INTRODUCTION
Salmonellosis is one of the most common gastrointestinal infections and a major public health burden in the United Kingdom and elsewhere in the world. In 2006 about 60% of all confirmed clinical cases of salmonellosis in England and Wales were due to Salmonella Enteritidis (SE), followed by S. Typhimurium (ST) which was responsible for about 12% of cases [1]. The predominance of SE and ST in the United Kingdom is in line with most European countries [2]. The main source of infection with SE in humans in Europe is thought to be contaminated eggs produced by infected laying hens (Gallus gallus) [Reference Gillespie3, Reference De Jong and Ekdahl4].
Throughout the European Union (EU) there has been a coordinated effort to reduce the incidence of human salmonellosis by reducing the prevalence of infection in sites of primary production of poultry. Commission Regulation (EC) No. 2160/2003 requires member states to put in place control plans so that targets for the reduction of the prevalence of Salmonella at farm level can be achieved [5]. A later Regulation (2004/665/EC) required member states to carry out national prevalence surveys of Salmonella in holdings of commercial laying hens using standardized methodology. Results from these surveys indicated that SE (followed at a distance by ST) was the most common serovar in laying flocks throughout the EU [6]. Because SE and ST are also the two most prevalent serovars in humans throughout the EU, it was decided that national control plans for laying flocks should include these two serovars only. Field experiments have shown that SE and ST have an affinity for the laying-hen reproductive tract [Reference Okamura7, Reference Gantois8], and both serovars are known to survive well in albumin [Reference Guan, Grenier and Brooks9]. However, other Salmonella serovars present in the environment of the chicken house also have the capacity to penetrate to the interior of the egg and grow during storage [Reference Schoeni10]. However, in the field a greater tendency for SE to persist in laying houses over subsequent flock cycles has been reported [Reference Wales11, Reference Carrique-Mas, Breslin and Snow12].
It has long been suspected that rodents play an important role in the persistence of SE in laying houses, with high isolation rates from their faeces and an association with infected flocks [Reference Henzler and Opitz13–Reference Henzler and Opitz17]. The high susceptibility of rodents to SE is reflected in: (1) the low infective dose required [Reference Krabisch and Dorn18]; (2) the high prevalence of infection in SE-positive houses [Reference Henzler and Opitz13, Reference Davies and Wray15, Reference Henzler and Opitz17]; and (3) the high prevalence of systemic infection in SE-infected mice [Reference Davies and Wray15]. Mouse faeces have been shown in some studies to contain a large number of SE organisms [Reference Henzler and Opitz13, Reference Davies and Wray15]. Field investigations of houses contaminated with SE have shown that mice are typically infected in a larger proportion than most other environmental samples [Reference Davies and Wray16, Reference Wales, Breslin and Davies19]. In spite of this evidence, it is unclear whether rodents are actively involved in the transmission of SE to birds, or whether they just reflect infection in the flock. The relationship between persistence/elimination of rodent populations in laying houses and clearance of infection from flocks was investigated in the present study.
Since 1998 research at the Veterinary Laboratories Agency (VLA) has been focused on the longitudinal observation of infected laying flocks in order to study the epidemiology of Salmonella infection and advise farmers on control measures. In this study we used survival analysis methodology on the cohort of layer holdings followed up in order to establish the time to clearance of infection. The aim was to investigate whether serovar, type of production (i.e. cage, non-cage), manure removal system, level of rodent infestation and number of birds in the flock/holding had any influence on clearance. These visits were not strictly observational, since the farmer generally received advice on measures to promote the clearance of infection, although this advice was frequently not taken. A second factor investigated in this study was whether clearance of infection from flocks occurs typically as a result of cleaning and disinfection (C&D), or during production.
MATERIALS AND METHODS
Farms, houses, flocks and incidents
An ‘incident’ was defined as an instance in which a particular Salmonella serovar/phage type was detected in a house, and this triggered subsequent sampling visits to the same laying house. For SE incidents, the phage type was ignored, since conversion of some phage types into other closely related types can occur [Reference Brown20, Reference Rankin and Platt21]. Incidents were identified as a result of: (1) routine producer monitoring; (2) microbiological investigations following clinical symptoms of the flock; (3) trace-back investigations following a human outbreak; (4) sampling of the flock during the EU baseline survey. Additional positive houses were also recruited after testing all laying houses on positive sites, or other farms under the same ownership.
Sampling method
Houses were visited by a VLA sampling team. From each occupied house 20 (10 faecal/litter and 10 dust) samples were collected from representative locations. Faecal/litter samples consisted of 25 g of naturally pooled material collected from different locations depending on the design of the house including end of scrapers, belts and from the deep pits (step-cage houses), and from slats and scratching areas in non-cage houses. Dust samples consisted of 15 g material from the floor and egg belt spillage trays (cage houses) and ledges and beams (non-cage houses) [Reference Carrique-Mas, Breslin and Snow12, Reference Wales, Breslin and Davies19]. Each sample was collected using a hand-held gauze swab (Readiwipes; Robinson Healthcare, Worksop, UK) impregnated with buffered peptone water (BPW; Merck, Poole, UK) and placed directly into 225 ml BPW.
Laboratory method
The Salmonella culture method consisted of pre-enrichment of the sample in BPW (37°C, 18 h), followed by selective enrichment in modified semi-solid Rappaport–Vassiliadis medium (MSRV; Difco, Oxford, UK: 1868–17) (41·5°C, 24/48 h) followed by plating onto Rambach agar (Merck, Hull, UK: 1·07500) [Reference Carrique-Mas, Breslin and Snow12]. The method described is a simplified version of the ISO 6579:2002 (Annex D) Salmonella isolation method in which only one plating medium (Rambach) is used, rather than the two prescribed by ISO 6579:2002 (Annex D). Internal validation of the methodology indicated no further advantage in using two plating media (data not shown).
Suspect Salmonella colonies were confirmed by serotyping using the Kauffmann–White typing scheme [Reference Popoff22]. Selected ST isolates from each house were phage-typed using the Colindale typing scheme.
Statistical analysis
The data were analysed using statistical survival methods [Reference Collett23]. In this study ‘survival’ refers to the time during which there is persistence of infection, and ‘failure’ refers to the clearance of infection from a house known to be contaminated. For each incident an observation period was defined. The ‘time zero’ was taken to be the first date of sampling a house with a positive outcome. The end of the incident was the last sampling visit in which the house tested positive. All incidents were left-censored, since infection was already present in the flocks at the start of the study. Some incidents were also ‘lost to follow-up’, when houses tested positive at the end of the study period, and thus the end of the incident was unknown. In houses that became clear, due to the variable sampling intervals, the precise date of clearance (end of incident) could not be established, and an intermediate point in time between the last positive test and the first clear test was taken as the end of the incident.
In a few houses more than one incident was defined, when a flock tested positive after there had been at least one negative flock in between. On a few occasions (<5%) where flocks were tested at least three times at least one of the intermediate sampling occasions resulted in a negative result. On these few occasions results were treated as positive.
The data were first displayed graphically ignoring the hierarchical structure using Kaplan–Meier plots, which estimate the survivor function [Reference Dohoo, Martyn and Stryn24]. However, due to the multilevel nested structure of the data (i.e. incidents within laying houses within farms), survival analysis using mixed models was carried out [Reference Goldstein25]. Because the outcome was time, an accelerated (log-duration) model was developed. This type of model estimates the log survival time and allows the estimation of effects of any explanatory variables.
The log survival time for the ith incident from the jth laying house and the kth farm [log(T ijk)] can be modelled as:
where we assume u jk~N(0, δu2) and wk~N(0, δw2) as the random effects for house and farm respectively and where X represents the vector of values for any set of covariates, β corresponds to the coefficients in the model, and t 0 is an event time sampled from the baseline distribution corresponding to values of zero for the covariates. The estimation method employed was quasi-likelihood under iterative generalized least squares (IGLS).
The variables investigated with this model were: (1) the system of manure removal, defined by type of house as house with a scraper, manure belt, step-cage (‘A-frame’) and non-cage (i.e. barn or free range); (2) Salmonella serovar; (3) the natural log of the number of birds in the flock; (4) the year of recruitment; and (5) the level of rodent infestation at each visit (scored as ‘0’, ‘1’, ‘2’, or ‘3’). This was assessed by the frequency of sightings of rodent populations by the farmer/sampler and the presence of signs of mice and rats on inspection of the house. Signs of rodent activity included rodent droppings, urine pillars, grease marks, tracks, structural damage, and uptake of bait/trapping results. The scoring was: ‘0’ (very infrequent or no sightings of rodents by the farmer, and very few visible signs of rodent activity); ‘1’ (few rodent signs); ‘2’ (moderate level of rodent signs); and ‘3’ (high level of rodent signs). Results from this model were used to estimate the mean survival time for each type of incident. Rodent scores were compared in different types of house using non-parametric Wilcoxon rank sum tests.
For the descriptive statistical analysis S-Plus 6.2 (Insightful Corp., Seattle, WA, USA) was used. The analytical survival analysis was carried out using MLWin 2.01 (Institute of Education, London, UK).
Estimation of clearance of SE from flocks during lay
In order to estimate clearance during lay we considered the data from positive flocks that were tested more than once during lay, and that were followed by a subsequent flock that tested negative. Clearance during lay was assumed when at least one of the later flock tests was negative. Houses were assumed to have become clear after terminal (C&D) when such flocks tested positive at all occasions during lay.
RESULTS
Farms, houses, flocks and incidents
A total of 264 incidents were recorded during the period 21 July 1998 to 16 August 2007. These incidents occurred in 152 laying houses in 42 farms. The median number of laying houses in each farm was five [interquartile range (IQR) 2–7]. These consisted of 54 cage-scraper, 44 cage-belt, 24 free-range, 17 step-cage and 13 barn houses. The average capacity of the houses (in thousands of birds) was 30·0 (s.d.±29·3), 12·5 (s.d.±7·4), and 3·9 (s.d.±3·7) for cage, barn and free-range houses, respectively.
The numbers of flocks sampled per house ranged from 1 to 6 (Table 1). On average flocks and houses were sampled 1·6 times (median 1, IQR 1–3) and 2·6 times, respectively.
Table 1. Frequency distribution of visits to laying houses and flocks

SE was responsible for the largest number of incidents (141). Although it represented over half of all incidents, SE had been isolated at some point from over 80% of the houses and farms investigated (Table 2). A total of 101 houses (66%) had one incident only, 24 had two incidents (16%) and the remainder more than two.
Table 2. Serovar distribution among incidents, houses and farms

* Other serovars involved in less than six incidents include: S. Cubana, S. Rissen, S. Anatum, S. Corvallis, S. Virchow, S. Ohio, S. Indiana, S. Derby, S. Yoruba, S. Newport, S. Ouakam, S. Senftenbeg, S. Binza, S. Ajibo, S. Braenderup, S. Havana, S. Jedburgh, S. Lexington, S. Meleagridis, S. Muenster, S. Schwarzengrund, S. Tennessee, S. Wagenia, S. Worthington, S. Kentucky.
Kaplan–Meier plots (univariable)
Kaplan–Meier plots for SE, ST, and ‘other’ serovars are shown in Figure 1. Clearance of incidents involving SE occurred much later than those involving ST and ‘other’ serovars. Separate Kaplan–Meier plots for SE stratified by type of house suggested that clearance occurred earlier in non-cage compared with cage houses (Fig. 2).
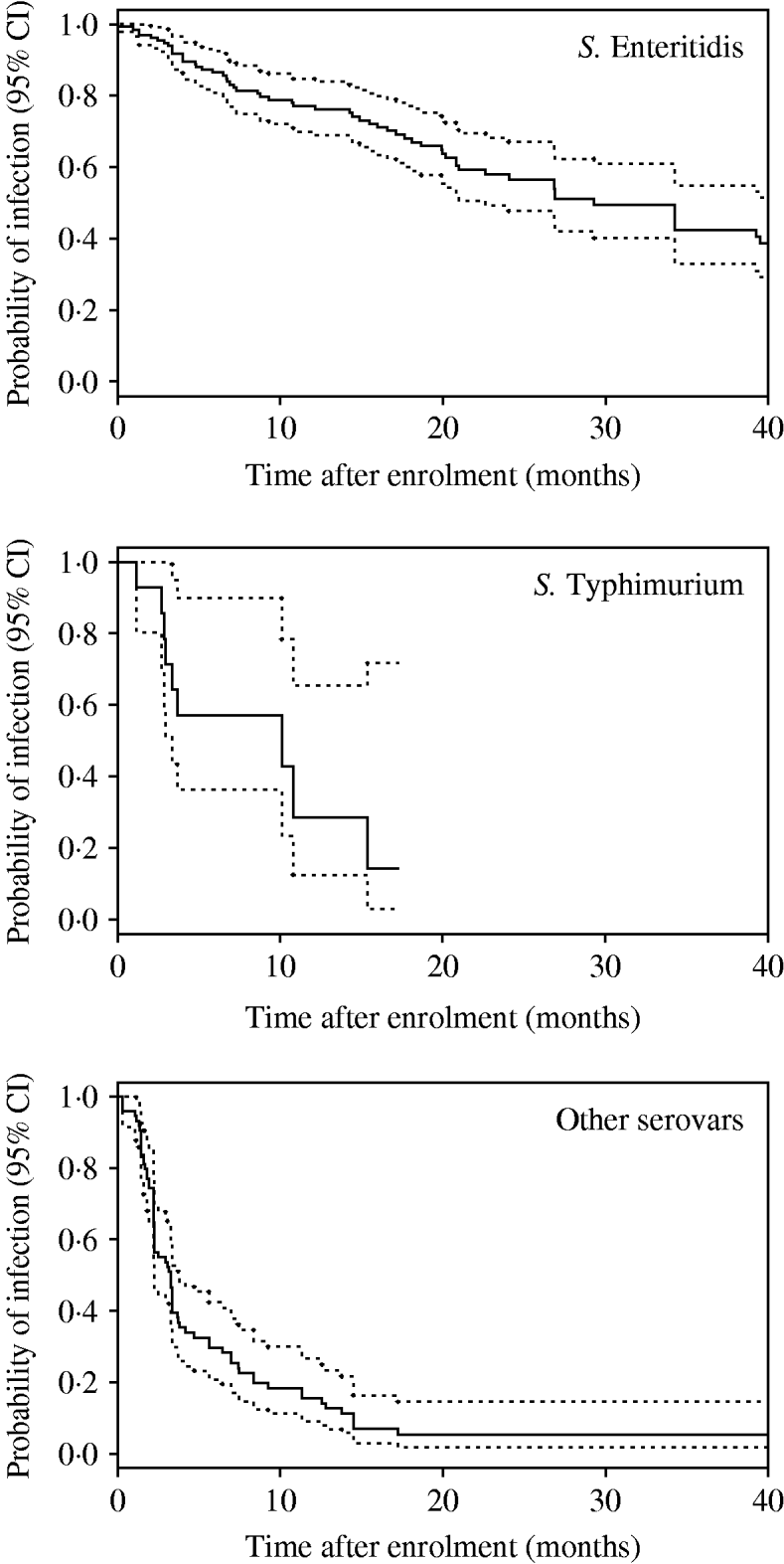
Fig. 1. Kaplan–Meier plots by Salmonella serovar (all types of house). Dotted lines indicate 95% confidence intervals (CI).
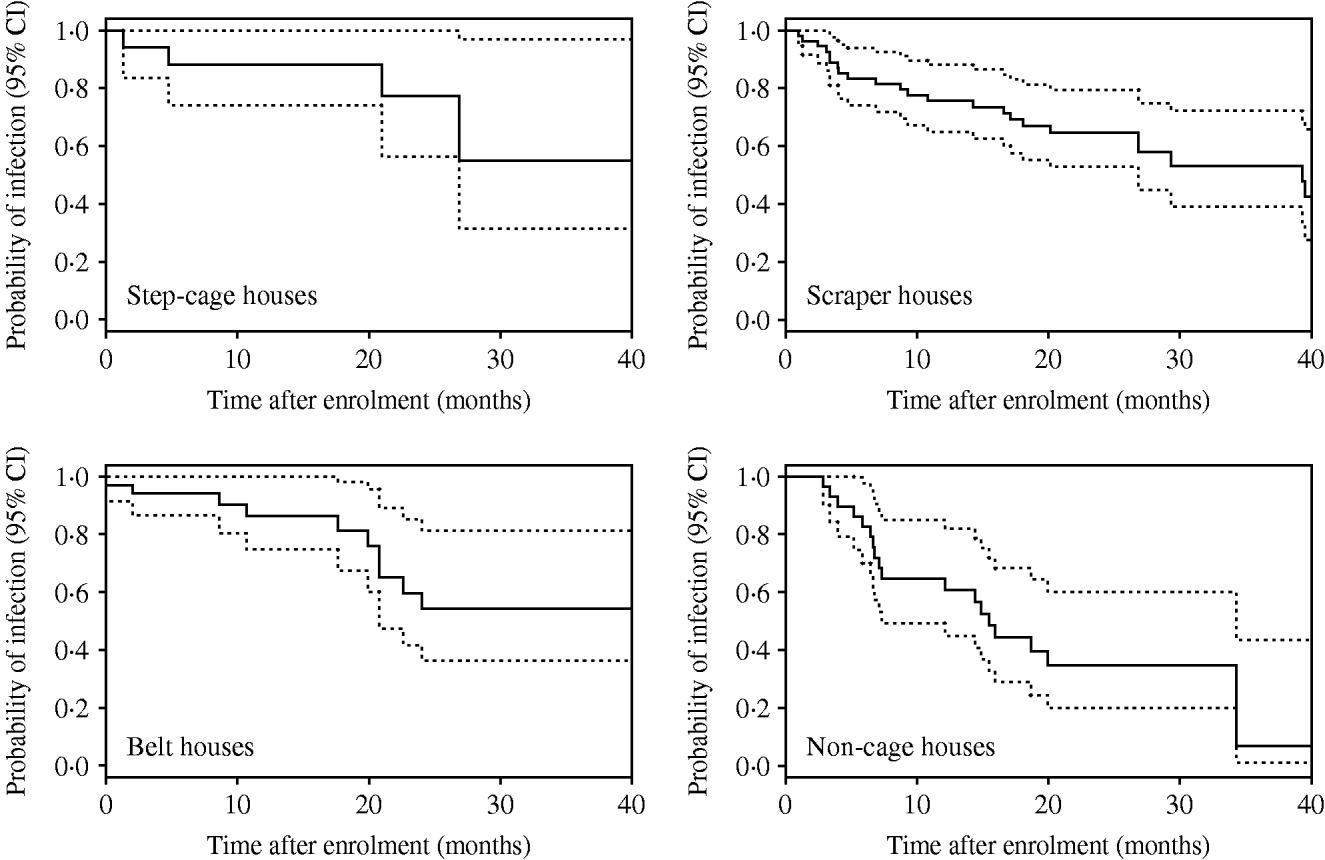
Fig. 2. Kaplan–Meier plots for Salmonella Enteritidis incidents by type of house. Dotted lines indicate 95% confidence intervals (CI).
Levels of rodent populations
Only 12 Salmonella incidents occurred in houses with a rodent score of ‘0’ (i.e. with no signs of rodents). These corresponded to six incidents of SE, two of S. Anatum, and one each of S. Livingstone, S. Kedougou, S. Agama and S. Infantis. In all other incidents rodents were always present at a variable level. The number of incidents and the median score of incidents by type of house and serovar is shown in Table 3. There was no significant difference in the overall rodent scores between cage-scraper and step-cage houses (Z=0·207, P=0·836), or between belt and non-cage houses (Z=0·221, P=0·825). However, there were significant differences in the rodent score between cage-scraper/step-cage houses and cage belt/non-cage houses (Z=−5·0637, P<0·001), as well as between incidents involving SE and ST (Z=2·9925, P=0·0028) and between incidents involving SE and other serovars (Z=2·015, P=0·044).
Table 3. Rodent score (RS) by type of laying house and serovar

IQR, Interquartile range.
Multilevel modelling of factors associated with persistence of Salmonella in laying houses
The year of recruitment was not significant, nor was the binary variable: ‘Recruited before/after 2005’. The rodent scores were subsequently grouped into three categories: (A) ‘0’; (B) between >0 and <1·5; and (C) ⩾1·5. However, the model was not stable and categories A and B were subsequently merged. There was no significant difference in survival of non-SE serovars if there were rodents present in the house (P=0·346), and therefore ‘serovar’ and ‘rodent score’ variables were subsequently combined into one single variable with category: (1) Non-SE with or without rodents; (2) SE, rodent score <1·5; (3) SE, rodent score ⩾1·5.
Results of the model indicate a significantly longer persistence for: (1) incidents involving SE and rodents (compared with non-SE incidents); (2) step-cage and cage-scraper houses (compared with belt houses and non-cage houses). Neither the flock nor the farm size were significant in the model and were therefore omitted. No interactions tested were significant. The multilevel model is presented in Table 4 and the model-estimated median times to clearance are presented in Table 5.
Table 4. Coefficients (β) derived from a multi-level hierarchical model investigating the log-time to clearance of infection in Salmonella-positive houses

SE, Salmonella Enteritidis; s.e., standard error.
Model intercept=0·693 (0·421).
Table 5. Model-derived median time to clearance (in months) for incidents of Salmonella in laying flocks in the UK

SE, Salmonella Enteritidis.
Values with a different superscript were statistically different at the 95% confidence level.
Clearance of SE during the life of a flock and rodent score
Clearance was observed in 62/141 SE incidents. In 47 incidents this was followed by a subsequent flock testing negative. In 26 of such incidents the last positive flock was tested more than once, and in 11 of these cases at least one of the last tests of the flock gave a negative result. This results in an estimated 11/26 (42%) incidents that cleared when the flock was still in lay and 15/26 (58%) incidents that cleared after the house was depopulated (i.e. during C&D). These proportions were similar for cage and non-cage incidents (data not shown).
In order to investigate any potential association of a reduction in rodents with SE clearance, incidents where houses were sampled more than once were classified into those with an increase, a decrease, or total absence of rodents over time. In 52% (32/62) of the SE incidents where clearance was observed there was either a decrease in rodents or absence of rodents vs. 32% (23/72) where clearance was not observed. Around 72% (8/11) of flocks that cleared in lay (as defined previously) appeared to either have no rodents or have reduced rodent scores, compared with 20% for those that cleared at the end of lay (χ2=96·88, P=0·009), and with 31% that did not clear over the whole study period (χ2=8·99, P=0·003) (Table 6).
Table 6. Number (and %) of SE, Salmonella Enteritidis incidents by the evolution of the rodent score (RS)

DISCUSSION
This paper is the first large population-based epidemiological study that estimates the persistence of the main Salmonella serovars in laying houses in the United Kingdom. The main findings were: (1) a longer persistence of SE where high numbers of rodents are present, compared with non-SE serovars, but also with SE when rodents are absent or present at a low level; (2) a longer persistence of SE in houses with a deep pit (i.e. step-cage houses or houses with a scraper manure disposal system); and (3) the estimation that 42% of SE incidents cleared during lay, and this was associated with a reduction or absence of rodent populations from laying houses.
This study is based on observational longitudinal data that involved sampling/testing laying houses repeatedly over time. After infection was originally detected in a flock, farmers received specific advice which typically consisted of an upgrade of procedures for terminal C&D and biosecurity, as well as pest control. This advisory input is theoretically likely to have led to a more pro-active intervention than in other situations where no advice is given (e.g. because no Salmonella has been detected), although on most occasions this advice was not taken at all or not fully implemented, except in a small proportion of the farms. The reason for this was economic considerations and a lack of a direct benefit to the farmer. This changed considerably in 2007, when fears of the effect of EU legislation in 2008/2009 prompted a more pro-active attitude in some farms. Persistence of infection is likely to be underestimated in this study. This is because houses were positive for Salmonella for an unquantifiable period of time before infection was detected. In addition, it is possible that incidents were declared ‘clear’ when a low-level infection was still present but undetected. For example in 12 cases there was more than one incident of SE in the same house. This may be due to infection no longer being detected (i.e. low sensitivity) or a result of re-introduction to new flocks as a result, for example, of the presence of Salmonella in a contiguous house. Although the sensitivity of the sampling methods used in the study is unknown, they are regarded to be considerably more sensitive than other environmental methods [Reference Carrique-Mas26].
All Salmonella serovars typically persisted for about twice as long in cage houses with scrapers and step-cage houses compared with cage houses with belt systems and non-cage houses. The common feature of step-cage houses and houses with a scraper is the presence of a deep pit which accumulates manure over the full life of the flock, and is often not fully cleaned and disinfected between production cycles. Houses with a scraper system and step-cages represent two thirds of all cage systems in the United Kingdom, similar to other EU countries (R. Davies, unpublished data). Therefore it is not surprising that a higher prevalence of SE in cage houses has also been reported in studies throughout the EU [Reference Methner27–Reference Much29]. However, in one study the opposite was found where hens within flocks were of different ages [Reference Mollenhorst30], which would be expected in multi-age flocks. A similar outcome was observed in houses detected to be positive for SE in the EU baseline layer survey, in which the status of subsequent flocks was investigated. In most cases, the same Salmonella serovar/phage type was recovered in the next flock, and this carry-over occurred in a higher proportion in cage houses compared to non-cage houses [Reference Carrique-Mas, Breslin and Snow12]. Another contributing factor for the longer persistence of infection in cage houses is the physical difficulty involved in cleaning the complicated structures (i.e. high stacks of cages, feeders, drinkers, manure belts, egg belts) in addition to the deep pits present in some houses.
However, it has been suggested that the observed greater prevalence in cage systems may in part be confounded by holding capacity (i.e. number of birds or number of flocks per holding), since cage laying houses (and holdings) are typically larger in size [Reference Snow31]. This is consistent with results from the risk factor analysis of the UK layer survey, in which cage systems were associated with a higher prevalence particularly for SE (but less so for non-SE serovars) (L. Snow, personal communication).
Because we did not find an association between number of birds in the house and persistence, we conclude it is more likely that the higher prevalence in large houses is a result of a greater risk of introduction of infection, compared with smaller houses. Introduction of SE to naive flocks is a relatively common occurrence in holdings that have other SE-positive flocks when biosecurity is poor or due to the migration of rodents. Introduction of SE (the most prevalent) serovar through feed and replacement birds is thought to be a rare event in the United Kingdom in recent years [Reference Davies and Mead32].
An interesting finding in our study was the quantitative interaction between rodents and SE, resulting in a longer persistence of SE in those houses with high levels of rodents, and faster clearance in houses where rodent levels were low or non-existent. The mechanism by which infected mice or rats infect hens with SE is not entirely understood, but it is thought that rodents acquire infection through contact with faeces or dust in positive houses [Reference Taylor33], as well as through horizontal transmission within the rodent colony [Reference Welch, Ostrolenk and Bartram34]. Infected rodents are capable of excreting high levels of Salmonella in their faeces [Reference Davies and Wray15] which contaminate poultry feed, the main vehicle of introduction of infection to birds. The rapid breeding of rodents means that there are always susceptible populations of juveniles that become infected and excrete large numbers of organisms, even though adult rodents may possibly acquire a certain degree of immunity to Salmonella with age. Because the terminal C&D rarely achieves elimination of rodents, infection of newly placed flocks by existing infected populations is very probable.
In general cage houses are a more attractive location for the establishment of rodent populations, since birds are restricted in their movements and rodents can then roam freely inside the house, where water, and feed are available ad libitum. The presence of a deep pit adds to the attractiveness of the house, since dry manure is an ideal nesting ground for rodents. Control of rodents in deep pits is complicated because of limited access to all parts of the pit to apply baits. The specific association between rodent infestation in the houses and infection with SE was also reflected in the EU baseline survey, in which no such association was found for incidents involving serovars other than SE (L. Snow, personal communication). In our final model step-cage and cage-scraper houses were a risk, independently of rodent score and serovar. Both types of cage houses are similar in the sense that they have deep pits, which may be more difficult to adequately clean and disinfect [Reference Wales, Breslin and Davies35]. In addition to this, some farmers do not include the deep pit as part of the C&D programme, since it may be more convenient to spread the manure directly onto fields after the grain harvest. This residual contamination clearly represents a risk for the next flock placed in the house.
Another reason for only partial cleaning of the pit is the desirability for many farmers to maintain litter beetles in order to control flies, a particular problem of deep pit houses. Flies can also carry high levels of contamination between flocks [Reference Wales, Breslin and Davies19, Reference Wales, Breslin and Davies35]. Some of the farms described were the same ones previously described by Davies & Breslin [Reference Davies and Breslin36] and it is in those farms where rodents have not been controlled that SE has succeeded in persisting over many production cycles. On the very few occasions where rodents were not a problem, SE infection did not typically carry-over from one flock to another provided that an adequate C&D procedure was carried out [Reference Wales, Breslin and Davies35].
In the present study most of the houses followed-up contained flocks vaccinated against SE at some point, and these represented the overwhelming majority of flocks. A range of vaccines were used, and in some houses different vaccination programmes have been over-used for subsequent flocks. We do not believe that the vaccination programmes have contributed to any important differences between the serovars (SE/ST/Other) or house type since the vaccine type distribution was similar for all types of incident (data not shown). However, it is possible that in the absence of rodents, the lack of difference in persistence between SE and other serovars might be due to the modulation as a result of prior vaccination of the flocks against SE.
We do not have a clear explanation for the SE/rodent interaction. Some experiments have highlighted a particular susceptibility of rodents to ST [Reference Kempelmacher37, Reference Poppe38]. In our experience the prevalence of infection of rodent faecal samples does not vary greatly depending on serovar, but there may be differences in the levels of Salmonella in these samples. It is also possible that the typically higher level of contamination in SE-infected flocks (data not shown) may explain why rodents played a more active role in such incidents.
The practical implications of these findings are important, since in the NCP the same restrictions will apply to ST- and SE-infected laying flocks. It is more likely that laying houses with SE will maintain the infection for long periods unless very vigorous action is undertaken to ensure elimination of rodents and effective disinfection, unlike in the case of ST incidents.
Effective control of rodents requires intensive baiting applied inside the houses, particularly in areas of harbourage and access to the houses and feed. The present study has provided an indication that the latter is possible, but studies using intensive and standardized sampling in parallel to intensive rodent control, coupled with adequate monitoring of the evolution of Salmonella in rodents and birds are necessary.
From January 2009 EU legislation will ban the sale of fresh eggs from both SE-positive and ST-positive flocks. This restriction will apply to flocks over their remaining lifespan. This study suggests that elimination of infection from flocks during lay is possible. Should this be validated by subsequent intensive sampling, there is a case for the relaxation of restrictions and a resumption of production.
ACKNOWLEDGEMENTS
We are grateful to all farmers participating in the study, and to all laboratory staff at the Food and Environmental Safety Department at VLA. This study was funded by Defra through Project OZ0321 and OZ0325.
DECLARATION OF INTEREST
None.










