The importance of diet and its relationship to human health was recognised by Hippocrates who stated ‘Leave your drugs in the chemist's pot if you can heal the patient with food’ and this view is now well respected in many branches of nutritional and clinical science. In the last 100 years we have seen a rapid rise in conditions with an inflammatory pathology including CVD, immune dysfunction, rheumatoid arthritis and bowel inflammation, among others(Reference Ruxton, Calder and Reed1–Reference Bjøkkaer, Brun and Valen4).
In the 1970s, studies on Greenland Inuit populations established that, despite high lipid intake, cardiac and other inflammatory illnesses were virtually absent in these populations and that the protective effect was linked to high fish intake(Reference Dyerberg, Bang and Hjorne5). Nowadays, the health benefits of consuming marine products, rich in n-3 highly unsaturated fatty acids (HUFA), most notably EPA (20 : 5n-3) and DHA (22 : 6n-3), are well recognised. In addition to their efficacy in treating the inflammatory conditions described above, there is also considerable evidence of n-3 HUFA benefits for a range of neural and behavioural disorders as well as for maternal and child health(Reference Young and Conquer6–Reference Hibbeln, Davis and Steer8).
The classical methods of blood fatty acid analysis have used a range of blood fractions including erythrocytes and plasma polar and total lipid (TL) fatty acids as well as whole blood(Reference Stark9, Reference Metherel, Armstrong and Patterson10). The drawbacks of collecting venous blood from the antecubital vein are the time and costs involved and these methods are not always applicable for young or vulnerable patients or for individuals that require blood collection on a frequent basis. Whole blood is usually taken by venepuncture, erythrocytes separated by centrifugation, lipids extracted by organic solvents, the polar lipid fraction isolated, methylated and purified to produce fatty acid methyl esters (FAME) before measuring the fatty acids by GLC. This process can take 4–5 d from sample delivery to reporting the FAME data.
Initial attempts to develop rapid analysis methods include an early study by Lands et al. (Reference Lands, Hamazaki and Yamazaki11), using plasma that was directly methylated without extraction but this was not widely adopted. By comparison, measuring whole blood fatty acids, collected by means of a fingertip lance, allows collection of high sample numbers that can be subjected to high-throughput automated procedures that allow results to be delivered in a few days at an economical price. A relatively small number of studies have investigated the use of fingertip whole blood to quantify fatty acid compositions to date(Reference Maragoni, Colombo and Galli12–Reference Rizzo, Montorfano and Negroni16). There is currently a need for an economical, rapid, minimally invasive, accurate and validated system to measure HUFA proportions that reflect and monitor an individual's dietary intake of essential fatty acids, which provides a valid health risk assessment for individual patients. Such assessments, based on the methodologies described in the present study, should be of benefit when screening larger population groups and could be utilised in both public health initiatives as well as in clinical practice.
The aims of the present study were to validate a simple and rapid method of blood collection using a fingertip whole blood sample and compare the fatty acid compositions obtained with erythrocyte polar lipid fatty acid compositions. Samples were collected from patients having variable n-3 HUFA intakes ranging from those consuming low-fish diets with no fish oil supplements to those consuming both fish and supplements. Fingertip samples were analysed following a direct methylation technique and compared with the erythrocytes that were extracted, the polar lipid fraction isolated and methylated by standard wet chemistry methods and analysed by conventional GC procedures.
Materials and methods
Study participants
A total of fifty-one patients attending the Essential Health Clinic, Rutherglen, Glasgow provided samples between May and July 2009. In the total sample of fifty-one patients, twenty-six had not taken any fish oil supplements in the preceding 3 months and this group was used as a fish oil-naïve control population.
Blood collection
Whole blood was collected using a blood lance (Accu-Chek®, Safe-T-Pro Plus, Roche Diagnostics GmbH, Mannheim, Germany). An additional 2–4 ml of whole blood was collected from each patient, to provide additional blood should a re-test be required, which was stored in glass tubes containing EDTA as an anticoagulant and sent to the Nutrition Group Laboratories at the University of Stirling, along with the blood spot cards, by next day delivery. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki. Verbal informed consent was obtained from all patients and verbal consent was witnessed and formally recorded.
Fatty acid stability test
Blood samples were obtained from four patients and the samples were pooled and thoroughly mixed. We prepared twenty-two Whatman 903 cards by adding butylated hydroxytoluene (BHT; 50 mg/100 ml in ethanol) to fully cover each collection spot. The cards were air-dried for 1 h and placed in a sealed polythene bag overnight. The following day the blood sample was mixed and 50 μl was applied to each of eighty-eight spots. The blood sample was mixed after every twelve spots. We placed forty-four blood spots in either a fridge at 4°C or a freezer at − 20°C. The samples were analysed at time 0 (3 h after spotting) and subsequent samples were removed for analysis after, 48 h, 7, 14, and 28 d in storage. The data for arachidonic acid (ARA), EPA, DHA, ARA:EPA ratio and percentage n-3 HUFA/total HUFA are shown in Table 1. A second test was conducted to determine whether pre-treating the Whatman collection cards with two drops of BHT in ethanol (50 mg/100 ml ethanol), prior to adding the blood sample, reduced fatty acid losses compared to cards with no addition of BHT. A single blood sample was mixed and spotted onto Whatman collector cards after the BHT solution was fully dry and the same blood sample was added to cards with no BHT. An initial sample was analysed for fatty acid compositions 3 h after blood addition and again after 1–4 weeks storage in Zip-Lock foil bags with desiccant at room temperature.
Table 1 Stability of blood spot fatty acid compositions (percentage of total fatty acids) stored at 4 and −20°C for 28 d
(Mean values and standard deviations, n 3)

a,b,c,d Mean values with unlike superscript letters were significantly different (P < 0·05).
* Increase.
Lipid extraction, preparation of phospholipid fraction and preparation of fatty acid methyl esters in erythrocytes and whole blood
The lipid extraction of erythrocytes was a modification of the method of Bligh & Dyer(Reference Bligh and Dyer17). The erythrocyte pellet was thawed and 0·25–0·50 ml placed in a 15 ml stoppered tube. Methanol (5 ml)–0·01 % BHT (w/v) was added and then mixed on a vortex mixer. After 20 min at room temperature 2·5 ml of chloroform–BHT was added, mixed and left at room temperature for a further 40 min. The tubes were centrifuged at 500 g for 5 min and the supernatant decanted into a clean 15 ml tube after filtering though Whatman No. 1 paper. A further 2·5 ml of chloroform–BHT and 2·5 ml of 0·88 % KCl (w/v) were added, mixed and centrifuged as above. The lower chloroform layer was removed and filtered into a 15 ml tube and evaporated under N2. The lipid was taken up in 0·8 ml of chloroform–methanol (2:1, v/v) and placed in a weighed glass vial. This was dried under N2 and desiccated for 16 h. After recording the lipid weight the sample was re-suspended in chloroform–methanol (2:1, v/v)+BHT, at a concentration of 10 mg/ml and stored at − 70°C until analysed.
A PL fraction was prepared from 0·5 mg of TL applied to a 20 × 20 cm silica gel 60 TLC plate (VWR, Lutterworth, Leicestershire, UK) and developed in isohexane–diethyl ether–acetic acid (80:20:1, by vol.) and dried for a few minutes at room temperature. The plate was sprayed lightly with 2,7-dichlorofluoricein (0·1 %, w/v) in 97 % methanol (v/v) and the PL bands on the origin scraped from the plate and placed in a 15 ml test-tube. FAME were prepared by acid-catalysed transesterification in 2 ml of 1 % H2SO4 in methanol at 50°C overnight(Reference Christie18). The samples were neutralised with 2·5 ml of 2 % KHCO3 and extracted with 5 ml isohexane–diethyl ether (1:1, v/v)+BHT. The samples were then re-extracted with 5 ml isohexane–diethyl ether (1:1) and the combined extracts were dried and dissolved in 0·3 ml of isohexane prior to fatty acid analysis.
The dried whole blood sample was detached from the collection device using forceps and placed into a screw-cap vial containing 1 ml of a methylating solution (1·25 m-methanol–HCl). The vials were placed in a hot block at 70°C for 1 h. The vials were allowed to cool to room temperature and then 2 ml of distilled water and 2 ml of saturated HCl solution were added. FAME were then extracted using 2 × 2 ml of isohexane.
Measurement of erythrocyte and whole blood fatty acids
FAME were separated and quantified by GLC (ThermoFisher Trace, Hemel Hempstead, Herts, UK) using a 60 m × 0·32 mm × 0·25 μm film thickness capillary column (ZB Wax; Phenomenex, Macclesfield, Cheshire, UK). H2 was used as a carrier gas at a flow rate of 4·0 ml/min and the temperature programme was from 50 to 150°C at 40°C/min then to 195°C at 2°C/min and finally to 215°C at 0·5°C/min. Individual FAME were identified compared to well-characterised in-house standards as well as commercial FAME mixtures (Supelco™ 37 FAME mix; Sigma-Aldrich Limited, Gillingham, Dorset, UK). The precision of the whole-blood fatty acid analysis was conducted by measuring triplicate samples over four successive days to provide mean and standard deviation and CV% (n 12).
Statistical analysis
The significance of the differences (P < 0·05) between groups was determined by the use of Student's t test or in the case of the storage-losses study one-way ANOVA was used. For the latter, significant differences were tested using Tukey's multiple comparisons test. Regression analysis was used to obtain correlation coefficients from plots of blood fatty acids from different fractions. ANOVA, t tests and regression analyses were performed using the Graphpad Prism™ (version 4.0) statistical package (Graphpad Software, San Diego, CA, USA).
Results
The data from the blood stability trial are shown in Table 1. For ARA stored at − 20°C for 28 d there was no change in the percentage values over the 28 d period. However, when stored at 4°C the percentage values were significantly reduced from 3 h values when sampled after 48 h and 7 d, and were reduced further following storage for 14 and 28 d with the reduction between 3 h and 28 d being 14·5 %. For EPA stored at − 20°C there was no reduction up to 7 d storage but a significant reduction was observed in samples stored for 14 and 28 d (9·1 % loss at 14 d). For EPA stored at 4°C there was an initial loss after 48 h and further reductions after 14 and 28 d (28·8 % reduction at 28 d). For DHA stored at − 20°C the reductions were similar to EPA at the same temperature with losses at 28 d being 4·7 %. As with EPA at 4°C DHA dropped significantly after 48 h and 7 d and then again after 14 and 28 d such that the overall reduction was 25·3 %. For the ARA:EPA ratio at − 20°C the ratio rose after 14 and 28 d in storage with the increase due to ARA being more stable than EPA and the increase between 3 h and 14 d was 8·7 %. The ARA:EPA ratio at 4°C increased from 4·33 at 3 h sampling to 5·17 after 28 d in storage representing a 16·3 % increase in the ARA:EPA ratio over 28 d. The percentage n-3 HUFA:total HUFA ratio at − 20°C was stable up to 7 d storage and declined significantly after 14 and 28 d storage. The reduction over 28 d was only 3 %. By comparison, the percentage n-3 HUFA:total HUFA ratio stored at 4°C showed a significant reduction after 7 d and further reductions after 14 and 28 d storage. The reduction over 28 d was 8 %.
Data obtained from the blood stability trial comparing Whatman sample collection cards pre-treated with BHT or with no added BHT showed that while DHA concentrations reduced steadily from 2·93 % in the initial samples to 1·95 % after 4 weeks at room temperature there were no significant differences, due to BHT addition, between the samples stored for the same time period. This suggested there was no benefit in adding BHT to the sample cards prior to adding the whole blood spot to the collector cards. Other HUFA responded in a similar way to DHA.
A comparison of the erythrocyte PL fatty acid compositions with whole blood fatty acid compositions, collected and analysed from a blood spot applied to a collection device, as described above, is shown in Table 2. Of the thirty-five individual fatty acids, fatty acid groups or ratios measured, all values were significantly different between the erythrocyte PL and whole blood fatty acids with the exception of 20 : 2n-6 and the percentage n-3 HUFA:total HUFA ratio. Whole blood fatty acids showed higher values for the SFA, 14 : 0, 15 : 0, 16 : 0 and all MUFA with the exception of 24 : 1n-9. In the PUFA and HUFA, higher values were seen in the whole blood for the PUFA, 18 : 2n-6, 18 : 3n-6 and 18 : 3n-3 while all HUFA of both the n-3 and n-6 series were significantly lower in whole blood samples compared with erythrocyte PL. All the dimethyl acetal fatty acids were significantly lower in whole blood compared with erythrocyte PL as was the case for the ARA:EPA ratio although the n-6:n-3 ratio was higher in the whole blood fatty acids.
Table 2 A comparison of the fatty acid compositions (percentage of total fatty acids) of the erythrocyte polar lipid fraction and whole blood fatty acid compositions in patients
(Mean values and standard deviations, n 51)

PL, phospholipids; DMA, dimethyl acetals; HUFA, highly unsaturated fatty acids.
From the total study population, twenty-seven patients who did not consume any fish oil supplements, were selected, and the comparison of the erythrocyte PL and whole blood fatty acid compositions is shown in Table 3. As was the case for the total study group only two fatty acids out of thirty-five were not significantly different between the erythrocyte PL and whole blood fatty acids. These included 20 : 2n-6, which was also the case in the whole study group in Table 3, and the total n-6 fatty acids, which differed from the whole study group.
Table 3 A comparison of the fatty acid compositions (percentage of total fatty acids) of the erythrocyte polar lipid fraction and whole blood fatty acid compositions in patients who did not consume fish oil supplements
(Mean values and standard deviations, n 27)

PL, phospholipids; DMA, dimethyl acetals; HUFA, highly unsaturated fatty acids.
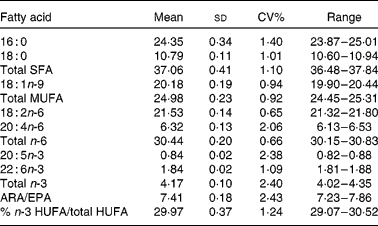
To assess the precision of the whole blood analysis method a single whole blood sample was analysed, in triplicate, over four consecutive days. The results for seven major whole blood fatty acids, total saturates, monounsaturates, total n-6 and n-3 fatty acids as well as the ARA:EPA ratio and the percentage n-3HUFA:total HUFA ratio are shown in Table 4. The results obtained show that the method precision for the major fatty acids, groups and ratios gave CV% values that were acceptable for all the fatty acids measured and were similar to those reported in the literature(Reference Maragoni, Colombo and Galli12).
Table 4 Precision of the new whole blood method when analysing the same sample in triplicate over 4 d
(Mean values and standard deviations, coefficient of variation (%) and ranges, n 12)
HUFA, highly unsaturated fatty acids.
Graphs showing the correlations between ARA, EPA, DHA, ARA:EPA ratio and the percentage n-3 HUFA:total HUFA, comparing fatty acid values from erythrocyte PL with corresponding values from whole blood spot data, are shown in Figs. 1–5. The correlations were generally good although the r 2 values for ARA and DHA were the lowest at 0·48 and 0·59, respectively, although there were much stronger correlations for EPA (r 2 0·89), ARA:EPA ratio (r 2 0·94) and the percentage n-3 HUFA:total HUFA (r 2 0·95).
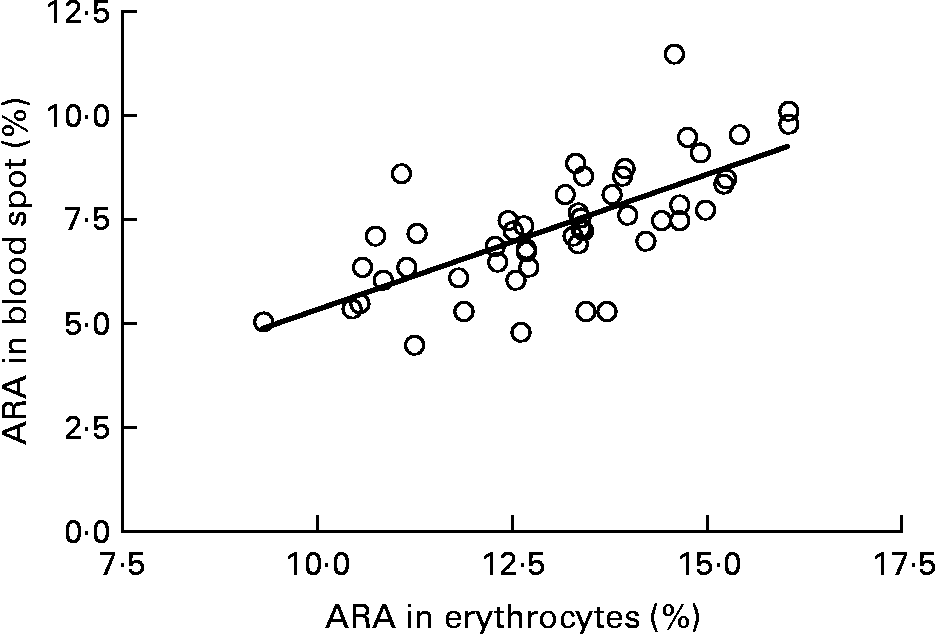
Fig. 1 Correlation between arachidonic acid (ARA) (percentage of total fatty acids; TFA) in erythrocyte polar lipid fraction (erythrocytes), collected by venepuncture, compared with the ARA (% TFA) in whole blood spot collected by finger prick from fifty-one patients. r 2 0·483.
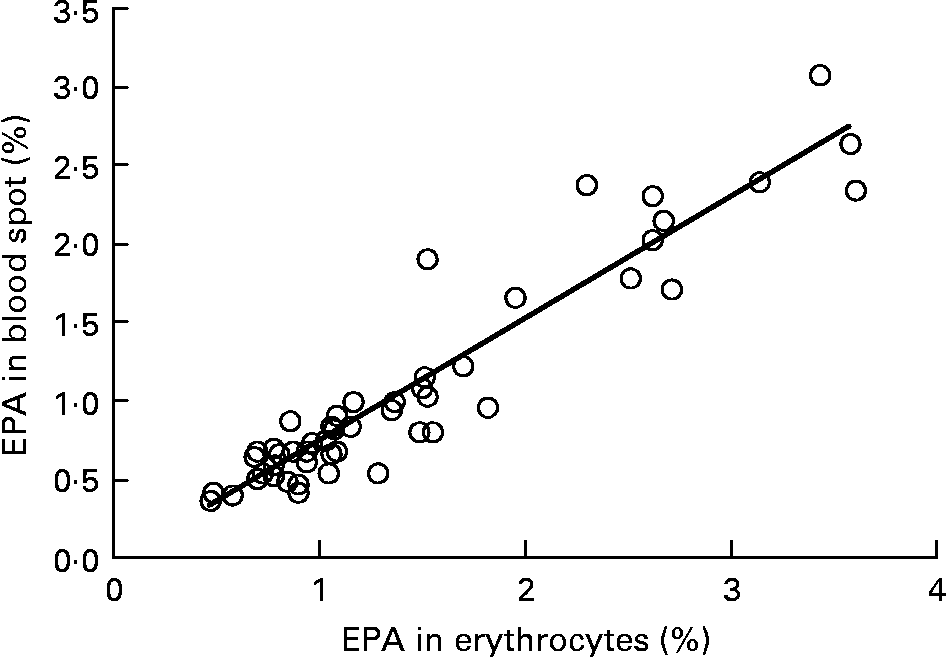
Fig. 2 Correlation between EPA (percentage of total fatty acids; TFA) in erythrocyte polar lipid fraction (erythrocytes), collected by venepuncture, compared with the EPA (% TFA) in whole blood spot collected by finger prick from fifty-one patients. r 2 0·887.
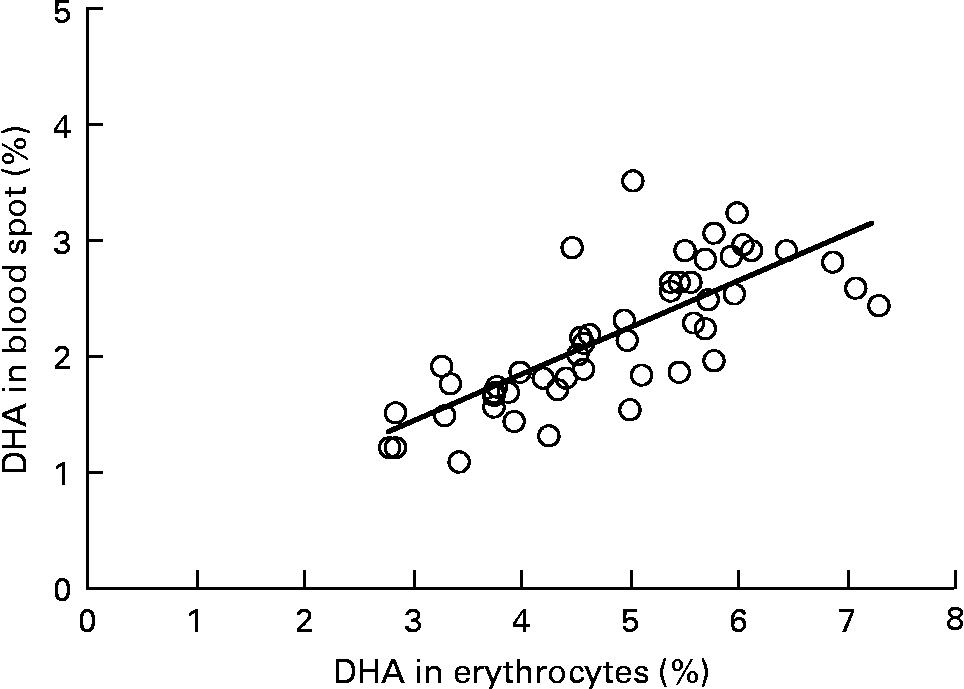
Fig. 3 Correlation between DHA (percentage of total fatty acids; TFA) in erythrocyte polar lipid fraction (erythrocytes), collected by venepuncture, compared with the DHA (% TFA) in whole blood spot collected by finger prick from fifty-one patients. r 2 0·579.
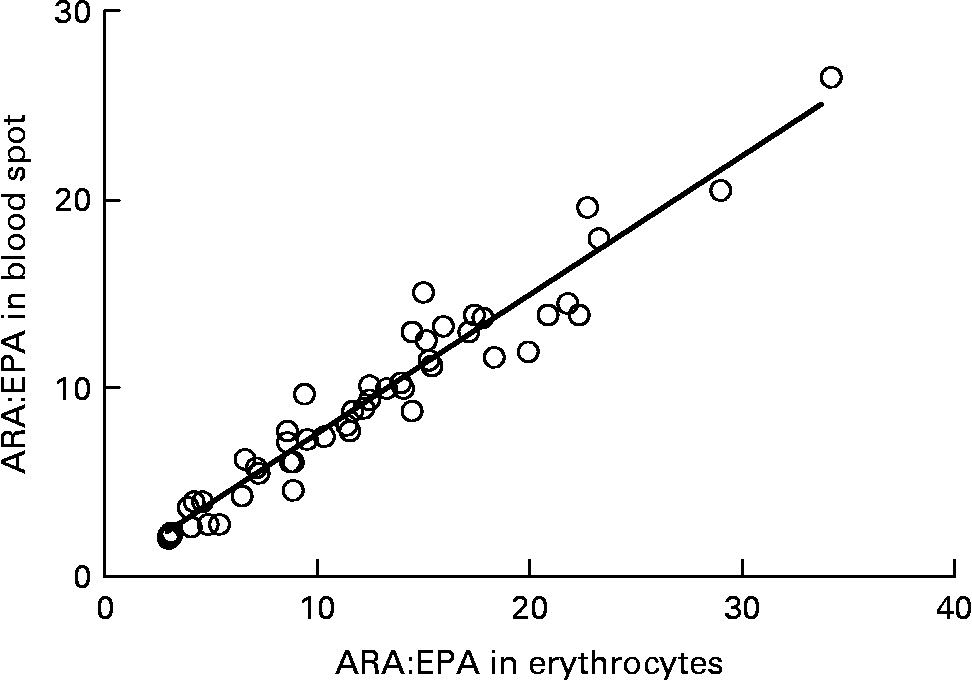
Fig. 4 Correlation between the ARA/EPA ratio in erythrocyte polar lipid fraction (erythrocytes), collected by venepuncture, compared with the ARA/EPA ratio in whole blood spot collected by finger prick from fifty-one patients. r 2 0·937.
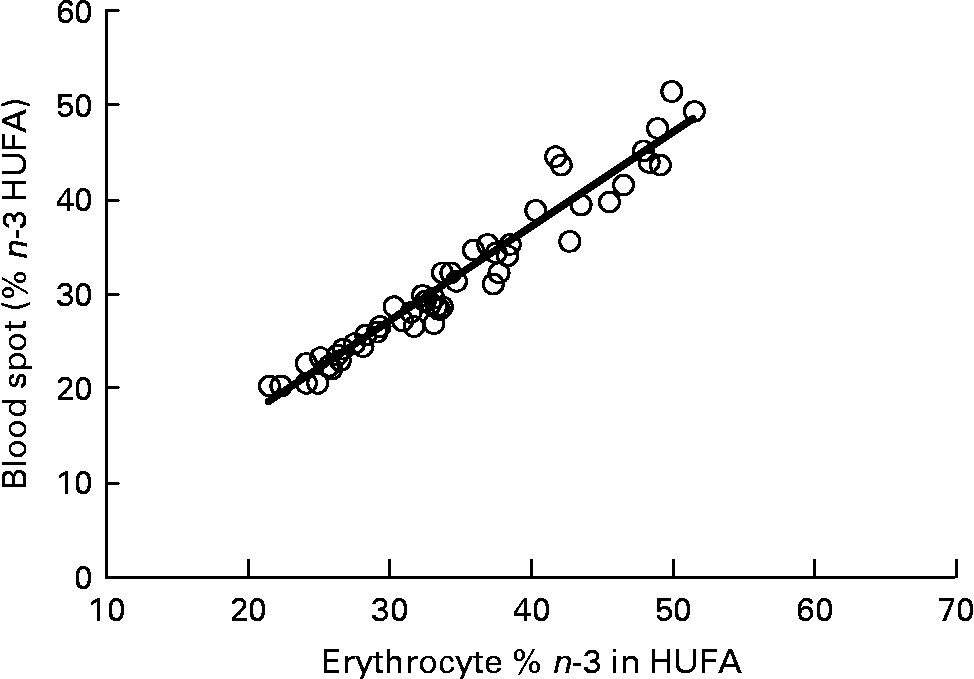
Fig. 5 Correlation between the percentage n-3 highly unsaturated fatty acid (HUFA)/total HUFA in erythrocyte polar lipid fraction (erythrocytes), collected by venepuncture, compared with the percentage n-3 HUFA/total HUFA in whole blood spot collected by finger prick from fifty-one patients. n-3 HUFA = EPA+22 : 5n-3+DHA and n-6 HUFA = 20 : 3n-6+ARA+22 :4n-6+22 : 5n-6. r 2 0·946.
Discussion
There is now conclusive evidence that increasing the intake of n-3 HUFA, in particular EPA and DHA, can prevent or attenuate CVD as well as other conditions with an inflammatory pathology, including type 2 diabetes, rheumatoid arthritis, asthma and some forms of cancer(Reference Maclean, Mojica and Morton3, Reference Siddiqui, Hravey and Ruzmetov19, Reference Schachter, Kourad and Merali20). There is also evidence of benefit in consuming n-3 HUFA for retinal function and for a range of neurological and neurodegenerative disorders(Reference Young and Conquer6, Reference Hodge, Barnes and Schachter21). Thus, the increasing interest in n-3 HUFA as a medical intervention for a range of conditions has simultaneously increased demand for the development of more rapid, accurate and cost-effective methods of blood analysis.
However, clinical studies have used a number of different blood fractions when measuring fatty acid compositions including erythrocyte TL and PL, plasma TL and PL, plasma TAG and platelet TL and among others(Reference Marangoni, Angeli and Colli22–Reference Katan, Deslypere and van Birgelen24). Among these different fractions, measurement of the erythrocyte PL fraction has been regarded by many investigators as the ‘gold standard’ that might estimate, long-term dietary HUFA intake influenced by the erythrocyte lifespan of approximately 125 d(Reference Harris and von Schacky25, Reference Poppitt, Kilmartin and Butler26). However, the observed similar proportions of n-3 in HUFA of plasma and erythrocytes may be reconciled when long-standing awareness of continual remodelling of acids in erythrocyte membrane phospholipids (PL)(Reference Waku and Lands27) is included in the consideration. In addition, extraction, methylation, purification and chromatography involved in this intensive analytical procedure do not allow the high sample throughput required when analysing large sample numbers from clinical trials. In addition, the collection of venous blood from some patients, particularly young infants and the elderly, can prove difficult and can raise ethical concerns.
In the present study, we have developed a rapid, accurate and inexpensive method that is minimally invasive and involves the collection of a drop of whole blood, using a disposable blood lance, which can be stored on absorbent paper, and subjected to direct methylation techniques and allows a high sample throughput providing compositional data within 1–2 d. For most fatty acids significant differences were observed when comparing erythrocyte PL with whole blood lipids and this is due to the contribution of several lipid classes including TAG, PL, steryl esters and NEFA in the latter compared to the largely PL input in the former. The SFA, 14 : 0 and 15 : 0, were relatively higher in the whole blood than in the erythrocyte PL as these are not found in PL to any great degree. However, the saturates that have a structural role in the PL, particularly 18 : 0 and 24 : 0, were higher in the erythrocyte PL while 16 : 0, which also has a structural role in PL, as well as being abundant in neutral lipids, was similar in both PL and whole blood. In the MUFA all were higher in whole blood than in erythrocyte PL, which reflects their abundance in neutral lipids, with the exception of 24 : 1, which has important structural roles in PL and sphingolipids. In the C18 PUFA higher values were recorded in the whole blood than in erythrocyte PL as these fatty acids are mainly found in the neutral lipids with lower concentrations located in the PL fraction. By comparison, the HUFA, namely 20 : 3n-6, ARA, 22 : 4n-6 and 22 : 5n-6 of the n-6 series and EPA, 22 : 5n-3 and DHA of the n-3 series were more abundant in the erythrocyte PL reflecting their structural importance relating to cell membrane function. This was particularly noticeable for ARA and DHA where the values in whole blood in the fifty-one patients represented 56 and 45 % of the values in erythrocyte PL, respectively. The abundance of ARA and DHA in membrane PL reflects not only their importance to erythrocyte function but also their roles in all cellular membranes, but especially so in neural tissues(Reference Hibbeln, Nieminen and Blasbalg7, Reference Schachter, Kourad and Merali20, Reference Maclean, Issa and Newberry28). An earlier study compared the HUFA compositions in erythrocyte PL and whole blood samples collected by finger prick(Reference Bailey-Hall, Nelson and Ryan15). The percentage values recorded for ARA in erythrocytes v. whole blood were 10·6/8·06, which is similar to the values of 13·0/7·3 observed in the present study. The values for DHA were in fact closer, being 4·36/2·76 and 4·84/2·18, respectively for the study by Bailey-Hall et al. (Reference Bailey-Hall, Nelson and Ryan15), and the present study, respectively.
The storage stability trial analysed samples at regular intervals from 3 h to 1 month following storage at either 4 or − 20°C. For ARA, EPA and DHA at 4°C, progressive reductions were seen from 48 h onwards with reductions of 15, 29 and 25 % observed after 1 month (Table 2). For the ARA:EPA ratio values increased over time due to EPA declining more quickly than ARA such that values at 1 month were 16 % higher than at the start. The percentage n-3 HUFA/total HUFA values were the most stable being reduced by only 8 % after storage for 1 month at 4°C. For samples stored at − 20°C for 1 month reduction in HUFA and ratios were much lower (Table 2). No reduction over time was seen for ARA while there was no loss of EPA or DHA until 14 d in storage and losses were 9·1 and 4·7 % after 1 month, respectively. As above the ARA:EPA ratio after 1 month at − 20°C was reduced by 9 % while the percentage n-3 HUFA/total HUFA values were reduced after 14 d but only by 3 % over the month.
In the present study, we compared the correlations between erythrocyte PL fatty acids and whole blood finger-prick assays in the fifty-one patients for ARA, EPA, DHA, ARA:EPA ratio and percentage n-3 HUFA/total HUFA. The poorest correlation was seen for ARA, which had an r 2 value of 0·48 in the present study, which was similar to the value of 0·41 recorded by Bailey-Hall et al. (Reference Bailey-Hall, Nelson and Ryan15). An earlier study by Ichihara et al. (Reference Ichihara, Waku and Yamaguchi29) suggested that the correlation of ARA could be improved if it was expressed as a ratio such as ARA:(EPA+DHA) and, as such was an indicator of n-6:n-3 status. Using this ratio increased the r 2 value to 0·93, for whole blood v. erythrocyte PL(Reference Bailey-Hall, Nelson and Ryan15) while in the present study the ARA:EPA ratio had an almost identical r 2 value of 0·94 although in the study by Rizzo et al. (Reference Rizzo, Montorfano and Negroni16) a slightly lower value of 0·87 was recorded. For EPA the correlation between erythrocyte PL and whole blood gave an r 2 value of 0·89, which is considerably higher than the value of 0·64 recorded by Bailey-Hall et al. (Reference Bailey-Hall, Nelson and Ryan15). This difference might relate to the wider ranges obtained in the present study compared with the Bailey-Hall et al. (Reference Bailey-Hall, Nelson and Ryan15) study (0·4–3·6 v. 0·2–2·4 in erythrocyte PL) and (0·4–3·0 v. 0·18–1·6 in the whole blood). Conversely, for DHA the opposite effect was seen when comparing the present trial with that of Bailey-Hall et al. (Reference Bailey-Hall, Nelson and Ryan15) with the r 2 values being 0·58 and 0·86, respectively. As with EPA, this might be explained by the wider ranges seen in the Bailey-Hall et al. (Reference Bailey-Hall, Nelson and Ryan15) study compared with the present study (1·7–7·0 v. 2·8–7·2 in erythrocyte PL) and (1·1–4·3 v. 1·1–3·5).
The percentage n-3 HUFA:total HUFA ratio that includes EPA, DPA and DHA of the n-3 series and 20 : 3n-6, ARA, 22 : 4n-6 and 22 : 5n-6 gave the best correlation between whole blood and erythrocyte PL in the present study with an r 2 value of 0·95. This value is very similar to that reported by Stark(Reference Stark9), of 0·93 and 0·94 for plasma and erythrocytes, respectively. The use of percentage n-3 HUFA/total HUFA was also shown to provide the most consistent estimates of n-3 fatty acid status with stronger correlations across different blood matrices when compared with only considering sum EPA+DHA(Reference Metherel, Armstrong and Patterson10, Reference Marangoni, Colombo and Martiello13, Reference Lands, Simopoulo and De Meester30). It is noteworthy that comparing the percentage n-3 HUFA/total HUFA between the present study in Scotland, with those by Marangoni et al. (Reference Marangoni, Colombo and Martiello13) (Italy) and Stark(Reference Stark9) (USA) show values of 26·43 in Scotland, 25·76 in Italy and 20·17 in the USA. These values are all relatively low and reflect the low fish intake in most Western diets(Reference Rizzo, Montorfano and Negroni16, Reference Innis and Elias31). The equations developed by Lands et al. (Reference Lands, Libelt and Morris32) suggested that adding 750 mg of EPA+DHA to the typical North American diet, without changing other fatty acid intake, would provide significant cardioprotection and would increase the ratio to 44 %. A value of 40 % has been predicted to reduce mortality from myocardial infarction and this approximates to an EPA+DHA value of 8 % of total erythrocyte fatty acids(Reference Dolecek33, Reference Lands34).
In conclusion, the increasing interest in the use of n-3 HUFA as beneficial interventions in a range of inflammatory disorders prevalent in Western populations requires the development of methods to assess efficacy of the intervention. The collection of whole blood that can be immobilised and stored on absorbent paper and directly derivatised to allow a cheap, rapid and reproducible analysis of fatty acid compositions in large cohorts is a valuable addition in the evaluation of disease risk and subsequent treatment. The use of the percentage n-3 HUFA:total HUFA ratio appears to be a robust measure of HUFA status across a range of blood fractions and HUFA concentrations. The evidence from the present study suggests that this methodology has the potential to relate blood HUFA with dietary intake and physiological biomarkers that could assist in fine-tuning therapeutic interventions. The present study demonstrates that individual HUFA, and their relative proportions, can be measured accurately from a whole blood spot. This provides an economical and rapid alternative to current labour-intensive methods that require centrifugation and complex analysis of erythrocyte lipid classes. The method described here eliminates the cost and time to isolate erythrocytes and offers researchers, clinicians and the public an alternative method that should become less costly as increased use provides economy of scale. The results obtained demonstrate the validity of simplified analytical methods that make them both easily available and understandable to clinicians and public user groups. The rapid blood test is currently marketed as the Ideal Omega Test™ (GHS Ltd, Poulton Le Fylde, Lancashire, UK). To assess this methodology fully will require further large-scale trials that should incorporate rapid methods of automated sample processing coupled with fast GC technology in an effort to reduce analysis times.
Acknowledgements
The present study was funded by Scottish Enterprise. J. G. B. wrote the manuscript with assistance from all other authors in particular J. R. D., T. G. and B. L.; T. G. was responsible for sample collection and J. R. D., E. E. M. and I. Y. were responsible for sample processing, GC analysis and data processing. All authors read and approved the findings of the study. T. G. is Clinical Director of Glasgow Health Solutions Limited. None of the other authors had a conflict of interest.











