Menopause is associated with unfavourable blood-cholesterol changes such as elevated total cholesterol (TC), TAG and LDL-cholesterol and reduced HDL-cholesterol( Reference Phan and Toth 1 ). Dyslipidaemia, especially elevated blood LDL-cholesterol, is a major risk factor for CVD( Reference Phan and Toth 1 ). Post-menopausal women have increased levels of LDL-cholesterol and TC as compared with pre-menopausal women, all of these contribute to an atherogenic lipid profile( Reference Phan and Toth 1 , Reference Moorthy, Yadav and Mantha 2 ). Post-menopausal women are, therefore, at increased risk for CVD.
Oestrogen injection has been shown to decrease blood levels of LDL-cholesterol by reducing hepatic cholesterol synthesis via down-regulation of sterol regulatory element-binding protein-2 (SREBP-2) and 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA reductase)( Reference Moorthy, Yadav and Mantha 2 – Reference De Marinis, Martini and Trentalance 4 ). As a new drug target for lowering LDL-cholesterol, proprotein convertase subtilisin/kexin type 9 (PCSK9) is also decreased by oestrogen, suggesting that oestrogen prevents the degradation of the LDL receptor( Reference Persson, Galman and Angelin 5 , Reference Ghosh, Galman and Rudling 6 ). However, oestrogen has also been suggested to induce cholestasis by inhibiting biliary cholesterol secretion and bile acid synthesis via down-regulating expression of cholesterol 7α-hydroxylase (CYP7A1)( Reference Davis, Elliott and Lattier 7 , Reference Cuevas, Mauriz and Almar 8 ).
Unlike oestrogen, n-3 PUFA, EPA (20 : 5n-3) and DHA (22 : 6n-3) have been shown to decrease cholesterol by increasing hepatic bile acid synthesis by up-regulation of CYP7A1, sterol 12α-hydroxylase (CYP8B1) and sterol 27-hydroxylase (CYP27A1)( Reference Berard, Dumon and Darmon 9 , Reference Kamisako, Tanaka and Ikeda 10 ). In addition to increasing synthesis of bile acids, n-3 PUFA decrease blood levels of cholesterol by down-regulating SREBP-2 and HMG-CoA reductase and, thus, decreasing hepatic cholesterol synthesis( Reference Boschetti, Di Nunzio and Danesi 11 , Reference Ramaprasad, Srinivasan and Baskaran 12 ). However, the effect of n-3 PUFA on hepatic expression of PCSK9 has not been studied.
Bravo et al. ( Reference Bravo, Cantafora and Cicchini 13 ) reported that supplementation with n-3 PUFA reduced plasma levels of LDL-cholesterol in male rats injected with oestrogen by increasing the number of LDL receptors, but hepatic cholesterol synthesis was not studied. Although the effect of n-3 PUFA on cholesterol concentration is not entirely clear, a recent meta-analysis reported an increase in LDL-cholesterol by n-3 PUFA( Reference Wei and Jacobson 14 ). The LDL-cholesterol-increasing effects of n-3 PUFA have been shown in patients with hypertriacylglycerolaemia( Reference Mori, Burke and Puddey 15 ) and hamsters consuming high-fat diets( Reference Ishida, Ohta and Nakakuki 16 ) but not in healthy volunteers( Reference Grimsgaard, Bonaa and Hansen 17 ). Therefore, the purpose of the present study was to determine the effects of the combination of n-3 PUFA supplementation and oestrogen injection on hepatic cholesterol synthesis and breakdown, including the effects on PCSK9 expression, in ovariectomised rats (OVX) fed a low-fat diet.
Methods
Animals
A total of forty-eight female Wistar rats aged 3 weeks (Jungang Lab Animal Inc.) were used for the present study. All experimental protocols were adhered to the institutional guidelines for the care and management of laboratory animals, and approved by the Institutional Animal Care and Use Committee of Hanyang University (registration no. HY-IACUC-12-076).
Study design
Rats were individually housed under conditions of constant temperature (22 (sem 1)°C) and humidity (47 (sem 1) %) and a 12 h light–12 h dark cycle. Food pellets and fresh tap water were available ad libitum and food intake was measured daily. The body weight of the rats was determined weekly for 12 weeks.
After 1 week of acclimation, rats were randomly divided into three isoenergetic diet groups (n 16 each) and fed a modified American Institute of Nutrition (AIN) 93G diet for 12 weeks. Soyabean oil in the AIN-93G diet was substituted with same amount of grape seed oil (CJ CheilJedang) and/or fish oil (Cenovis Health Company) in order to achieve an EPA+DHA content of 0, 1 or 2 % of the total energy. Grape seed oil contained very low levels of α-linolenic acid (18 : 3n-3), EPA and DHA. Per kg of diet, there was 42·94 g total fat (10 % of energy) and 0, 1 or 2 % n-3 PUFA (EPA+DHA) equal to 0, 8·09 or 16·21 g of fish oil, respectively. The fatty acid composition of the diets is shown in Table 1.
Table 1 Fatty acid composition of the diets
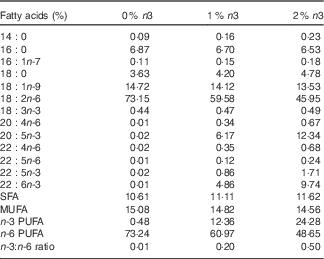
0, 1 and 2 % n3, % n-3 PUFA relative to the total energy intake in the diet.
At week 8, rats were surgically ovariectomised under anaesthesia using a combination of tiletamine/zolazepam (Zoletil, 25 ml/kg; Virbac S.A.) and xylazine (Rumpun, 10 ml/kg; Bayer Korea). Starting 1 week after ovariectomisation, rats (n 8/group) were randomly assigned to groups injected with either 17β-oestradiol-3-benzoate (E2) or the vehicle only (maize oil; Sigma-Aldrich) for the last 3 weeks of the study (week 10–12); the six groups were: 0 % n-3 PUFA diet with maize oil injection (0 % n3), 0 % n-3 PUFA diet with E2 injection (0 % n3+E2), 1 % n-3 PUFA diet with maize oil injection (1 % n3), 1 % n-3 PUFA diet with E2 injection (1 % n3+E2), 2 % n-3 PUFA diet with maize oil injection (2 % n3) and 2 % n-3 PUFA diet with E2 injection (2 % n3+E2). At 12 week of the experiment, rats were fasted overnight and euthanised with an intraperitoneal injection of Zoletil (25 ml/kg) and Rumpun (10 ml/kg). Blood was collected, and serum was obtained after centrifugation. Organs were collected, washed with saline solution and weighed. Serum and tissue samples were stored at –80°C until analysis.
Determination of serum and liver lipid
Hepatic lipid was extracted according to the method of Folch et al. ( Reference Folch, Lees and Sloane Stanley 18 ). Hepatic and serum concentrations of TC, TAG and serum HDL-cholesterol were measured using commercial kits (Asan Pharmaceutical) according to manufacturer’s instructions. The concentration of LDL-cholesterol was calculated using the formula of Friedewald et al. ( Reference Friedewald, Levy and Fredrickson 19 ).
GC analysis
Serum fatty acid composition was measured as previously reported( Reference Harris, von Schacky and Park 20 ). Briefly, 12·5 µl of serum was methylated with 500 µl of boron trifluoride methanol-benzene (Sigma-Aldrich) for 45 min at 100°C. Fatty acid methyl esters were extracted with 500 µl of hexane and analysed by GC (Shimadzu 2010) using a SP2560 capillary column (100 m×0·25 mm i.d., 0·2 µm film thickness, Supelco). Fatty acids were identified by comparing retention times with standards (GLC-727; Nu-Check Prep). Every batch was analysed with a quality control sample, and the CV was <5 %.
Western blot analysis
For analysis, extracts were prepared by homogenising the liver tissue in lysis buffer (0·25 m-sucrose, 20 mm-N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid, 2 mm-dithiothreitol, 1 mm-phenylmethanesulfonylfluoride, 0·5 mm-EDTA, 10 µg/ml aprotinin, 10 µg/ml leupeptin and 1 mm-Na3VO4, pH 7·5) plus a phosphatase inhibitor cocktail tablet (Roche Life Science). After centrifugation at 20 000 g for 1 h at 4°C (Eppendorf 5417R; Eppendorf), the supernatant was collected as a cytosolic fraction. To obtain the nuclear fraction, the pellets were re-suspended in lysis buffer with 1 % (v/v) Triton X-100, and centrifuged at 20 000 g for 30 min at 4°C. The supernatant was used for analysis of nuclear proteins. For protein quantification, the Bradford method was applied using bovine serum albumin (Bio-Rad) as a standard. Individual lysates (30–80 µg of protein) were separated on an 8 % SDS-PAGE and transferred to polyvinylidene fluoride membranes (0·45 µm, Merck Millipore, Billerica, MA, USA).
Antibodies used in this study included the following: AMP activated protein kinase (AMPK) and phosphorylated AMPK (p-AMPK) from Cell Signaling Technology; HMG-CoA reductase, CYP7A1 and CYP8B1 from Santa Cruz Biotechnology; and SREBP-2, PCSK9, CYP27A1, oestrogen receptor-α (ER-α) and oestrogen receptor-β (ER-β) from Abcam.
After blocking with 5 % skimmed milk or 5 % bovine serum albumin in tris-buffered saline with 0·1 % tween for 1 h at room temperature, membranes were incubated overnight at 4°C with the following primary antibodies: AMPK (1:500), p-AMPK (1:1000), HMG-CoA reductase (1:100), CYP7A1 (1:250), CYP8B1 (1:250), SREBP-2 (1:1000), PCSK9 (1:1000), CYP27A1 (1:1000), ER-α (1:500) and ER-β (1:500). Secondary antibody incubation was performed using horseradish peroxidase-conjugated anti-mouse IgG (1:5000; Enzo Life Science), anti-goat (1:10 000; Santa Cruz Biotechnology) or anti-rabbit (1:10 000; Cell Signaling Technology) for 1 h at room temperature. The immunoreactive proteins were developed using the enhanced chemiluminescence kit (GE Healthcare Life Sciences) and quantified using the Chemidoc MP Imaging System (Bio-Rad). The relative amounts of the proteins were calculated by normalising to the quantity of β-actin (1:1000; BD Transduction Laboratories).
Statistical analyses
All data are expressed as mean values with their standard errors. Statistical differences were calculated with a two-way ANOVA. All the statistical analyses were performed using SPSS for Windows, version 18.0 (SPSS Inc.). P values<0·05 were considered statistically significant.
Results
Food intake, body weight and organ weight
Supplementation with n-3 PUFA had no significant effect on dietary intake, body weight or organ weight (Table 2; see online Supplementary Table S1). E2 injection, however, significantly decreased dietary intake, final body weight and visceral fat weight, whereas it increased liver, uterus and kidney weight regardless of n-3 PUFA supplementation. E2 injection had no significant effect on the weight of the liver or kidneys.
Table 2 Dietary intake, body weight and organ weight of rats (Mean values with their standard errors)

0, 1 and 2 % n3, 0, 1 and 2 % n-3 PUFA diet with maize oil injection; 0, 1 and 2 % n3+17β-oestradiol-3-benzoated (E2), 0, 1 and 2 % n-3 PUFA diet with E2 injection.
* Values are significantly different between maize oil and (E2) injection for diets containing the same amount of n-3 PUFA (P<0·05).
Serum and hepatic lipid
Supplementation with n-3 PUFA significantly and dose-dependently decreased the serum levels of TC, LDL-cholesterol and TAG, and the hepatic levels of TC and TAG (Table 3; see online Supplementary Table S2). Similarly, E2 injection significantly decreased the serum levels of LDL-cholesterol and TAG and the hepatic levels of TC and TAG. However, serum levels of TC were not significantly affected by E2 injection. Serum levels of HDL-cholesterol were significantly increased by E2 injection but not by n-3 PUFA supplementation. There was a significant synergistic effect of n-3 PUFA supplementation and E2 injection on serum LDL-cholesterol and hepatic TC levels.
Table 3 Serum and hepatic lipid profile (Mean values with their standard errors)

0, 1 and 2 % n3, 0, 1 and 2 % n-3 PUFA diet with maize oil injection; 0, 1 and 2 % n3+17β-oestradiol-3-benzoated (E2), 0, 1 and 2 % n-3 PUFA diet with E2 injection; TC, total cholesterol.
* Values are significantly different between maize oil and E2 injection for diets containing the same amount of n-3 PUFA (P<0·05).
† Values are significantly different among 0, 1 and 2 % n3 within the maize oil and E2 injected groups (P<0·05).
‡ Values are significantly different between 1 and 2 % n3 within the maize oil and E2 injected groups (P<0·05).
Serum fatty acid composition
Supplementation with n-3 PUFA significantly increased serum levels of total n-3 PUFA, 20 : 5n-3, 22 : 5n-3 and 22 : 6n-3, while decreasing the serum levels of total n-6 PUFA 20 : 4n-6 in a dose-dependent manner (see online Supplementary Table S3). E2 injection decreased the serum levels of 14 : 0, 16 : 0, 16 : 1n-7 and 18 : 1n-9 but increased the concentration of 18 : 0. In addition, E2 injection significantly increased the serum levels of 22 : 5n-3 and 22 : 6n-3 in rats fed n-3 PUFA. There was a significant interaction between n-3 PUFA supplementation and E2 injection on the serum levels of 22 : 6n-3, total n-3 PUFA, 22 : 5n-6 and total n-6 PUFA.
Hepatic protein expression related with cholesterol metabolism
Supplementation with n-3 PUFA and E2 injection significantly decreased the expression of HMG-CoA reductase, SREBP-2 and PCSK9, whereas it increased the ratio of the expression of p-AMPK:AMPK (Fig. 1). Hepatic expressions of p-AMPK and AMPK were also increased both by n-3 PUFA supplementation and E2 injection (see online Supplementary Fig. S1).

Fig. 1 The effect of n-3 PUFA supplementation and 17β-oestradiol-3-benzoated (E2) injection on expression of (a) 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA reductase), (b) sterol regulatory element-binding protein-2 (SREBP-2), (c) phosphorylated AMP activated protein kinase (p-AMPK):AMP activated protein kinase (AMPK) ratio and (d) proprotein convertase subtilisin/kexin type 9 (PCSK9) in the liver of ovariectomised rats. Values are means (n 8 rats/group), with their standard errors represented by vertical bars. * Mean values are significantly different between maize oil and E2 injection for diets containing the same amount of n-3 PUFA (P<0·05). † Mean values are significantly different among 0, 1 and 2 % n3 within the maize oil and E2 injected groups. 0, 1 and 2 % n3, 0, 1 and 2 % n-3 PUFA diets with maize oil injection; 0, 1 and 2 % n3+E2, 0, 1 and 2 % n-3 PUFA diets with E2 injection (P<0·05).
Supplementation with n-3 PUFA significantly increased the expression of CYP7A1, CYP8B1 and CYP27A1 (Fig. 2). However, E2 injection significantly decreased the expression of CYP8B1 but not of CYP27A1 (Fig. 2). In addition, E2 injection significantly increased the hepatic expression of ER-α and ER-β, but n-3 PUFA did not (Fig. 3).

Fig. 2 The effect of n-3 PUFA supplementation and 17β-oestradiol-3-benzoated (E2) injection on the expression of (a) cholesterol 7 α-hydroxylase (CYP7A1), (b) sterol 12 α-hydroxylase (CYP8B1) and (c) sterol 27-hydroxylase (CYP27A1) in the liver of ovariectomised rats. Values are means (n 8 rats/group), with their standard errors represented by vertical bars. * Mean values are significantly different between maize oil and E2 injection for diets containing the same amount of n-3 PUFA (P<0·05). † Mean values are significantly different among 0, 1 and 2 % n3 within the maize oil and E2 injected groups. 0, 1 and 2 % n3, 0, 1 and 2 % n-3 PUFA diets with maize oil injection; 0, 1 and 2 % n3+E2, 0, 1 and 2 % n-3 PUFA diets with E2 injection (P<0·05).

Fig. 3 The effect of n-3 PUFA supplementation and 17β-oestradiol-3-benzoated (E2) injection on expression of (a) oestrogen receptor-α (ER-α) and (b) oestrogen receptor-β (ER-β) in the liver of ovariectomised rats. Values are means (n 8 rats/group), with their standard errors represented by vertical bars. * Mean values are significantly different between maize oil and E2 injection for diets containing the same amount of n-3 PUFA (P<0·05). 0, 1 and 2 % n3, 0, 1 and 2 % n-3 PUFA diets with maize oil injection; 0, 1 and 2 % n3+E2, 0, 1 and 2 % n-3 PUFA diets with E2 injection (P<0·05).
Discussion
This was the first report to suggest that E2 injection and n-3 PUFA supplementation have a synergic hypocholesterolaemic effect through inhibited hepatic cholesterol synthesis and increased breakdown of hepatic cholesterol in OVX. Consistent with our results, Bravo et al. ( Reference Bravo, Cantafora and Cicchini 13 ) reported that E2 injection and n-3 PUFA supplementation decreased LDL-cholesterol by increasing the amount of LDL receptors in male rats. The LDL receptor binds with PCSK9, which enhances LDL receptor degradation and results in increased plasma levels of LDL-cholesterol( Reference Zaid, Roubtsova and Essalmani 21 , Reference Rashid, Curtis and Garuti 22 ). The present study showed that E2 injection and n-3 PUFA supplementation synergistically reduced hepatic expression of PCSK9, which inhibited LDL receptor degradation and decreased LDL-cholesterol. Previously, E2 injection decreased PCSK9 expression in male rats( Reference Persson, Galman and Angelin 5 ), but the effect of n-3 PUFA had not been investigated.
PCSK9 has been shown to be regulated by SREBP-2, which communicates with hepatic proteins involved in cholesterol synthesis such as p-AMPK, AMPK and HMG-CoA reductase( Reference Horton, Cohen and Hobbs 23 ). Previous preclinical studies have suggested that E2 injection( Reference De Marinis, Martini and Trentalance 4 , Reference Trapani, Violo and Pallottini 24 ) or n-3 PUFA supplementation( Reference Boschetti, Di Nunzio and Danesi 11 , Reference Suchankova, Tekle and Saha 25 ) reduce cholesterol synthesis by increasing AMPK phosphorylation, reducing expression of HMG-CoA reductase and reducing expression of SREBP-2. Consistently, the present study shows that the reduced hepatic cholesterol synthesis was due to an up-regulation of p-AMPK and AMPK, an increase in the p-AMPK:AMPK ratio, and a down-regulation of HMG-CoA reductase and SREBP-2. However, reports on the effects of n-3 PUFA on LDL-cholesterol have been contradictory; some have proposed that rapid TAG clearance by lipoprotein lipase can promote the conversion of VLDL to LDL and thus increase circulating LDL-cholesterol( Reference Park and Harris 26 ). The LDL-cholesterol-increasing effects of n-3 PUFA have been reported in hypertriacylglycerolaemia( Reference Mori, Burke and Puddey 15 ), but not in normotriacylglycerolaemia( Reference Grimsgaard, Bonaa and Hansen 17 ), as in the present study.
Another mechanism that has been shown to reduce blood levels of cholesterol is increased hepatic cholesterol breakdown. Bile acids promote hepatic cholesterol removal, and thus an increase in bile acid synthesis results in a reduction of blood cholesterol( Reference Kamisako, Tanaka and Ikeda 10 ). Previous studies reported that n-3 PUFA supplementation increased hepatic expression of CYP7A1, CYP8B1 and CYP27A1 ( Reference Berard, Dumon and Darmon 9 , Reference Kamisako, Tanaka and Ikeda 10 ) in mice, suggesting enhanced bile acid synthesis and reduced hepatic levels of cholesterol. On the other hand, oestrogen has been shown to decrease hepatic synthesis of bile acids through a reduction of CYP7A1 and CYP8B1 but not CYP27A1, in rats and mice( Reference Cuevas, Mauriz and Almar 8 , Reference Yamamoto, Moore and Hess 27 ). Similarly, the present study showed that E2 injection suppressed hepatic expression of CYP7A1 and CYP8B1 but not CYP27A1. The effect of oestrogen could be related to the expression of endoplasmic reticulum-localised enzymes such as CYP7A1 but not the mitochondria-localised enzyme CYP27A1 ( Reference Koopen, Post and Wolters 28 ). CYP7A1 regulates CYP8B1, which mediates bile acid synthesis through cholic acid synthesis( Reference Chiang 29 ), and the suppression of CYP8B1 (e.g. by E2) causes a reduction of CYP7A1 expression. Although E2 injection did not increase the hepatic synthesis of bile acids in this study, it suppressed hepatic cholesterol synthesis that, in turn, decreased hepatic and blood levels of cholesterol. Previous studies have suggested that oestrogen induces cholestasis through reducing hepatic CYP7A1 and biliary cholesterol secretion( Reference Cuevas, Mauriz and Almar 8 , Reference Yamamoto, Moore and Hess 27 ). Therefore, it is worth noting that the combination of n-3 PUFA and E2 in the present study could have decreased blood and hepatic levels of cholesterol by decreasing hepatic cholesterol synthesis and increasing hepatic synthesis of bile acids.
In the liver, E2 binds to oestrogen receptors (ER), specifically ER-α and ER-β subtypes, to modulate cholesterol metabolism. The binding of E2 to ER results in the recruitment of co-activators and the displacement of co-repressors at DNA binding sites and thereby the modulation of gene and protein expression( Reference Moolman 30 ) including LDL receptors in liver. Thus, oestrogen can up-regulate the expression of the LDL receptor through binding to ER and increasing the clearance of blood LDL-cholesterol( Reference Parini, Angelin and Rudling 31 ). In the present study, E2 injection increased the hepatic expression of ER-α and ER-β, but n-3 PUFA did not. This result is consistent with a previous study in which oestrogen increased the amount of hepatic ER in OVX rats( Reference Sahlin, Elger and Hedden 32 ). However, the effect of n-3 PUFA on ER has not yet been reported.
In the present study, E2 injection decreased serum levels of 14 : 0, 16 : 0, 16 : 1n-7 and 18 : 1n-9 but increased 18 : 0. The elongase 6 enzyme elongates 16 : 0 to 18 : 0; this was demonstrated in elongase 6 knockout mice who exhibited greater concentrations of 16 : 0 but less 18 : 0 compared with wild-type mice( Reference Matsuzaka, Shimano and Yahagi 33 ). In addition, elongase 6 expression was higher in females than males and was increased in oestrogen-treated HepG2 cells( Reference Marks, Kitson and Stark 34 ). Consistently, the present study showed that E2 injection increased 18 : 0 concentrations through activation of elongase 6. Additionally, oestrogen has been shown to inhibit stearoyl-CoA desaturase-1( Reference Paquette, Wang and Jankowski 35 ), the rate-limiting enzyme in the synthesis of MUFA. In the present study, concentrations of MUFA 16 : 1n-7 and 18 : 1n-9 were depressed in rats injected with E2. Furthermore, E2 injection and n-3 PUFA supplementation increased serum levels of 22 : 5n-3 and 22 : 6n-3 synergistically. E2 injection increased serum levels of 22 : 5n-6 in the 0 % n3 diet, as observed previously in plasma in rats( Reference Kitson, Marks and Shaw 36 ). Previous studies reported that dietary n-3 PUFA( Reference Nakanishi, Iitsuka and Tsukamoto 37 , Reference Mohamed, Hussein and Bhathena 38 ) and oestrogen( Reference Kitson, Marks and Shaw 36 ) increased blood levels of n-3 PUFA and decreased levels of n-6 PUFA. Oestrogen increases Δ6-desaturase, which converts 18 : 3n-3 to 20 : 5n-3 and 22 : 6n-3( Reference Kitson, Marks and Shaw 36 ).
This study had a few limitations. Although rats are the most commonly used model for lipoprotein research, lipoprotein metabolism differs between rats and human. Differences are the efficient mechanisms for clearance of remnant-removal pathways and absence of cholesteryl ester transfer reaction( Reference Oschry and Eisenberg 39 ). There was no sham surgery group in the present study, but previous studies compared cholesterol metabolism between OVX and sham rats( Reference Nigro, Santos and Barthem 3 , Reference van Lenten, Melchior and Roheim 40 ). In addition, the present study did not measure bile acid concentrations of faecal or biliary secretion.
In conclusion, n-3 PUFA supplementation and E2 injection significantly reduced hepatic and blood levels of cholesterol by inhibiting hepatic cholesterol synthesis through increasing AMPK phosphorylation, decreasing PCSK9, SREBP-2 and HMG-CoA reductase expression, and enhancing hepatic cholesterol breakdown through CYP7A1, CYP8B1 and CYP27A1. Further research is needed to determine the effects of n-3 PUFA supplementation and hormone replacement therapy on cholesterol metabolism in post-menopausal women.
Acknowledgements
The present study was supported by a Korean Research Foundation grant funded by the Korean Government (grant no. NRF-2012R1A1A2040553).
The authors’ contributions are as follows: Y. O. carried out the experimental work and prepared the manuscript; Y. J. conducted laboratory and statistical analysis; Y. P. edited manuscript and has primary responsibility for the final content. All authors read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114515003517








