Chronic obstructive pulmonary disease (COPD), the third leading cause of mortality in the USA( Reference Minino, Murphy and Xu 1 ) and among the top ten leading causes of total years of life lost in the world( 2 ), is characterised by progressive airway obstruction. Pulmonary function tests (PFT), as performed by spirometry, are used to quantify pulmonary function parameters including forced expiratory volume in the 1st second (FEV1) and forced vital capacity (FVC). Pulmonary function increases throughout childhood, plateaus in the 20s, and thereafter adults experience an age-related decline( Reference Weiss 3 ). The majority of COPD cases (85 %) are related to smoking( Reference Forey, Thornton and Lee 4 ), which alters the trajectory in pulmonary function, by hindering growth, reducing peak function and accelerating age-related decline( Reference Guerra, Stern and Zhou 5 ).
Vitamin D is proposed to have protective effects in the lungs via gene regulation( Reference Fletcher, Basdeo and Allen 6 ). In vitro studies found that 1,25-dihydroxyvitamin D (1,25-(OH)2D), the active vitamin D metabolite, induced antimicrobial peptides for host defence in the lung and modulated airway remodelling( Reference Litonjua 7 ). In humans, 25-hydroxyvitamin D (25(OH)D) is the major vitamin D metabolite in serum, most of which forms a complex with vitamin D binding protein (DBP) (approximately 85–90 % is DBP-bound)( Reference Bikle, Gee and Halloran 8 ), and then is metabolised to 1,25-(OH)2D, the active steroid hormone form( Reference Bikle, Gee and Halloran 8 , Reference Nykjaer, Dragun and Walther 9 ). Total 25(OH)D is the commonly used biomarker of vitamin D status, and it is preferred to other vitamin D metabolites, such as non-DBP-bound 25(OH)D and 1,25-(OH)2D, given that it is a comprehensive indicator for vitamin D stores, has a longer half-life (approximately 3 weeks) and is less affected by Ca( Reference Zerwekh 10 , Reference Nielson, Jones and Chun 11 ). On average, African ancestry (AA) populations have lower serum 25(OH)D concentrations, due to multiple factors including genetics and skin pigmentation( Reference Litonjua 7 ), but evidence exists that AA populations have higher 1,25-(OH)2D levels and greater bone mineral density compared with European ancestry (EA) populations( Reference Freedman and Register 12 ).
Previous observational cross-sectional studies of the vitamin D– pulmonary function association in the general population reported mixed findings. Most of these studies reported a positive association between 25(OH)D and pulmonary function( Reference Black and Scragg 13 – Reference Tolppanen, Williams and Henderson 19 ), although some reported a null or inverse association( Reference Shaheen, Jameson and Robinson 20 – Reference Tolppanen, Sayers and Granell 22 ), and two others reported a positive association under certain conditions, such as only in male current smokers( Reference Lange, Sparrow and Vokonas 23 ) or only in overweight and obese males( Reference Khan, Mai and Chen 24 ). The largest previous cross-sectional study, which included two Danish cohorts (total 18 507), reported positive associations of 25(OH)D with pulmonary function( Reference Afzal, Lange and Bojesen 16 ). Only one prior cross-sectional study investigated serum 25(OH)D and pulmonary function in an ancestry group other than European, and it confirmed similar positive associations in the 3957 AA participants studied( Reference Black and Scragg 13 ).
The current study investigated the hypothesis that serum 25(OH)D level is positively associated with pulmonary function. We leveraged the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium to include population-based data on serum 25(OH)D and pulmonary function in a harmonised analysis. In addition, we compared the association of serum 25(OH)D and pulmonary function across EA and AA groups and investigated effect modification by cigarette smoking.
Methods
Cohorts and participants
Nine prospective cohorts in the CHARGE Consortium were included (Table 1). All cohorts had EA participants, and five of the cohorts had AA participants. Only one cohort (Multi-Ethnic Study of Atherosclerosis (MESA)) has participants with other ancestries, and these other ancestries were not included in this study. Among the nine cohorts, the Framingham Heart Study (FHS) had two sub-cohorts analysed separately: the Offspring and the Third-Generation (Gen3) cohorts. Our analysis pipeline harmonised the outcome and exposure definitions, the units on all variables and the statistical modelling. The same exclusion criteria were applied to each cohort: missing PFT, unacceptable PFT using the American Thoracic Society and European Respiratory Society criteria for acceptability, missing serum 25(OH)D, serum 25(OH)D >374·4 nmol/l (or 150 ng/ml, leading to removal of a single outlier)( Reference O’Neal 25 ) or missing on other covariates (online Supplementary Table S1).
Table 1 Cross-sectional participant characteristics of each cohort in the Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium (n 27 128)Footnote *(Mean values and standard deviations)
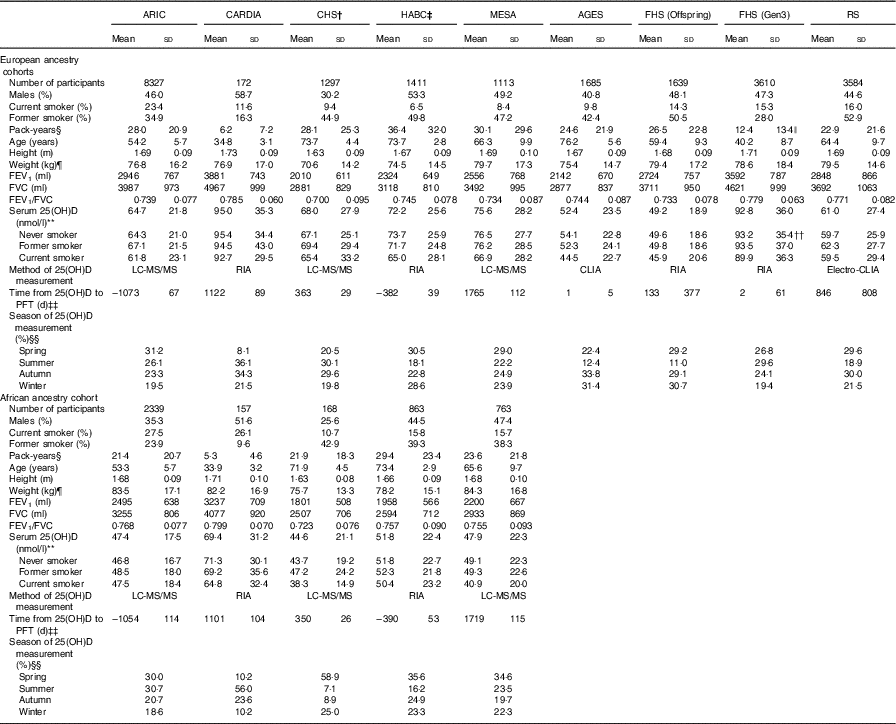
ARIC, Atherosclerosis Risk in Communities Study; CARDIA, Coronary Artery Risk Development in Young Adults Study; CHS, Cardiovascular Health Study; HABC, Health, Aging, and Body Composition Study; MESA, Multi-Ethnic Study of Atherosclerosis; AGES, Age, Gene, Environment, Susceptibility Study − Reykjavik, Iceland; FHS (Offspring), Framingham Heart Study − Offspring Cohort; FHS (Gen3), Framingham Heart Study − Generation 3 Cohort; RS, Rotterdam Study (Netherlands); FEV1, forced expiratory volume in the 1st second; FVC, forced vital capacity; 25(OH)D, 25-hydroxyvitamin D; LC-MS/MS, liquid chromatography in tandem with MS; CLIA, chemiluminescence immunoassay; RIA, radioimmunoassay.
*AGES, RS and FHS only have participants of European ancestry; n 22 838 for EA, n 4290 for AA, total n 27 128.
† The number of participants used to compute descriptive statistics in CHS excluded those who had residual outliers based on the preliminary models (n 8 for EA and n 6 for AA); whereas other cohorts used the number of participants before applying residual exclusion for the descriptive statistics.
‡ Numbers vary slightly for different outcomes in HABC (for the FVC outcome, n 1385 for EA and n 821 for AA; for the ratio outcome, n 1382 for EAs and n 817 for AAs). The numbers of participants for the FEV1 outcome are used. However, the descriptive statistics is similar across different outcomes.
§ Pack-years is calculated only among current and former smokers in each cohort.
|| We used 1554 ever smokers here, instead of a total of 1561 ever smokers in the Gen3 cohort, because the pack-years of seven ever smokers were so small that they were coded as 0. Therefore, these seven ever smokers do not contribute to the pack-years descriptive statistics here.
¶ The number of participants who have weight data is slightly different from the total number of participants in each cohort. However, the descriptive statistics of weight stays similar.
** Mean (sd) of serum 25(OH)D level for all the participants in each cohort, and mean (sd) of 25(OH)D level in participants with each smoking status are shown here, stratified by ancestry.
†† We used 2046 never smokers, rather than a total of 2049 never smokers in the Gen3 cohort, to compute the 25(OH)D level in never smokers.
‡‡ The time difference is the interval between the time when pulmonary function was measured and the time when serum vitamin D was measured. The difference is positive, if the serum vitamin D was measured before the pulmonary function test; whereas the value is negative, if the serum vitamin D was measured after the pulmonary function test.
§§ The proportion of participants in each season when their serum was measured was rounded (thus rounding errors mean sums may not be exactly 100 %).
Outcome and exposure assessment
Pre-bronchodilator pulmonary function outcomes (FEV1, FVC and FEV1/FVC), which have similar accuracy as post-bronchodilator measures for long-term outcomes( Reference Mannino, Diaz-Guzman and Buist 26 ), were measured in each cohort using standardised methods defined by the American Thoracic Society/European Respiratory Society criteria (online Supplementary Table S2). The methods used to measure 25(OH)D varied by cohort (online Supplementary Table S2). Three cohorts, including MESA, the Atherosclerosis Risk In Communities (ARIC) study, and the Cardiovascular Health Study (CHS), used the current reference method, liquid chromatography in tandem with mass spectrometry (LC-MS/MS); three cohorts, including FHS, the Coronary Artery Risk Development in Young Adults (CARDIA) study, and the Health, Aging, and Body Composition (HABC) study, used radioimmunoassay (RIA); one cohort, the Age, Gene, Environment, Susceptibility Study − Reykjavik, Iceland (AGES), used chemiluminescence immunoassay (CLIA); and one cohort (the Rotterdam Study (RS)) used electro-CLIA. Only MESA calibrated the serum 25(OH)D measurement against the standard reference material 972( Reference Phinney 27 ), which reflects the calendar time of the measurements in the cohorts, most of which occurred before the availability of the standard reference material (online Supplementary Table S3). Measurements of the outcome and exposure variables were planned for either the full cohort (ARIC, CHS, FHS, HABC and RS) or a subset of the cohort if the outcome or the exposure was only measured in an ancillary study (AGES, CARDIA and MESA)( Reference Lederer, Enright and Kawut 28 – Reference Runarsdottir, Gudmundsson and Aspelund 31 ) (online Supplementary Table S1). Continuous variables were used for serum 25(OH)D and pulmonary function to capture the association of 25(OH)D on PFT across the broad distribution of ranges in the cohorts.
As shown in Table 1, among nine cohorts, four (AGES, CHS, FHS-Offspring and FHS-Gen3) had a mean time difference of <1 year in the PFT measurements and the preceding 25(OH)D measurement, and the greatest mean time difference between 25(OH)D and PFT measurement was <5 years (MESA). Participants in ARIC and HABC had blood drawn for serum 25(OH)D after their PFT measure, but within 3 years.
Other covariates, including smoking status, pack-years (number of packs of cigarettes smoked per d times the number of years smoked), height, weight and age, were measured concurrently with pulmonary function, except for CHS, which assessed covariates concurrent with the serum 25(OH)D measure, but within 1 year of the PFT measurement (online Supplementary Table S3). All data collection and analysis was approved by the Institutional Review Board at each cohort’s respective institution. Spirometry measures are available on the database of Genotypes and Phenotypes via accession numbers as follows: ARIC (phs000280), CARDIA (phs000285), CHS (phs000287), FHS (phs000007) and MESA (phs000209). Serum vitamin D measures are also available at the same accession numbers for CHS, FHS and MESA.
Statistical analysis in individual cohorts
All analyses were first conducted independently in each cohort, stratified by ancestry, given the lower mean serum 25(OH)D level in AA participants( Reference Litonjua 7 ). For FEV1 and FEV1/FVC, models were adjusted for smoking status, pack-years, height, height squared, age, age squared, sex, season of blood draw and study centre (if applicable); for FVC, the model was further adjusted for weight. Residual outliers, identified using the studentised residuals of the linear models (online Supplementary methods for more details), were excluded from all models (about 0·3 % of the total sample size). The model was extended to test the interaction between 25(OH)D and smoking status (never (reference group), former and current smokers).
Meta-analysis
We tested the association of serum 25(OH)D on each PFT outcome among individuals in each ancestry group and each cohort, separately, and then combined the effect estimates (also referred to as two-stage meta-analysis( Reference Morris, Fisher and Kenward 32 )), using inverse variance weighting and assuming fixed-effects, with heterogeneity assessed via the I 2 statistic( Reference Higgins, Thompson and Deeks 33 ). Random-effects meta-analysis was performed as a sensitivity analysis if there was potential heterogeneity (I 2>30 %). The comparison of meta-analysed coefficients of the 25(OH)D–PFT associations for the two ancestry groups was conducted using a Z test( Reference Borenstein 34 ). Meta-analysis of the interaction terms of 25(OH)D with smoking status was also performed (online Supplementary methods for more details, online Supplementary Tables S4 and S5 for cohort-specific findings and online Supplementary Table S6 for meta-analysed results).
Meta-regression was conducted to explore the potential causes of moderate heterogeneity in the meta-analysis of 25(OH)D on FEV1 and FVC in the EA cohorts. Modifiers were tested individually in the meta-regression models to investigate heterogeneity; modifiers included factors that could vary between cohorts, such as proportion of ever, current and former smokers, mean 25(OH)D level, assay method for serum 25(OH)D, time between 25(OH)D and PFT measures, and mean age of participants in each cohort. The two-sided type I error was examined at 0·05 for all analyses. Meta-analysis and meta-regression were conducted using the metafor package (version 1.9-8) in R (version 3.2.3.; R Foundation for Statistical Computing).
Regression coefficients (β) with their standard errors calculated within each cohort per 1 nmol/l 25(OH)D are presented in the figures. In addition, to put the magnitude of the 25(OH)D–PFT associations in terms relevant to public health, the meta-analysed regression coefficients were multiplied by 10 nmol/l 25(OH)D, which is about half of the standard deviation of the 25(OH)D distribution.
Results
We studied 22 838 EA and 4290 AA participants. EA participants had higher FEV1, FVC and serum 25(OH)D than AA participants in each cohort, whereas FEV1/FVC was similar across ancestry groups (Table 1 and online Supplementary Fig. S1). CARDIA and FHS-Gen3 were younger than the seven other cohorts, with consequently lower pack-years smoked in ever smokers. Across all cohorts, among EA participants, 17 % were current smokers and 40 % were former smokers; among AA participants, 22 % were current smokers and 30 % were former smokers. The mean serum 25(OH)D level was highest among never smokers (70 (sd 30) nmol/l), followed by former smokers (67 (sd 29) nmol/l) and current smokers (64 (29) nmol/l) in EAs, whereas the trend was less obvious in AA (49 (sd 21) nmol/l in current smokers, 50 (sd 21) nmol/l in former smokers and 48 (sd 21) nmol/l in never smokers). The mean of serum 25(OH)D for EA participants across nine cohorts was 68 (sd 29) nmol/l and for AA participants across five cohorts the mean was 49 (sd 21) nmol/l.
Fixed-effects meta-analysis (Fig. 1) revealed a consistently positive association of serum 25(OH)D with the PFT outcomes, FEV1 and FVC, in both ancestry groups. To put these findings into context, a 10 nmol/l (approximately 0·5 sd) higher 25(OH)D was associated with 11·1 ml higher FEV1 in EA (P<0·0001) and 17·9 ml higher FEV1 in AA (P<0·0001). Similarly, for a 10nmol/l higher 25(OH)D, FVC was higher by 12·9 ml in EA (P<0·0001) and by 15·4 ml in AA (P=0·0001). The magnitudes of the 25(OH)D–PFT associations did not differ significantly between the two ancestry groups (P=0·06 and P=0·56 for FEV1 and FVC, respectively). The association of serum 25(OH)D with FEV1/FVC reached statistical significance only in EA (P=0·0013), and the magnitude was negligible; a 10 nmol/l higher 25(OH)D was associated with a ratio being lower by 0·055 % (online Supplementary Table S7 and Supplementary Fig. S2 for ancestry- and cohort-specific findings).
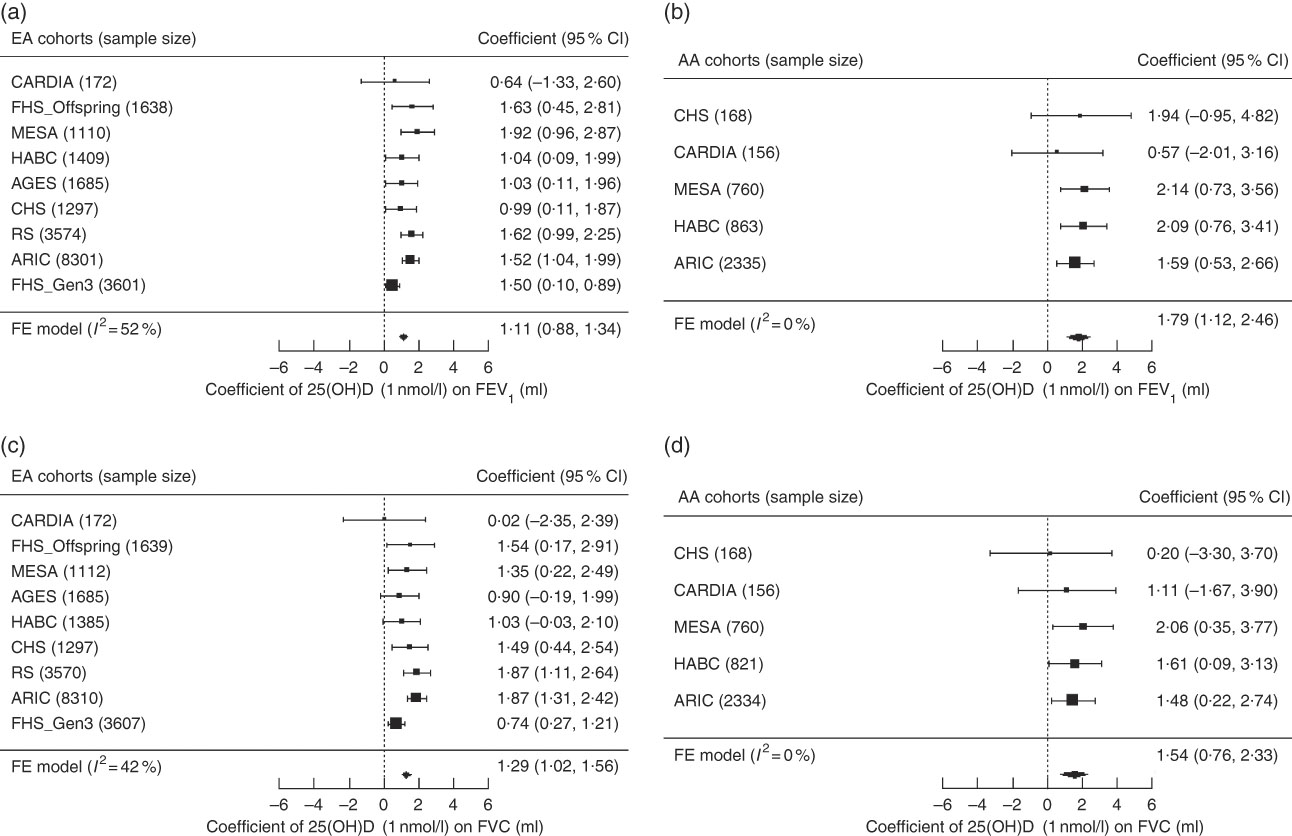
Fig. 1 Forest plots of the meta-analysis of serum 25-hydroxyvitamin D (25(OH)D) on forced expiratory volume in the 1st second (FEV1) and forced vital capacity (FVC) across cohorts in the Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium, stratified by participant ancestry. Associations are presented for serum 25(OH)D on (a) FEV1 in European ancestry cohorts (n 22 787). (b) FEV1 in African ancestry cohorts (n 4282). (c) FVC in European ancestry cohorts (n 22 777). (d) FVC in African ancestry cohorts (n 4239). β (unit: ml) denotes the coefficient from the fixed-effects meta-analysis for serum 25(OH)D on the pulmonary function outcome per 1 nmol/l increment of 25(OH)D, with its 95 % CI. Cohorts findings were ordered from the least to the most precise, and heterogeneity is presented (I 2). EA, European ancestry; AA, African ancestry; CARDIA, Coronary Artery Risk Development in Young Adults Study; FHS (Offspring), Framingham Heart Study – Offspring Cohort; AGES, Age, Gene, Environment, Susceptibility Study – Reykjavik, Iceland; CHS, Cardiovascular Health Study; RS, Rotterdam Study (Netherlands); ARIC, Atherosclerosis Risk in Communities Study; FHS (Gen3), Framingham Heart Study – Generation 3 Cohort; FE, fixed-effects; HABC, Health, Aging, and Body Composition Study; MESA, Multi-Ethnic Study of Atherosclerosis.
In the main-effect meta-analysis of serum 25(OH)D on pulmonary function, EA cohorts had low to moderate heterogeneity, whereas AA cohorts had low heterogeneity (Fig. 1, online Supplementary Fig. S2). We did a sensitivity analysis using random-effects meta-analysis among EA cohorts for the FEV1 and FVC outcomes, and no substantial change was found in the meta-analysed effect estimates and corresponding se (coefficient of 1 nmol/l 25(OH)D on the FEV1 outcome was 1·11 (se 0·12) ml in the fixed-effects model and 1·21 (se 0·19) ml in the random-effects model; coefficient on the FVC outcome was 1·29 (se 0·14) ml in the fixed-effects model and 1·31 (se 0·20) ml in the random-effects model). Meta-regression was also performed in the EA cohorts and we found that among these cohorts, cohorts with lower mean 25(OH)D concentration had stronger 25(OH)D–PFT associations (Fig. 2). The proportion of ever smokers and of former smokers had significant linear associations with the 25(OH)D–PFT coefficients (online Supplementary Fig. S3), and these two variables were both highly correlated with mean 25(OH)D levels (Pearson’s r<−0·75 for all pairwise correlations). The 25(OH)D–PFT association in EA cohorts varied by 25(OH)D assay method (meta-regression P=0·0059); the association was attenuated in cohorts using RIA compared with cohorts using liquid chromatography in tandem with MS (pairwise P<0·005, online Supplementary Fig. S4). Mean age of each cohort was a significant positive modifier of the 25(OH)D–FEV1 association, while time difference between 25(OH)D and spirometry measures did not affect the 25(OH)D–PFT association (online Supplementary Fig. S3).
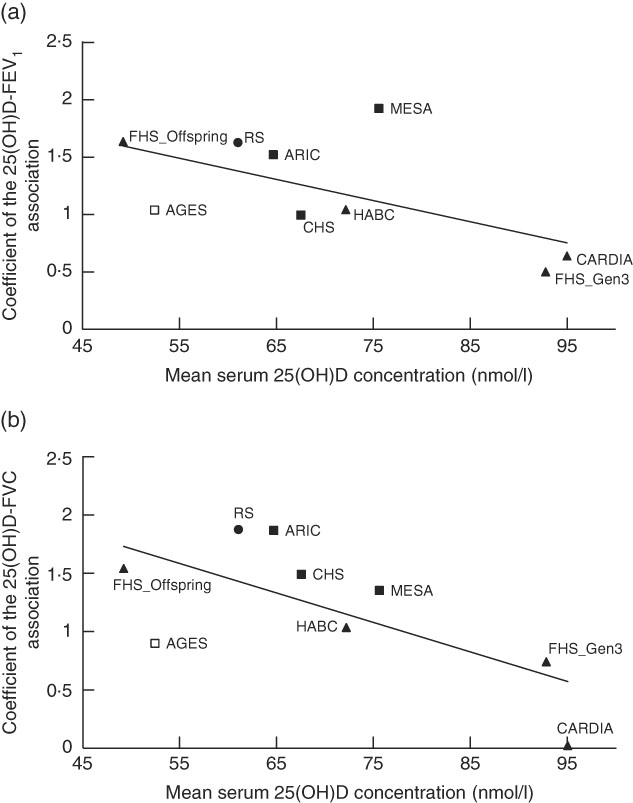
Fig. 2 Meta-regression of mean serum 25-hydroxyvitamin D (25(OH)D) levels against the association estimates of 25(OH)D with pulmonary function test in nine European ancestry cohorts in the Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium. (a) Forced expiratory volume in the 1st second (FEV1) outcome (coefficient unit: ml per 1 nmol/l 25(OH)D), and (b) forced vital capacity (FVC) outcome (coefficient unit: ml per 1 nmol/l 25(OH)D). The modifier is mean serum 25(OH)D level of each nine cohorts. A linear regression line is present for each sub-figure, with a meta-regression P value of 0·0006 for the FEV1 outcome, and 0·005 for the FVC outcome. The figure also shows the measurement method for the serum 25(OH)D assay (legend shows symbols for each of the four assay methods). ![]() , LC-MS/MS;
, LC-MS/MS; ![]() , RIA;
, RIA; ![]() , CLIA;
, CLIA; ![]() , Electro-CLIA. LC-MS/MS, liquid chromatography in tandem with mass spectrometry; RIA, radioimmunoassay; CLIA, chemiluminescence immunoassay; MESA, Multi-Ethnic Study of Atherosclerosis; FHS (Offspring), Framingham Heart Study – Offspring Cohort; RS, Rotterdam Study (Netherlands); ARIC, Atherosclerosis Risk in Communities Study; AGES, Age, Gene, Environment, Susceptibility Study – Reykjavik, Iceland; CHS, Cardiovascular Health Study; HABC, Health, Aging, and Body Composition Study; CARDIA, Coronary Artery Risk Development in Young Adults Study; FHS (Gen3), Framingham Heart Study – Generation 3 Cohort.
, Electro-CLIA. LC-MS/MS, liquid chromatography in tandem with mass spectrometry; RIA, radioimmunoassay; CLIA, chemiluminescence immunoassay; MESA, Multi-Ethnic Study of Atherosclerosis; FHS (Offspring), Framingham Heart Study – Offspring Cohort; RS, Rotterdam Study (Netherlands); ARIC, Atherosclerosis Risk in Communities Study; AGES, Age, Gene, Environment, Susceptibility Study – Reykjavik, Iceland; CHS, Cardiovascular Health Study; HABC, Health, Aging, and Body Composition Study; CARDIA, Coronary Artery Risk Development in Young Adults Study; FHS (Gen3), Framingham Heart Study – Generation 3 Cohort.
To examine the potential impact of family relatedness between the FHS-Gen3 and the FHS-Offspring cohorts on the meta-analysis, sensitivity analysis confirmed that the findings were unchanged when either cohort was excluded (results not shown). In addition, the meta-analysis findings were not sensitive to exclusion of residual outliers.
In the EA cohorts, 25(OH)D had a greater positive association with FVC in current smokers than in never smokers (β current×25(OH)D= 7·5 ml for 10 nmol/l increment of 25(OH)D, P=0·047). Similarly, 25(OH)D had a greater positive association with FVC in former smokers than in never smokers (β former×25(OH)D=7·9 ml for 10 nmol/l increment of 25(OH)D, P=0·0065) (Fig. 3). For the FEV1 outcome in the EA cohorts, the interaction coefficients for 25(OH)D and smoking status had the same positive direction as the coefficients for FVC, but were not statistically significant for either current (P=0·14) or former smokers (P=0·14). No statistical evidence of interaction of 25(OH)D and cigarette smoking was found in the AA cohorts for either outcome. To put the interaction finding into context, a 10 nmol/L higher serum 25(OH)D was associated with a 17·3ml higher FVC in current smokers and a 16·6ml higher FVC in former smokers, which was more than double the association magnitude in never smokers (β=7·8 ml). A similar trend was found for the FEV1 outcome in the EA cohorts. For 10 nmol/l higher serum 25(OH)D, FEV1 was higher by 14·0 ml in current smokers, 12·0 ml in former smokers and 8·0 ml in never smokers (Fig. 4).
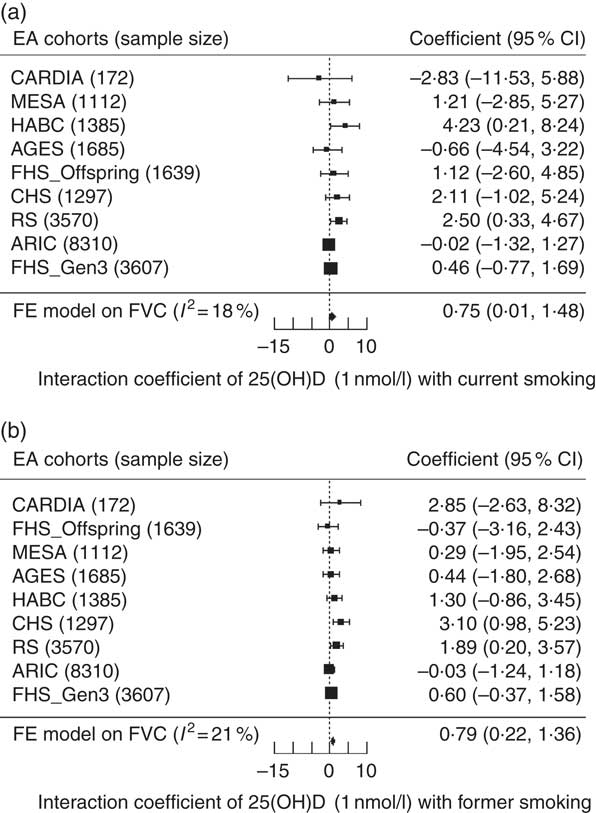
Fig. 3 Forest plots of the interaction meta-analysis of serum 25-hydroxyvitamin D (25(OH)D) and smoking status on forced vital capacity (FVC) in the European ancestry cohorts in the Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium (n 22 777). (a) Current smokers and (b) former smokers. β (unit: ml) denotes the interaction term coefficient of 25(OH)D and smoking status on FVC from the fixed effects meta-analysis, per 1 nmol/l increment of 25(OH)D, with its 95 % CI. Cohorts were ordered from the least to the most precise, and heterogeneity is presented (I 2). EA, European ancestry; CARDIA, Coronary Artery Risk Development in Young Adults Study; MESA, Multi-Ethnic Study of Atherosclerosis; HABC, Health, Aging, and Body Composition Study; AGES, Age, Gene, Environment, Susceptibility Study – Reykjavik, Iceland; FHS (Offspring), Framingham Heart Study – Offspring Cohort; CHS, Cardiovascular Health Study; RS, Rotterdam Study (Netherlands); ARIC, Atherosclerosis Risk in Communities Study; FHS (Gen3), Framingham Heart Study—Generation 3 Cohort; FE, fixed-effects.
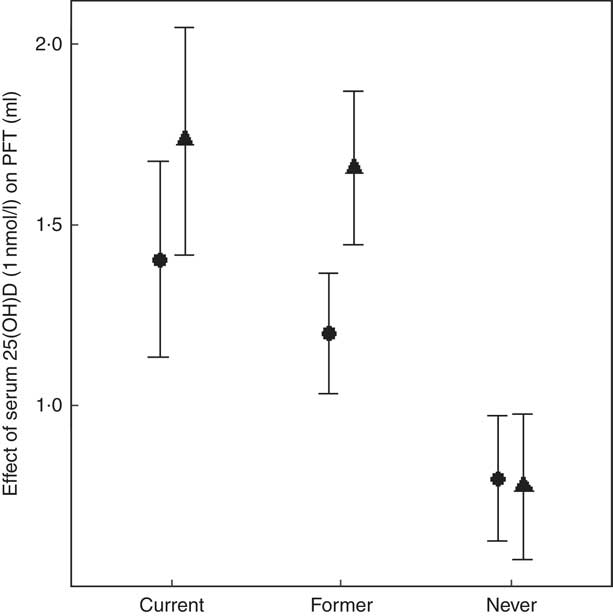
Fig. 4 Meta-analysis of the association of serum 25-hydroxyvitamin D (25(OH)D) – pulmonary function test outcomes among current, former and never smokers in the European ancestry cohorts in the Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium. Forced expiratory volume in the 1st second (FEV1, ![]() ) and forced vital capacity (FVC,
) and forced vital capacity (FVC, ![]() ) are presented for each smoking status. β (unit: ml) denotes that 1 nmol/l higher serum 25(OH)D was associated with a β mL higher FEV1 (or FVC), calculated from an analysis including the interaction of serum 25(OH)D and smoking status. The error bar represents ±1 standard error. We used 22 786 European ancestry (EA) participants for the FEV1 outcome and 22 777 EA participants for the FVC outcome.
) are presented for each smoking status. β (unit: ml) denotes that 1 nmol/l higher serum 25(OH)D was associated with a β mL higher FEV1 (or FVC), calculated from an analysis including the interaction of serum 25(OH)D and smoking status. The error bar represents ±1 standard error. We used 22 786 European ancestry (EA) participants for the FEV1 outcome and 22 777 EA participants for the FVC outcome.
Discussion
This study investigated the association of serum 25(OH)D with pulmonary function using multiple cohorts of different ancestries. We found a consistently positive association of serum 25(OH)D with FEV1 and FVC across both EA and AA groups. In addition, in the EA group, a significantly stronger association was observed for current and former smokers, compared with never smokers.
A previous cross-sectional study in a EA population (two Copenhagen cohorts: n 10 116 and n 8391, respectively) similarly reported positive associations of 25(OH)D with FEV1 percentage predicted and FVC percentage predicted, but not with FEV1/FVC( Reference Afzal, Lange and Bojesen 16 ). The magnitude of the association was about four times greater in the Copenhagen study, which may be due to the difference in the mean serum 25(OH)D (Danish median approximately 42 nmol/l v. CHARGE median of approximately 65 nmol/l) given our finding that the 25(OH)D–PFT association was stronger in cohorts with lower serum 25(OH)D. Our finding for the serum 25(OH)D–FEV1 association was similar in magnitude to the association reported in a British cohort of 6789 participants with an average age of 45 years( Reference Berry, Hesketh and Power 17 ), but weaker than a previous report from the FHS cohort( Reference Hansen, Gao and Dupuis 15 ). Given that the rate of decline in FEV1 at age 45 years is increased by approximately 15 ml/year in current smokers( Reference Mirabelli, Preisser and Loehr 35 ), we estimate that a 10 nmol/l higher 25(OH)D is similar to approximately 1 year of current smoking-related decline in FEV1 for both ancestries, but in the opposite direction. Several putative biological mechanisms may support a causal association between low 25(OH)D levels and worse pulmonary function. First, lung tissue cells can locally convert 25(OH)D to 1,25-(OH)2D( Reference Hansdottir, Monick and Hinde 36 ), the active form of vitamin D, which could improve the immune and anti-inflammatory responses in lungs via gene regulation( Reference Hansdottir, Monick and Hinde 36 – Reference Dimeloe, Richards and Urry 38 ). If there is not enough circulating 25(OH)D, it is likely that the resolution of inflammation in lungs would be slower, which could have a negative impact on pulmonary function. In addition, 1,25-(OH)2D in lungs, converted locally from 25(OH)D, can regulate the extracellular matrix homeostasis via the ERp60-mediated pathway( Reference Boyan, Wong and Fang 39 ), and this is important for maintenance of lung structure. Furthermore, low vitamin D status could decrease circulating Ca status, which in turn can adversely affect thoracic skeleton mobility and respiratory muscle performance( Reference Herr, Greulich and Koczulla 40 , Reference Schlaich, Minne and Bruckner 41 ).
Our findings show that the association of serum 25(OH)D with FEV1 and FVC were stronger in magnitude in AA v. EA participants, although the difference by race did not reach statistical significance. The finding may reflect the lower serum 25(OH)D in AA participants, which is consistent with the meta-regression finding and with a previous study reporting attenuated associations at higher serum 25(OH)D( Reference Hansen, Gao and Dupuis 15 ). Future studies that investigate genetic variation in EA and AA in the context of serum 25(OH)D may help explain the differences.
In EA participants, the positive interaction terms between serum 25(OH)D and smoking status supported a stronger magnitude of association of serum 25(OH)D with FVC in current and former smokers than in never smokers, with a consistent, but not statistically significant, difference for FEV1. The interaction finding is consistent with a prior cross-sectional National Health and Nutrition Examination Survey (NHANES) study, which reported a stronger 25(OH)D–FEV1 association in current and former smokers than in never smokers that was near statistical significance (P=0·06)( Reference Black and Scragg 13 ). Given smokers have a higher level of oxidative stress and lower pulmonary function than never smokers, partly due to chronic inflammation in lung tissue, the stronger protective association of 25(OH)D on pulmonary function in smokers suggests a benefit for smokers. To explore this interaction, estimates of the 25(OH)D–PFT association were computed within each smoking category. In EA participants, the 25(OH)D–FEV1 (or FVC) associations were statistically significant in all strata. Generally, in ever smokers of EA, the coefficients for 25(OH)D were greater for FVC than for FEV1.
Meta-regression provided additional evidence for effect modification by smoking. The proportion of ever smokers was a significant modifier of the association of serum 25(OH)D with FEV1 and FVC. The higher the proportion of ever smokers, the greater the 25(OH)D–PFT association. More specifically, the proportion of former smokers explained the heterogeneity in the 25(OH)D–PFT association across cohorts more fully than the proportion of current smokers; this may be explained by a survival bias in older participants who were current smokers. The meta-regression, based on mean age of the cohorts, showed that cohorts with a higher mean age had a greater association magnitude of 25(OH)D with FEV1. Given that meta-regression analysis uses cohort-level factors (e.g. mean age rather than age of each individual), ecological bias is possible( Reference Thompson and Higgins 42 ). Nevertheless, the age-related meta-regression finding was consistent with a prior NHANES study that showed the association of 25(OH)D and FEV1 was stronger in people over age 60 compared with younger individuals( Reference Black and Scragg 13 ).
Several methodological considerations should be taken into account in interpreting the findings of this study. First, the meta-regression showed stronger 25(OH)D–PFT associations in cohorts with lower mean serum 25(OH)D, indicating a non-linear 25(OH)D–PFT association. This finding is consistent with a prior study in the FHS cohort, which reported a non-linear association and a stronger 25(OH)D–FEV1 association in participants at risk of vitamin D deficiency (<30 nmol/l)( Reference Hansen, Gao and Dupuis 15 ). Second, serum 25(OH)D was measured by four different methods across the cohorts. For example, two cohorts with high mean 25(OH)D (>90 nmol/l) used RIA methods. These same cohorts had a lower magnitude estimate of the 25(OH)D–PFT association; if the higher mean represents the ‘truth’ (and is not caused by measurement error in the RIA assay), then the lower 25(OH)D–PFT association may be primarily driven by the vitamin D distribution and not by the RIA method. Whether the assay method itself directly influences the estimate of the 25(OH)D–PFT association requires further data. Third, in this cross-sectional meta-analysis, minor differences were found in the time separation between the measurement of serum 25(OH)D and pulmonary function, but the meta-regression test for heterogeneity confirmed that time separation between measurements did not affect the 25(OH)D–PFT associations. Indeed, past studies with longitudinal measurements of serum 25(OH)D reported a high correlation of 25(OH)D measurements over a long period of time, with a correlation coefficient of 0·7 for measurements separated by 1 year, 0·5 for measurements separated by 5 years( Reference Hofmann, Yu and Horst 43 ), and 0·42–0·52 for measurements separated by 14 years( Reference Jorde, Sneve and Hutchinson 44 ), which supports the use of a single 25(OH)D measurement to represent usual level. Fourth, residual confounding was unlikely given the consistent results across multiple cohorts in various settings. Weight was adjusted for the FVC outcome, given that higher weight and adiposity negatively affects lung volume (i.e. FVC)( Reference Amara, Koval and Paterson 45 ); weight was not adjusted in the FEV1 models, given FEV1 is a measure of airways obstruction and not physical restriction of lung volume. Physical activity was not adjusted because it is not a confounder in estimating the serum 25(OH)D–PFT association; while physical activity is known to contribute to O2 utilisation in lungs( Reference Burri, Gehr and Muller 46 ), little evidence and no biological rationale exists for a causal association of physical activity with either FEV1 or FVC( Reference Cheng, Macera and Addy 47 ), which are markers for airways obstruction and lung volume, respectively. Finally, even though three cohorts (AGES, CARDIA, MESA) had the outcome or the exposure only measured in an ancillary study (random subset of the entire cohort), we do not expect selection bias to affect the estimate of the serum vitamin D–PFT association in this meta-analysis; indeed, the association magnitude and direction was consistent across all cohorts, regardless of the proportion of the original cohort contributing to the analysis. Thus, selection bias is expected to be negligible and would likely lead to an underestimated association, given the participants retained in the cohorts are expected to be, on average, healthier than those who were lost to follow-up.
This study meta-analysed the serum 25(OH)D–PFT association across nine cohorts, according to a common pipeline that harmonised the variables and statistical analysis. The sample size comprised 17 569 EA participants from the USA; 5269 EA participants from Iceland and the Netherlands; and 4290 AA participants from the USA, all of whom were 19–95 years old. The sample provided excellent representation of the US population, based on comparisons of demographic factors including sex, height, weight, smoking status and COPD prevalence (about 6·1 %) to national surveys( Reference Jamal, Homa and O’Connor 48 – Reference Ward, Nugent and Blumberg 50 ), which strengthens the external validity of the study’s findings.
In summary, using meta-analysis, we estimated a positive association of serum 25(OH)D with the pulmonary function parameters FEV1 and FVC in both EA and AA participants. Associations varied by smoking status in the EA group, with stronger serum 25(OH)D–PFT associations seen in current and former smokers. The observational design means we cannot infer a causal association, and future studies, such as randomised controlled trials or Mendelian randomisation studies, are needed to further investigate the causality of 25(OH)D on pulmonary function.
Acknowledgements
The authors thank Professor James Booth in the Department of Biological Statistics and Computational Biology at Cornell University for providing advice on meta-analysis. The authors thank the staff (Lynn Johnson and Francoise Vermeylen) in Cornell Statistical Consulting Unit for their role in providing advice on methods. The authors also thank Hanfei Xu, PhD student in the research group of Dr Josée Dupuis for his assistance on residual model testing in the Framingham Heart Study.
This work was supported by National Institutes of Health (NIH) grant no. R21 HL125574 funded by the National Heart, Lung, and Blood Institute (NHLBI) and the NIH Office of Dietary Supplements (ODS) (multiple principal investigators (MPIs): D. B. H. and P. A. C.). The corresponding author (P. A. C.) had full access to the data for the meta-analysis, and had final responsibility for the decision to submit for publication. No funding source had any role in the analysis of the data, the writing of the manuscript or the decision to submit it. This work was also supported in part by R01HL077612 (PI: R. G. B.) and by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (ZO1 ES043012, PI: S. J. L.). S. J. L. is supported by the Intramural Research Program of NIH, National Institute of Environmental Health Sciences. Infrastructure for the CHARGE Consortium is supported in part by the NHLBI grant R01HL105756. The Age, Gene, Environment, Susceptibility (AGES)–Reykjavik Study has been funded by NIH contracts N01-AG-1-2100 and 271201200022C, the National Institute on Aging (NIA) Intramural Research Program, Hjartavernd (the Icelandic Heart Association) and the Althingi (the Icelandic Parliament). The study is approved by the Icelandic National Bioethics Committee, VSN: 00-063. The researchers are indebted to the participants for their willingness to participate in the study. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by NHLBI contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C and HHSN268201100012C. 25(OH)D measurements were conducted with the support of R01 HL103706 from the NHLBI and R01 HL103706-S1 from the NIH ODS. The authors thank the staff and participants of the ARIC study for their important contributions. This Cardiovascular Health Study (CHS) research was supported by NHLBI contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086 and NHLBI grants U01HL080295, R01HL085251, R01HL087652, R01HL105756, R01HL103612, R01HL120393 and R01HL130114 with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided through R01AG023629 from NIA. A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Vitamin D measurements were made possible by NHLBI (R01HL084443-01A2). This work in Framingham Heart Study was supported by NHLBI’s Framingham Heart Study contract (N01-HC-25195 and HHSN268201500001I). Vitamin D measurements in the Framingham study were made possible by NIA (R01 AG14759 to S. L. B.). The Health Aging and Body Composition cohort study was supported by NIA contracts N01AG62101, N01AG2103 and N01AG62106, NIA grant R01-AG028050, National Institute of Nursing Research grant R01-NR012459, and in part by the Intramural Research Program of the NIA, NIH. This research was further supported by RC1AG035835 and the serum vitamin D assays were supported by R01AG029364. The Multi-Ethnic Study of Atherosclerosis (MESA) study is conducted and supported by NHLBI in collaboration with MESA investigators. Support for MESA is provided by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from NHLBI, UL1-TR-000040, UL1-TR-001079 and UL1-TR-001881 from the National Center for Research Resources and DK063491 from the National Institute of Diabetes and Digestive and Kidney Diseases. The MESA Lung study was supported by grants R01 HL077612, RC1 HL100543 and R01 HL093081 from NHLBI. Support for the Mineral Metabolite dataset was provided by grant HL096875. The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, Rotterdam, the Netherlands; the Organization for the Health Research and Development (ZonMw); the Research Institute for Diseases in the Elderly; the Dutch Ministry of Education, Culture, and Science; the Dutch Ministry for Health, Welfare, and Sports; the European Commission (DG XII), and the Municipality of Rotterdam. L. L. was a postdoctoral fellow of the Research Foundation—Flanders (FWO) in Brussels, Belgium. Part of this work was supported by a FWO-grant G035014N. DSM Nutritional Products AG, Kaiseraugst, Switzerland, sponsored the Vitamin D serum analyses. The authors are grateful to the study participants, the staff from the Rotterdam Study, and the participating general practitioners and pharmacists. The Coronary Artery Risk Development in Young Adults Study (CARDIA) is supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C and HHSN268200900041C from the NHLBI, the Intramural Research Program of the NIA and an intra-agency agreement between NIA and NHLBI (AG0005).
P. A. C., D. B. H. and J. X. conceived and designed the study. R. G. B., J. L., J. D., S. A. G., L. L., S. J. L., K. E. N., A. V. S., B. M. P. and L. M. S. provided the data and supervised the data analysis in each cohort. J. X., T. M. B., R. R. R., A. V. S., A. W. M., F. S., N. T. and X. Z. analysed data within each cohort. J. X., P. A. C. and D. B. H. meta-analysed and interpreted the data, co-wrote and edited the first draft of the manuscript and had primary responsibility for final content. All authors provided data, analytic support and/or study design suggestions at all stages, critically reviewed the manuscript and read and approved the final version.
Dr B. M. P. serves on the DSMB of a clinical trial funded by the manufacturer (Zoll LifeCor) and on the Steering Committee of the Yale Open Data Access Project funded by Johnson & Johnson. All other authors have no conflicts of interest. There is no commercial support or financial interest from the tobacco industry for the research presented.
The study sponsors were not involved in study design, data collection, data analysis, data interpretation, report writing or decisions to submit the paper for publication. P. A. C. and D. B. H. had final responsibility for the decision to submit for publication.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518002180








