INTRODUCTION
Streptococcus agalactiae, or Lancefield group B streptococcus (GBS), is a leading cause of neonatal infections with high mortality and morbidity rates [Reference Holt1, Reference Schuchat2]. GBS infections may be divided into early-onset disease (EOD), which occurs within the first 6 days after birth, and late-onset disease (LOD), which occurs from days 7 to 89 after birth [3]. EOD is caused by vertical transmission from mothers to neonates during childbirth, and presents most frequently as sepsis, followed by meningitis [Reference Hansen4–Reference Tsolia6]. LOD mostly presents as meningitis due either to transmission from the mother or contact with environmental sources [Reference Schuchat2].
The initial guidelines issued in 1996 [3] and the revised guidelines of 2002 [Reference Schrag7] for the prevention of neonatal GBS infections in the USA proposed the use of a prophylactic intrapartum antimicrobial agent in pregnant carriers. These preventive measures were associated with a significant decline in the incidence of EOD over the past decade in the USA, while the incidence of LOD remained unchanged [8, Reference Schrag9]. The guidelines were again revised in 2010 [Reference Verani10]. Active Bacterial Core Surveillance (ABCs) recently estimated the incidence of invasive GBS as 0·25 EOD and 0·26 LOD cases/1000 live births in 2010 (http://www.cdc.gov/abcs/reports-findings/survreports/gbs10).
In 2008, the Japan Society of Obstetrics and Gynecology (JSOG) adopted similar guidelines, recommending the universal screening of pregnant women at 33–37 weeks gestation and the administration of a prophylactic intrapartum antibiotic to those who test positive for GBS. The incidence of neonatal GBS infections was estimated to be 0·4/1000 live births in the Hokkaido area in 2010 [Reference Sakata11]. A nationwide surveillance study in Japan between 2004 and 2010 reported a decrease in mortality due to EOD and LOD, but the incidence of GBS infection remained unchanged [Reference Matsubara, Hoshina and Suzuki12].
In the 10 capsular polysaccharide serotypes, types Ia, Ib, and III most frequently cause neonatal disease [Reference Davies13–Reference Phares16]. In particular, about 80% of LOD is caused by type III GBS [Reference Davies13]. Current PCR methods can readily identify GBS serotypes [Reference Poyart17, Reference Yao18]. Multilocus sequence typing (MLST), developed by Jones et al. [Reference Jones19] is also used to distinguish genetic lineages to probe the associations between specific GBS genotypes and diseases. A lineage with enhanced invasive capacity expressing serotype III has been characterized as sequence type 17 (ST17) by most MLST studies [Reference Davies20–Reference Lin22]. Another report indicates that capsular type Ia of ST23 and ST24 demonstrates enhanced potential for invasive diseases, especially EOD [Reference Martins23].
In the present study we aimed to clarify the genetic diversity of GBS isolates responsible for invasive infections in neonates in Japan from 2006 to 2011. The GBS isolates sent to our laboratory by medical institutions participating in the surveillance group underwent serotype characterization by real-time PCR and MLST. Susceptibility to antimicrobials and the presence of macrolide (ML) resistance genes were also investigated.
MATERIALS AND METHODS
Strains
The study population was limited to patients aged <1 year with invasive GBS infections including sepsis, meningitis, pneumonia, and others such as purulent arthritis. GBS strains isolated from normally sterile clinical specimens such as blood, cerebrospinal fluid, and joint fluid, were collected from the 351 medical institutions participating in the Active Surveillance of Invasive Pneumococcal and Streptococcal Infections in Japan. During 2006–2011, our laboratory at Kitasato Institute for Life Sciences received a total of 150 GBS strains accompanied by an anonymous survey form completed by the attending physician.
Capsular serotyping
To identify the capsular serotypes of GBS by real-time PCR, we constructed nine sets of primers and molecular beacon (MB) probes, according to Poyart et al. [Reference Poyart17] and Igarashi & Mitsuhashi [Reference Igarashi and Mitsuhashi24]. One colony was picked from each agar plate and placed in 50 μl lysis solution containing 2 U mutanolysin (Sigma Aldrich, Germany). The lytic reaction was performed for 10 min at 37°C and 10 min at 60°C, followed by 5 min at 94°C. Lysate was added to each of six tubes containing the PCR mixtures for the individual capsular types. The combinations were as follows: types Ia and Ib in tube A, type II in tube B, types III and IV in tube C, types V and VI in tube D, types VII and VIII in tube E, and the dltS gene in tube F.
The PCR reaction mixture (total volume, 50 μl) consisted of 20 pmol of each primer, 25 pmol of each probe, 2 × multiplex power mix (Bio-Rad, USA), and DNase- and RNase-free distilled water. DNA amplification was performed for 40 cycles at 95°C for 10 s, 50 °C for 30 s, and 72°C for 20 s.
MLST analysis
Primer sets corresponding to seven housekeeping genes (adhP, atr, glcK, glnA, pheS, sdhA, tkt) used for MLST analysis were constructed in reference to the MLST website (http://pubmlst.org/sagalactiae/). MLST was applied to the sequence for these seven genes according to previously described methods [Reference Jones19], with alleles and sequence type (ST) assignments determined using the S. agalactiae MLST database. Alleles and sequence types not previously posted were entered into the S. agalactiae MLST database.
Antibiotic susceptibility and resistance mechanism
Susceptibility testing of GBS strains was performed using an agar dilution method conforming to the standards of the Clinical and Laboratory Standards Institute (www.clsi.org). Oral antimicrobial agents employed in this study were penicillin G (PEN), ampicillin (AMP), amoxicillin (AMX), and erythromycin (ERY). Parenteral agents were cefotaxime (CTX), panipenem (PAM), meropenem (MEM), and vancomycin (VAN).
ML resistance genes of erm(A), erm(B), and mef(A/E) were identified by real-time PCR. The primer and probe sets and PCR conditions required modification of previously described methods [Reference Wajima25].
RESULTS
Relationship between serotype and GBS diseases according to age at onset
The 150 cases of invasive GBS infection we collected between 2006 and 2011 are summarized in Table 1 according to the serotype of the GBS strain and the age at onset as follows: EOD, within 6 days after birth (n = 23, 15·3%); LOD, from 7 to 89 days after birth (n = 115, 76·7%); and from 90 days to 1 year (n = 12, 8·0%). GBS diseases are categorized as meningitis, sepsis (including a few bacteraemic cases), or other infections including purulent arthritis.
Table 1. Correlation between capsular serotypes and diseases according to age at onset
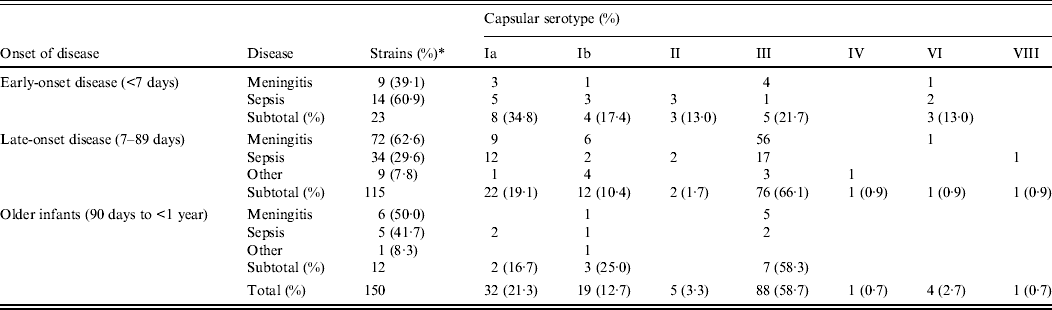
* Percentage of each subtotal.
In EOD cases, sepsis (60·9%) was more frequent than meningitis (39·1%), but in LOD cases, meningitis (62·6%) exceeded sepsis (29·6%) as well as other infections (7·8%). This difference between EOD and LOD was statistically significant (P = 0·011), while the findings of LOD did not differ significantly from those in older infants (P = 0·669).
Infants with low birth weight or underlying congenital diseases were substantially represented in both EOD cases (26·1%) and LOD cases (19·1%). Patients with outcomes of death and neurological sequelae, respectively, were 13·0% and 8·7% for EOD, and 0·9% and 7·0% for LOD.
Of the EOD cases, serotype Ia was most frequent (34·8%), followed by serotype III (21·7%) and serotype Ib (17·4%). In contrast, serotype III was most prevalent in LOD cases (66·1%) and in older infants (58·3%), especially for meningitis and sepsis. Serotype distribution differed significantly depending on the disorders in LOD (P < 0·001), but not in EOD (P = 0·213) or older infants (P = 0·206).
Of all tested strains, the most frequent serotype was III (58·7%), which was followed by Ia (21·3%) and Ib (12·7%); other types had lower incidences.
Association between serotype and MLST
Associations between capsular serotype and ST or clonal complex (CC) for all GBS strains were analysed by MLST using the GBS website. As shown in Table 2, strains were classified into 13 STs and five CCs. New STs identified in this study included ST144 and ST145, belonging to CC23; and ST335, belonging to CC19. Correlations between the serotype and ST were evident, such as serotype Ia with ST23, serotype Ib with ST10, and serotype III with ST17, ST19, and ST335.
Table 2. Relationships between clonal complex, sequence type, and serotype in 150 invasive GBS isolates
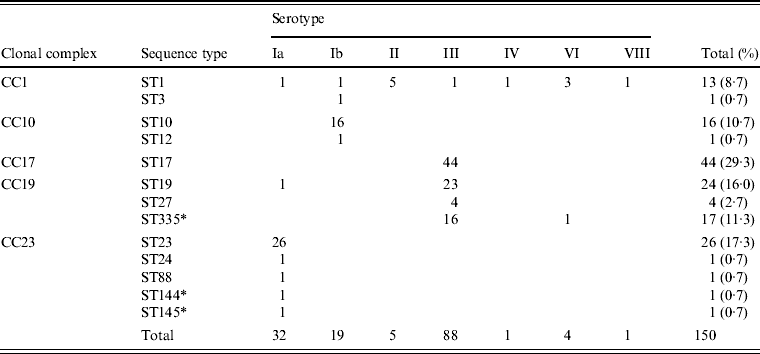
* New sequence types identified in this study.
Phylogenic relationship between ST and CC
Figure 1 is a dendrogram of STs based on the results of MLST using an unweighted pair-group method with average linkages (UPGMA). One half of serotype III isolates, identified as ST17 of CC17 (n = 44), represented known high-virulence strains; the remainder belonged to ST19, ST27, and ST335 of CC19 (n = 43), except for a single strain of ST1. Notably, CC17 was plotted near CC61 (ST61, ST76, ST91), a bovine-derived GBS strain.
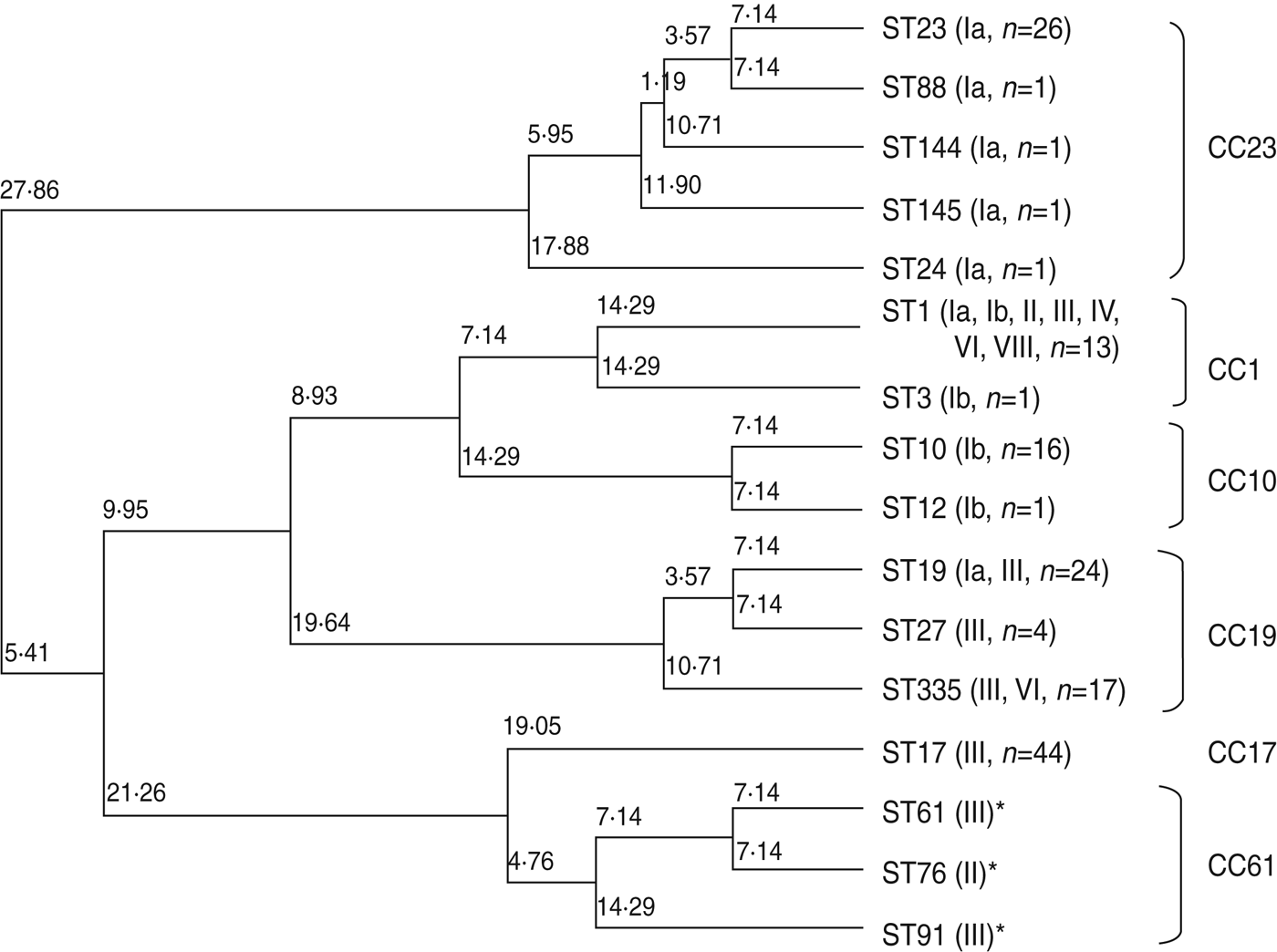
Fig. 1. Neighbour-joining analysis of allelic identities of 13 sequence types (STs) in clinical isolates of GBS and three reference bovine-derived strains. Numbers at nodes are the percentages of 1000 bootstrap replicates in which these nodes appeared. Only nodes with percentages exceeding 50% were included. * Known bovine-derived strains. CC, Clonal complex
Five STs, including the frequently occurring ST23, which belongs to CC23 in serotype Ia, clearly differed from other CCs. ST10 and ST12, accounting for most of serotype Ib, belonged to CC10. ST1, belonging to CC1, was distributed in seven serotypes.
Antimicrobial susceptibility and ML resistance
The minimum inhibitory concentration (MIC) range, MIC50–MIC90, for GBS of eight antimicrobial agents including three penicillins, cefotaxime, two carbapenems (MEM and PAM), VAN, and ERY are shown in Table 3. No β-lactam-resistant strains were identified. The MIC90 for ML-resistant strains was ⩾64 μg/ml.
Table 3. Susceptibility of invasive GBS isolates to eight antimicrobial agents
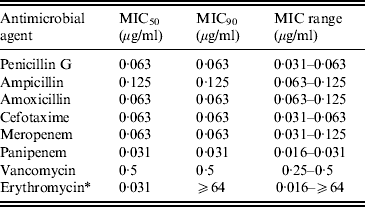
MIC, Minimum inhibitory concentration.
* Strains possessing macrolide resistance genes are as follows: erm(B), 8·7%; erm(A), 12·0%; mef(A/E), 1·3%.
Table 4 shows the correlation between the ML resistance gene, serotype, and ST. Strains possessing the erm(B) gene mediating high ML resistance, the erm(A) gene mediating inducible high ML resistance, and the mef(A/E) gene mediating intermediate ML resistance (2–16 μg/ml), were 8·7%, 12·0%, and 1·3%, respectively, totalling 22·0% of all strains. ML resistance was particularly prevalent in serotype Ia belonging to ST23 and in serotype III belonging to ST335, ST17, and ST19.
Table 4. Relationships between macrolide resistance gene, serotype, and sequence type
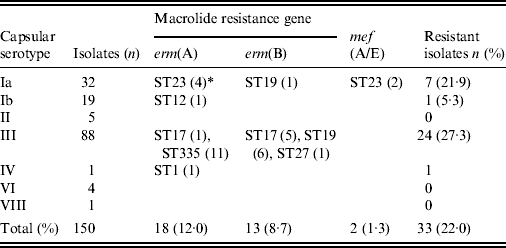
* Numbers of isolates with a given gene and sequence type are given in parentheses.
DISCUSSION
In 1996, guidelines for the prevention of neonatal GBS infections using antimicrobial prophylaxis first were issued in the USA [3]. The guidelines were subsequently revised to include universal screening for vaginal GBS colonization in pregnant women at 35–37 weeks gestation [Reference Schrag7]. Epidemiological studies later found that the recommendations had contributed to a decrease in EOD, but not in LOD [Reference Verani10].
In 2008, JSOG recommended universal GBS screening of pregnant women at 33–37 weeks gestation as well as intrapartum prophylaxis with an antimicrobial agent for GBS-positive cases [26]. The results of continued study of meningitis indicate that prophylactic intrapartum administration of antimicrobial agents after the introduction of the JSOG guidelines led to a decrease in the incidence of EOD, but the incidence of LOD remained unchanged [Reference Shinjoh27, Reference Sunakawa28].
In response to this problem, revised US guidelines were issued in 2010. The application of a rapid diagnostic test for GBS identification was added, and the antimicrobials used for intrapartum prophylaxis were changed [Reference Verani10]. In Japan, similar revised guidelines were also published in 2011 [29].
As previously reported [Reference Davies20, Reference Poyart30], our results also show that meningitis is overwhelmingly caused by capsular type III. GBS colonization of pregnant Japanese women in an advanced gestational stage was reported as 15% according to bacterial culture, but increased to 22% with real-time PCR [Reference Igarashi and Mitsuhashi24]. Capsular types V, Ia, and Ib were predominant; type III accounted for only 12% of colonization cases in Japan.
The ability of type III GBS to frequently cause meningitis has been attributed to neuraminidase activity [Reference Milligan31], the presence of Spbl surface proteins involved in adhesion to and invasion of epithelial cells by GBS [Reference Adderson32], fibrinogen receptor FbsA [Reference Schubert33], and pili that are involved in the invasion of brain microvascular endothelial cells [Reference Maisey34]. CspA serine protease-like surface protein has been reported to inactivate chemokines through its anti-phagocytotic action [Reference Bryan and Shelver35]. If strains of highly pathogenic capsular type can be rapidly identified, the prevention of neonatal GBS infections should be enhanced.
The use of multiplex PCR to rapidly detect capsular type has been reported by Poyart et al. [Reference Poyart17]. We also developed a real-time PCR method to identify GBS and capsular types. A rapid PCR method with high sensitivity and specificity is most useful as a screening to prevent neonatal GBS infections.
Relationships between capsular type, virulence, and MLST have been studied by many investigators [Reference Jones19–Reference Martins23, Reference Gherardi36, Reference Martins37]. The capsular type III strain of ST17 belonging to CC17 has been linked to high pathogenicity [Reference Jones19–Reference Martins23, Reference Gherardi36, Reference Martins37] as a high-virulence clone [Reference Al38, Reference Seifert39]. Our results showed that half of the capsular type III strains were ST17 and the remainder were ST19 or its new subtype, ST335, belonging to CC19. Based on MLST analysis, CC17 is placed near lineage CC61, a bovine pathogen [Reference Springman40]. Our results also placed CC61 near CC17 on the phylogenic tree. However, analysis of 15 housekeeping genes did not favour such a conclusion [Reference Sørensen41]. Although cps gene recombination or capsular switching has been suggested [Reference Gherardi36], it remains to be investigated whether such gene recombination occurs between human and bovine-derived GBS strains.
β-lactam-resistant GBS isolates have not explicitly been confirmed anywhere in the world. However, in 2008, Kimura et al. reported that penicillin-resistant GBS (PRGBS) strains possessing a few amino acid substitutions adjacent to conserved motifs of Ser-Ser-Asn or Lys-Ser-Gly in PBP2x had been isolated from adult sputum [Reference Kimura42]. The capsular types were Ib, III, VI, and VIII. The PRGBS of capsular type III, belonging to ST19, was also isolated from elderly patients in the USA [Reference Dahesh43].
CDC guidelines [Reference Verani10] have recently been revised, increasing the dose of PEN used for prophylaxis. From now on, it will be necessary to monitor the β-lactam susceptibility of GBS isolates – especially those derived from neonates. An additional important point is that 22·0% of our Japanese strains had genes mediating ML resistance. ML-resistant strains are increasing worldwide. When isolates from pregnant women who are allergic to penicillin are resistant to ML, VAN should be used for intrapartum antibiotic prophylaxis.
To control invasive neonatal GBS infection most effectively, the development of a GBS vaccine is an extremely important goal.
ACKNOWLEDGMENTS
Our study was partly funded by a grant under the category of ‘Research on Emerging and Re-emerging Infectious Diseases’ (no. H22-013) of the Japanese Ministry of Health, Labour and Welfare (to Dr K. Ubukata).
DECLARATION OF INTEREST
None.







