherwise, the masks could lose their filtering efficiency, which could lead to a failure of protection against infection. A decontaminated mask is not a fresh mask. After each decontamination, a seal check should always be conducted before wearing the mask. The mask must fit with the face with no leaking point for letting the air out. The straps should be intact and must not be loose. If a mask loses its structure, it should be discarded immediately. Touching the inside surface of the mask should be avoided. After touching the mask, the hands must be washed with soap and water for at least 20 seconds or sanitized using a hand rub with at least 60% alcohol. Reference Desai and Mehrotra6 The reused mask should be worn by the same person.
The limitation of this study was the quality of the included studies. No clinical study has proven that the studied methods are clinically effective. We detected heterogeneity among the included studies, with considerable variation in decontamination equipment and techniques. The volume of masks was not addressed. Currently, no data are available on methods for disinfection of SARS-CoV-2. Instead, studies investigating influenza virus and bacteria spores were included here. High-quality studies investigating SARS-CoV-2 decontamination from used surgical masks are required for a higher level of evidence in future research.
In conclusion, decontamination of surgical masks and N95 FFRs is necessary to prepare them for reuse in the shortage crisis during the COVID-19 pandemic. The selection of decontamination methods should be considered based on the data in which the effectiveness of virus and bacterial disinfection, the filtration efficiency, and the intact physical structure of the masks and FFRs after the decontamination process. Based on the influenza virus and bacterial inactivation, the UVGI, moist heat, MGS, and HPV methods were recommended as options. When these decontamination methods are used in practice, the techniques described in the literature should be strictly followed.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2020.379





