Introduction
Some of the greatest conservation concern for marine life arises among species whose habitat most overlaps with that of humans: the neritic species that inhabit shallow coastal zones facing multiple simultaneous pressures (Lotze et al., Reference Lotze, Lenihan, Bourque, Bradbury, Cooke and Kay2006; Crain et al., Reference Crain, Kroeker and Halpern2008, Reference Crain, Halpern, Beck and Kappel2009). Anthropogenic impacts on the oceans are often most concentrated here, including exploitation through industrial and artisanal fishing, climate change, coastal development, land-based effluents and pollution, shipping and recreational traffic, habitat destruction from fishing and aquaculture practices, sea-filling and dredging, coastal eutrophication, invasive species and sedimentation. Such pressures have direct impacts on habitats such as estuaries (Waltham & Connolly, Reference Waltham and Connolly2011), mangroves (Polidoro et al., Reference Polidoro, Carpenter, Collins, Duke, Ellison and Ellison2010), coral reefs (Carpenter et al., Reference Carpenter, Abrar, Aeby, Aronson, Banks and Bruckner2008), kelp forests (Krumhansl et al., Reference Krumhansl, Okamoto, Rassweiler, Novak, Bolton and Cavanaugh2016), sponges (Harasti et al., Reference Harasti, Martin-Smith and Gladstone2014), and seagrasses (Waycott et al., Reference Waycott, Duarte, Carruthers, Orth, Dennison and Olyarnik2009; Short et al., Reference Short, Polidoro, Livingstone, Carpenter, Bandeira and Bujang2011). Unsustainable fishing combined with habitat degradation and loss have a significant and often synergistic impact on fishes, and can lead to local extinctions and increased global risk (Dulvy et al., Reference Dulvy, Sadovy and Reynolds2003; Hutchings & Reynolds, Reference Hutchings and Reynolds2004; Reynolds et al., Reference Reynolds, Dulvy, Goodwin and Hutchings2005; Crain et al., Reference Crain, Kroeker and Halpern2008; Webb & Mindel, Reference Webb and Mindel2015).
The IUCN Red List of Threatened Species is the most widely used method for assessing the extinction risk of species. It has been published since 1964, with regular updates, and is used globally by governments, businesses, management agencies, international environmental agreements, and NGOs to inform conservation action. Its quantitative methods, which include explicit analyses of a species' intrinsic biological features and the pressures bearing on them, have been used to assess > 120,000 species (IUCN, 2020).
For each taxon, the first IUCN species assessments serve as baselines against which future reassessments will be compared. Such comparisons are used to produce the Red List Index, which measures aggregate genuine changes in the Red List category of species within a taxonomic group (Butchart et al., Reference Butchart, Stattersfield, Baillie, Bennun, Stuart and Akçakaya2005, Reference Butchart, Akçakaya, Chanson, Baillie, Collen and Quader2007, Reference Butchart, Walpole, Collen, van Strien, Scharlemann and Almond2010; Stuart et al., Reference Stuart, Wilson, McNeely, Mittermeier and Rodríguez2010). The Red List Index is used to highlight concerns about species that are declining in status and, conversely, highlight successes where conservation action has improved the status of species (Hoffmann et al., Reference Hoffmann, Hilton-Taylor, Angulo, Böhm, Brooks and Butchart2010, Reference Hoffmann, Duckworth, Holmes, Mallon, Rodrigues and Stuart2015).
Taxonomic comprehensiveness of the IUCN Red List and improvements in the Red List Index contribute directly to progress on several of the Aichi Biodiversity Targets (CBD, 2010) and UN Sustainable Development Goals (Aichi Targets 6 and 12 on sustainable fisheries and improving species conservation status, and UN Sustainable Development Goal 14 on conserving the oceans, respectively), and are essential for effective species conservation planning and action.
Some of the first marine fishes assessed on the IUCN Red List, in 1996, were the iconic seahorses (Hippocampus spp.). Apart from their intrinsic worth, flagship species such as seahorses have been shown to draw attention and finance towards conservation initiatives (Bowen-Jones & Entwistle, Reference Bowen-Jones and Entwistle2002; Walpole & Leader-Williams, Reference Walpole and Leader-Williams2002; Rodrigues & Brooks, Reference Rodrigues and Brooks2007; Shokri et al., Reference Shokri, Gladstone and Jelbart2009; Caro, Reference Caro2010; Bennett et al., Reference Bennett, Maloney and Possingham2015; Carrizo et al., Reference Carrizo, Jähnig, Bremerich, Freyhof, Harrison and He2017; Stiller et al., Reference Stiller, Wilson, Donnellan and Rouse2017).
Seahorses and their relatives are charismatic fishes that have garnered attention and reverence in human cultures for many centuries (Scales, Reference Scales2009; Lourie, Reference Lourie2016), and thus have the potential to help drive contemporary conservation efforts for coastal ecosystems (Shokri et al., Reference Shokri, Gladstone and Jelbart2009; Vincent et al., Reference Vincent, Foster and Koldewey2011; Stiller et al., Reference Stiller, Wilson, Donnellan and Rouse2017). Seahorses and many pipefishes are much sought after for traditional medicine, aquarium display and curios. A better understanding of the conservation status of these flagship animals will allow the mobilization of conservation action for a diversity of coastal ecosystems. The Syngnathiformes, as a group, have a circumglobal range that spans coasts everywhere except for polar regions. They live in an array of habitats, including estuaries, mangroves, seagrasses, coral and rocky reefs, kelp forests, sand or mud substrates, streams, lakes and rivers (Dawson, Reference Dawson1985; Foster & Vincent, Reference Foster and Vincent2004; Kuiter, Reference Kuiter2009; Lourie, Reference Lourie2016; De Brauwer et al., Reference De Brauwer, Harvey, McIlwain, Hobbs, Jompa and Burton2017). Effective conservation of syngnathiform fishes entails a range of conservation actions in coastal areas, many of which are under substantial human pressure (Halpern et al., Reference Halpern, Frazier, Potepenko, Casey, Koenig and Longo2015; McClanahan et al., Reference McClanahan, Allison and Cinner2015).
Here we synthesize assessments of the global extinction risk of the Syngnathiformes, drawing on individual species assessments based on the IUCN Red List Categories and Criteria (IUCN, 2020). This is the first time the order has been treated comprehensively, with only 18% of known species having been evaluated prior to 2017. These assessments will be of value in guiding conservation efforts and provide a baseline for a future Red List Index analysis of Syngnathiformes.
Methods
Taxa included in this study
The order Syngnathiformes (Class Actinopterygii) comprises five (formerly six) families of uniquely specialized fishes: Aulostomidae (trumpetfishes), Centriscidae (shrimpfishes), Fistulariidae (cornetfishes or flutemouths), Solenostomidae (ghost pipefishes), and Syngnathidae (seahorses, pipefishes and seadragons; Plate 1) (Longo et al., Reference Longo, Faircloth, Meyer, Westneat, Alfaro and Wainwright2017). Our phylogenetic and taxonomic paradigm for the order largely follows that of Nelson et al. (Reference Nelson, Grande and Wilson2016). We do not include in this assessment the Dactylopteridae (flying gurnards), Callionymidae (dragonets), Mullidae (goatfishes) or Pegasidae (seamoths) as evidence for their inclusion within the Syngathiformes is mixed (Kawahara et al., Reference Kawahara, Miya, Mabuchi, Lavoué, Inoue and Satoh2008; Wilson & Orr, Reference Wilson and Orr2011; Betancur-R. et al., Reference Betancur-R., Broughton, Wiley, Carpenter, López and Li2013; Near et al., Reference Near, Dornburg, Eytan, Keck, Smith and Kuhn2013; Song et al., Reference Song, Mabuchi, Satoh, Moore, Yamanoue, Miya and Nishida2014; Sanciangco et al., Reference Sanciangco, Carpenter and Betancur-R.2016). Longo et al. (Reference Longo, Faircloth, Meyer, Westneat, Alfaro and Wainwright2017) consider these groups to belong to a benthic-associated sister clade that is closely related to, but not a part of, the order Syngnathiformes. In addition, we do not include the Gasterosteiformes (sticklebacks), a distinct order that formerly included families now within the Syngnathiformes (Sanciangco et al., Reference Sanciangco, Carpenter and Betancur-R.2016; Longo et al., Reference Longo, Faircloth, Meyer, Westneat, Alfaro and Wainwright2017).
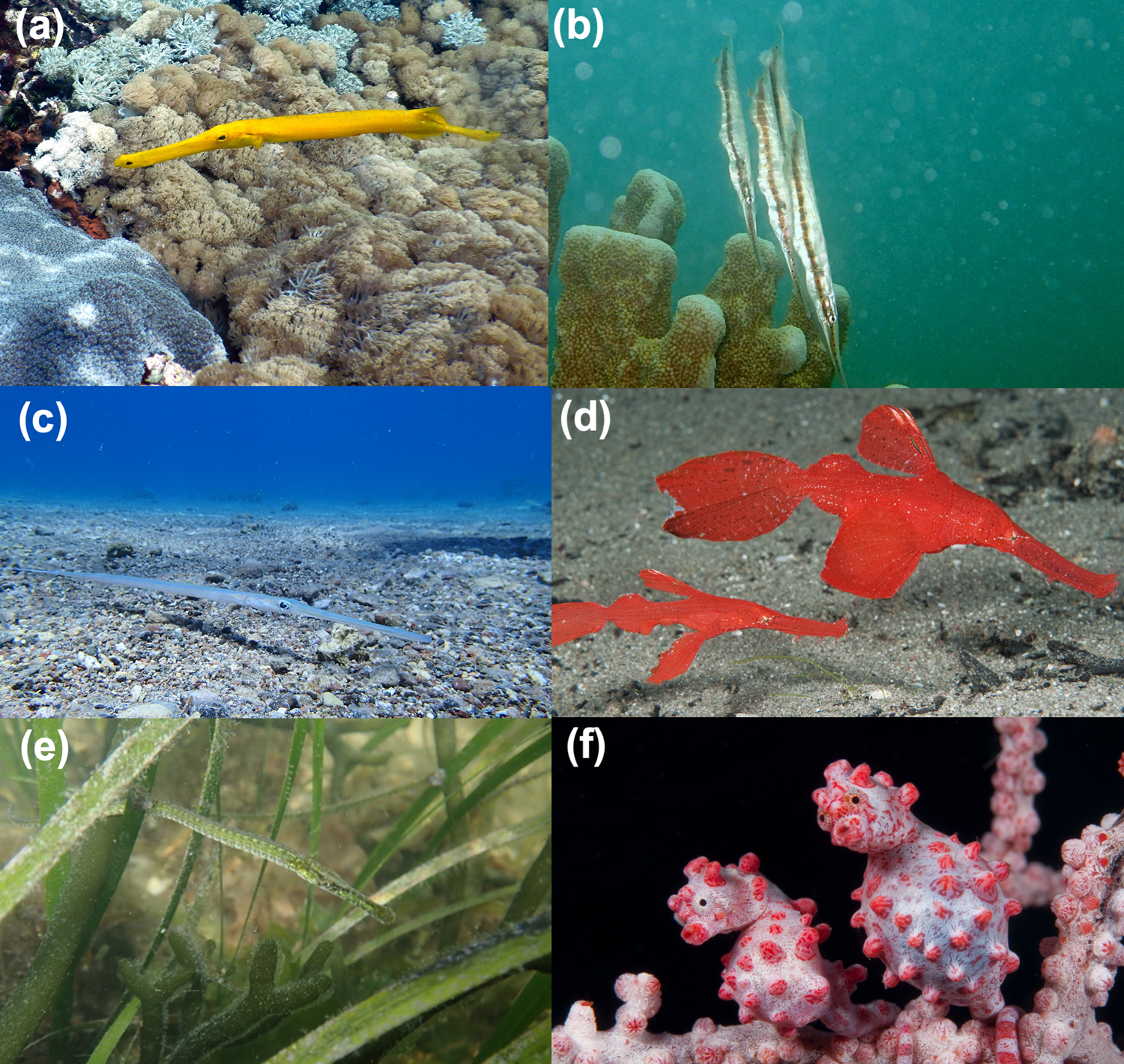
Plate 1 Representatives of all five extant syngnathiform families. (a) Aulostomidae: Pacific trumpetfish Aulostomus chinensis, northern Nusa Penida, Bali, Indonesia (bja2800dk/iNaturalist). (b) Centriscidae: speckled shrimpfish Aeoliscus punctulatus, Toliara, southwest Madagascar (Carmelo López Abad/iNaturalist). (c) Fistulariidae: bluespotted cornetfish Fistularia commersonii, northern Gulf of Aqaba (Rachel Andres-Beck/iNaturalist). (d) Solenostomidae: robust ghost pipefishes Solenostomus cyanopterus, Komodo, East Nusa Tenggara, Indonesia (Richard Smith/OceanRealmImages.com). (e) Syngnathidae: broadnosed pipefish Syngnathus typhle, Faro, Portugal (Carmen B. de los Santos/iNaturalist). (f) Syngnathidae. Bargibant's pygmy seahorse Hippocampus bargibanti, Wakatobi, Southeast Sulawesi, Indonesia (Richard Smith/OceanRealmImages.com).
We arrived at a final list of syngnathiform fishes that included 300 extant species at the time of project completion (early 2017; Supplementary Table 1). Of these, 258 are largely restricted to marine waters and 42 inhabit brackish and/or freshwater. Considerable taxonomic uncertainty remains within the order. We used morphological and/or molecular evidence to define valid species, and rejected putative species differentiated only by variations in colour pattern. During this research several new syngnathid species have been described and their Red List assessments are forthcoming (e.g. Short et al., Reference Short, Claassens, Smith, De Brauwer, Hamilton, Stat and Harasti2020). Comprehensive treatments of taxonomy are available for the Aulostomidae (Bowen et al., Reference Bowen, Bass, Rocha, Grant and Robertson2001) and the Syngnathidae at the generic level (Hamilton et al., Reference Hamilton, Saarman, Short, Sellas, Moore and Hoang2017). The most recent reviews of the majority of pipefish genera (Syngnathidae) were undertaken by Dawson (Poss & Heal, Reference Poss and Heal1994), and we used his subsequent book on the Indo-Pacific pipefishes to inform taxonomic decisions for the pipefishes in that region (Dawson, Reference Dawson1985). The genus Hippocampus (seahorses) was recently revised at the species level (Lourie et al., Reference Lourie, Pollom and Foster2016).
The IUCN Red List assessment process
Assessments for all species were carried out by the globally designated expert group for this taxon, the IUCN Species Survival Commission Seahorse, Pipefish and Seadragon Specialist Group. Project Seahorse, which acts as the core of this Specialist Group, carried out the assessments, with undergraduate volunteers assisting with data collection. We consulted experts, including fisheries and conservation biologists, government fisheries managers and members of the Specialist Group during the assessment process, and many are either assessors or reviewers of the species assessments. Information for the assessments was obtained from peer-reviewed and grey literature, online databases (Froese & Pauly, Reference Froese and Pauly2019; Fricke et al., Reference Fricke, Eschmeyer and van der Laan2020; GBIF, 2020; Biodiversity Heritage Library, 2021; Encyclopedia of Life, 2021), and from a diversity of experts, including fisheries managers, seahorse traders, biologists, citizen scientists and divers.
The evidence used to inform each assessment includes data on the species' taxonomy, geographical range, population size and trends in abundance, habitats and ecology (especially life history characteristics), anthropogenic threats, conservation actions (those that have been taken as well as those that are needed) and research needs. The latter two items are particularly important for follow-up conservation and research initiatives for threatened and Data Deficient species, respectively.
The IUCN Red List requires species to be assessed as Least Concern (species that are widespread and abundant), Data Deficient (insufficient data available to properly assess extinction risk), Near Threatened (close to qualifying for a threatened category), or threatened. Threatened species fall into one of three categories: Vulnerable (facing a high risk of extinction in the wild), Endangered (facing a very high risk of extinction in the wild), or Critically Endangered (facing an extremely high risk of extinction in the wild). Species can also be assessed as Extinct in the Wild or Extinct (IUCN, 2012). Species assessed as Data Deficient may or may not be of conservation concern, and further research and/or analyses are required to make a valid assessment of extinction risk. Species are assessed as Data Deficient if there are plausible threats but insufficient data to categorize the species reliably as threatened or Near Threatened. Species with no known threats can be assessed as Least Concern regardless of a lack of population data.
Species are assessed for inclusion in one of the IUCN Red List categories using a series of quantitative thresholds that are embedded within five Red List criteria (IUCN, 2012). These include (1) estimates of population size reduction (criterion A); (2) geographical range size (in the form of either extent of occurrence, EOO, or area of occupancy, AOO) and fragmentation or few locations, continuing decline and/or extreme fluctuation (criterion B); (3) small population size and continuing decline (criterion C); and (4) very small or restricted population (criterion D). Assessors can also use (5) quantitative analysis (criterion E) to project future risk of extinction (such as with a population viability analysis; Boyce, Reference Boyce1992; Frankham et al., Reference Frankham, Bradshaw and Brook2014), but as data were not sufficient to undertake this task for syngnathiformes, we did not use criterion E. IUCN regularly updates its Guidelines for Using the IUCN Red List Categories and Criteria as new scenarios of extinction and risk are encountered (Mace et al., Reference Mace, Collar, Gaston, Hilton-Taylor, Akçakaya and Leader-Williams2008; IUCN Standards and Petitions Subcommittee, 2017).
In our assessments we created species distribution maps by drawing a polygon around known occurrences and trimming this layer to a base data layer that extends to the 200 m depth contour or a 100 km buffer from the coastline, to encompass all of the coastal areas potentially inhabited by syngnathiform fishes (e.g. Sanciangco et al., Reference Sanciangco, Carpenter, Etnoyer, Moretzsohn and Duff2013; Comeros-Raynal et al., Reference Comeros-Raynal, Polidoro, Broatch, Mann, Gorman and Buxton2016). These maps are intended only for visualization on the IUCN Red List and represent the area along the coastline where a species occurs, rather than depicting the actual areas and depths inhabited by the species; this approach may exaggerate the extent of species' distribution in some areas and was not used for calculations of EOO to determine extinction risk under criterion B.
Once completed, assessments were then further reviewed by the IUCN Marine Biodiversity Unit, which conducts the Global Marine Species Assessment, and returned to us for revision. Finally, assessments went through a consistency check with the IUCN Red List Unit, before being published. All of these assessments took place during 2013–2017 and are available along with associated spatial data (IUCN, 2020).
Results
This study provides a first comprehensive synthesis of extinction risk for 300 species of syngnathiform fishes. Globally, 18 of these species are threatened with extinction but many more may also be at risk. A single species, Syngnathus watermeyeri, is assessed as Critically Endangered (0.3%), three as Endangered (1%), and 14 as Vulnerable (5%; Tables 1 & 2), and the most threatened species occur in the Indo West Pacific (Table 3). However, 97 species (32%) were assessed as Data Deficient, indicating the total number of Syngnathiformes that are threatened will almost certainly be higher than the currently known 6%. If all Data Deficient species are threatened, this would be 38% of syngnathiform species. If Data Deficient species are as threatened, proportionately, as species currently known to be threatened, then a mid-point of c. 7.9% of Syngnathiformes would be threatened. In addition, two species were assessed as Near Threatened. The remaining 183 species (61%) were assessed as Least Concern; for these species, there were either no known threats affecting the species, or reductions in population size that were not severe enough to meet thresholds under criterion A despite the presence of known threats. Overall, the primary threats to syngnathiform fishes are incidental capture in industrial trawl fisheries (marine species) and habitat loss and degradation through natural system modifications and pollution (freshwater species) (Fig. 1). Pollution is only a minor, secondary threat for the marine species assessed here.

Fig. 1 Threats affecting threatened syngnathiform fishes, with the number of species affected. Numbers correspond to the hierarchical IUCN, Threats Classification Scheme (IUCN, 2012). Most species are affected by multiple threats. Marine species are primarily affected by exploitation; fresh- and brackish-water species by habitat loss and degradation.
Table 1 Syngnathids assessed as threatened or near threatened, the regions they inhabit, and their Red List category and the criteria (IUCN, 2012) used for assessment.

1 ECA, Eastern Central Atlantic; ECP, Eastern Central Pacific; EIO, Eastern Indian Ocean; NWA, Northwest Atlantic; NWP, Northwest Pacific; SWA, Southwest Atlantic; SWP, Southwest Pacific; WCA, Western Central Atlantic; WCP, Western Central Pacific. FW, species that predominantly inhabit freshwater.
2 CR, Critically Endangered; EN, Endangered; VU, Vulnerable; NT, Near Threatened.
Table 2 Examples of syngnathiform fishes assessed in each of the IUCN Red List categories, with rationale.

1 CR, Critically Endangered; EN, Endangered; VU, Vulnerable; DD, Data Deficient; NT, Near Threatened; LC, Least Concern.
2 Surveys after we concluded our assessment have located the species (L. Claassens, unpubl. data, 2020).
Table 3 Number of syngnathiform species in each IUCN Red List category for all UN Food and Agriculture Organization (FAO) fishing areas and for Hippocampus, Microphis and freshwater species.

1 CR, Critically Endangered; EN, Endangered; VU, Vulnerable; DD, Data Deficient; NT, Near Threatened; LC, Least Concern.
The threatened and Data Deficient species were concentrated in two groups: the marine/estuarine seahorses (Hippocampus spp.) and the predominantly fresh/brackish-water pipefishes of the genus Microphis (Table 1). Fourteen of the 42 seahorse species (one-third) were assessed as threatened, and a maximum of 31 species could be threatened (two Endangered, 12 Vulnerable and 17 Data Deficient). Among the pipefishes, two of the 18 Microphis species were considered to be threatened, with a potential of nine species at risk (one Endangered, one Vulnerable and seven Data Deficient). All but one of the threatened and Near Threatened marine seahorses (14/15) are assessed as such under criterion A (population size reduction), with only one species considered threatened under criterion B (geographical range size). Three of five of the threatened or Near Threatened freshwater pipefishes were assessed as such under criterion B, and one of these species qualified under both criteria A and C (Table 1). Other genera for which data are not yet available but that may include threatened species are the Aeoliscus centriscids (2/2 species Data Deficient) and the pipefishes in Doryichthys (5/5), Minyichthys (3/4), Siokunichthys (6/6) and Solegnathus (4/5), and the speciose Syngnathus (15/32).
Discussion
This first comprehensive assessment of extinction risk in syngnathiform fishes shows that 39% of species are likely to require attention in the form of management and conservation intervention (for threatened and Near Threatened species) and/or further research (for Data Deficient species), and 61% are categorized as Least Concern. The most threatened species fall into two groups: (1) marine species under high pressure from fishing, and (2) species in fresh- and transitional waters facing habitat degradation. This pattern is common for aquatic species, with fishing being the biggest threat to generalist, wide-ranging marine taxa, and habitat degradation threatening specialist, endemic freshwater species (Arthington et al., Reference Arthington, Dulvy, Gladstone and Winfield2016).
For syngnathids, the first group of concern comprises a suite of marine species (most notably the seahorses) that are extracted primarily as bycatch in indiscriminate fisheries (although sometimes also targeted specifically), at levels that drive declines in the number of mature individuals (Perante et al., Reference Perante, Pajaro, Meeuwig and Vincent2002; Baum & Vincent, Reference Baum and Vincent2005; Meeuwig et al., Reference Meeuwig, Hoang, Ky, Job and Vincent2006; O'Donnell et al., Reference O'Donnell, Pajaro and Vincent2010; Perry et al., Reference Perry, Lunn and Vincent2010; Vincent et al., Reference Vincent, Foster and Koldewey2011). These declines are exacerbated by habitat loss through coastal development, destructive fishing practices and the impacts of climate change (Hughes et al., Reference Hughes, Williams, Duarte, Heck and Waycott2009; Vincent et al., Reference Vincent, Foster and Koldewey2011; Harasti, Reference Harasti2016). Habitat degradation and loss tend to worsen the effects of fishing pressure (e.g. Harasti, Reference Harasti2016). As is indicated by the predominance of the threatened species in this group being assessed as such under criterion A, marine seahorses suffer population declines from fishing pressure and habitat degradation, despite their sizeable ranges (with patchy populations).
The second group of concern comprises many fresh- and brackish-water species that are threatened or Data Deficient. Many of these species are restricted to a few rivers, watersheds or estuaries (Reynolds et al., Reference Reynolds, Dulvy, Goodwin and Hutchings2005; Kopf et al., Reference Kopf, Shaw and Humphries2017) and such systems are often among the most degraded and threatened from anthropogenic pressures (Dudgeon et al., Reference Dudgeon, Arthington, Gessner, Kawabata, Knowler and Lévêque2006; Carpenter et al., Reference Carpenter, Stanley and Vander Zanden2011). The often close proximity of people to rivers and lakes results in eutrophication, industrial and domestic pollution, damming and flow alteration, riparian housing and commercial developments, dredging and canalization (Carvajal-Quintero et al., Reference Carvajal-Quintero, Januchowski-Hartley, Maldonado-Ocampo, Jézéquel, Delgado and Tedesco2017). Syngnathiform fishes that are restricted to rivers and lakes tend to have smaller range sizes than their marine counterparts, and are thus exposed to multiple, often acute stressors. It is, therefore, perhaps not surprising that three of the five species of conservation concern in this group were recognized as threatened under criterion B. Only one freshwater pipefish is of concern as a result of population reduction (criterion A). The only seahorse assessed as threatened under criterion B is the Knysna seahorse Hippocampus capensis, which is associated with estuaries and restricted to a small area of South Africa. The most threatened syngnathiform fish is the freshwater pipefish Syngnathus watermeyeri, which occurs in the same region and faces similar threats; it is assessed as Critically Endangered under criterion C because of its small population size, a decline in the number of mature individuals, and extreme fluctuations.
Although threatened species occur in most regions, we note the higher numbers of threatened syngnathiform fishes in East and South-east Asia, a region with a high number of species that experiences intense fishing pressure and high demand for local and international trade (Table 3). The proportion of threatened species is also high in the South-east Atlantic. Despite this region's lack of diversity in syngnathiform fishes, a few species are threatened by restricted range size and estuarine degradation in South Africa.
Action is needed to determine the conservation status of the one-third of Syngnathiformes species that are currently assessed as Data Deficient. Although there are only limited data for all of the species assessed here, those species assessed as Data Deficient are of special concern. We generally assessed species as Least Concern when we could not identify major threats to the species, even when the available data were poor. We labelled species as Data Deficient when they were little studied and (1) subject to threats that may be causing declines (potential to be assessed as threatened under criterion A upon further investigation), or (2) their range could be small enough for the species to qualify as threatened under a restricted-range criterion (criteria B and D2) but they are under-surveyed, rare, and/or hard to detect. We can infer that some Data Deficient species are likely to be threatened. For example, the threats to the freshwater Doryichthys species assessed as Data Deficient are similar to those affecting the freshwater Microphis species that we know to be threatened (riparian habitat degradation, pollution and flow diversion), and their life history is comparable. Other Data Deficient species are assessed as such because little was known about the level of mortality that fisheries cause across their range. Research is urgently needed to properly evaluate the conservation status of the Data Deficient species; work could also be done to predict the status of Data Deficient species in the absence of additional data (Bland et al., Reference Bland, Bielby, Kearney, Orme, Watson and Collen2017; Kindsvater et al., Reference Kindsvater, Dulvy, Horswill, Juan-Jordá, Mangel and Matthiopoulos2018; Zhang & Vincent, Reference Zhang and Vincent2019).
The Syngnathiformes fall towards the middle of the extinction risk spectrum compared to other orders of fishes (Supplementary Fig. 1). It is likely their life history traits (i.e. low fecundity, extreme parental care and high site fidelity) make them more susceptible to threats than broadcast spawners such as the sardines and herrings (Clupeiformes) or the cods (Gadiformes). On the other hand, some of the larger, less fecund species such as the sawfishes (Pristidae), angel sharks (Squatiniformes), and sturgeons (Acipenseriformes) have life histories that make them more susceptible to threats, in particular targeted fisheries and bycatch.
Our assessment suggests that only 60% of species are secure (i.e. Least Concern). The vast capture of seahorses, which supplies trade for traditional medicines, aquarium display and souvenirs, is a cause for concern. Our assessments provide a baseline against which to compare future assessments, which will eventually facilitate the determination of an IUCN Red List Index for this group. Because of the high number of species assessed as Data Deficient and the absence of genuine shifts in status for species that had previously been assessed, the calculation of the Index is not currently feasible.
Two classes of remedial action are needed for syngnathids: limiting fishing pressure and protecting habitat, with monitoring and evaluation. The IUCN Red List flags species that require attention and helps in setting priorities. Although it has no automatic implications for management and policy, the Red List provides information for managers and policy-makers. In this context, periodic reassessment of species will facilitate development of a Red List Index for the taxon, and for assessments under the new IUCN Green Status of Species, the criteria of which are used to measure conservation legacy and recovery potential (Akçakaya et al., Reference Akçakaya, Bennett, Brooks, Grace, Heath and Hedges2018).
For the seahorses affected by fishing, effective conservation will require a concerted international effort to reduce exploitation in both non-selective and targeted fisheries, along with efforts to mitigate the effects of these fisheries on syngnathid habitats. A particular focus should be on curtailing bottom trawling; its indiscriminate and destructive nature leads to the extraction of tens of millions of seahorses annually and the destruction of benthic habitat (Lawson et al., Reference Lawson, Foster and Vincent2017). Spatial and temporal fisheries closures are key to avoiding such destructive fishing (Dunn et al., Reference Dunn, Boustany and Halpin2011; Dichmont et al., Reference Dichmont, Ellis, Bustamante, Deng, Tickell and Pascual2013), with a particular focus on establishing well-implemented zones or marine protected areas that exclude trawling. Regulating target fisheries for seahorses will require dialogue with small-scale and subsistence fishers. An adaptive management framework that emphasizes learning and refinement would be appropriate in this regard (Walters, Reference Walters2007).
For the seahorses and pipefishes affected by habitat destruction, the focus needs to be on reducing and ameliorating destruction and degradation of critical habitats such as seagrasses, sponges and corals. For the freshwater and estuarine pipefishes, in particular, conservation requires a combination of naturalizing flow regimes, addressing point-source pollution and nutrient influx, managing riverine and coastal development, and appropriately siting protected areas established within a whole-watershed framework. Such work is already underway in some areas, for example, in the Knysna Estuary, South Africa (de Villiers et al., Reference de Villiers, Barker, Claassens and Hodgson2019; Claassens et al., Reference Claassens, Barnes, Wasserman, Lamberth, Miranda, van Niekerk and Adams2020).
It is vital to evaluate the impact of all conservation actions, and to modify them responsively. Robust long-term monitoring programmes are needed to evaluate population dynamics, fisheries, trade and habitat quality. Dedicated coastal surveys are needed, especially for those species with small ranges. Such surveys should occur over different seasons and times of day, and should start in localities with confirmed specimens or sightings, working outwards to adjacent areas with suitable habitat. All must be controlled for effort, whether hours invested or distance surveyed. Species that are not currently assessed as threatened should also be monitored closely to ensure that known pressures of exploitation and habitat damage—married to ecological shifts arising from the increasingly urgent threat resulting from climate change—do not lead them to become threatened. Community science is a valuable tool that can and is assisting in this endeavour (Haywood et al., Reference Haywood, Parrish and Dolliver2016; Project Seahorse, unpubl. data, 2019).
Conservation action for syngnathiform fishes has the potential to benefit other species (Rodrigues & Brooks, Reference Rodrigues and Brooks2007; Shokri et al., Reference Shokri, Gladstone and Jelbart2009). Limiting fishing mortality, in particular by constraining bottom trawling and other non-selective fisheries, and ensuring healthy habitats is important both for the syngnathids and for other aquatic species. Given that the order is nearly global, there is potential for syngnathiformes, many of which are highly charismatic, to act as flagship species for ocean conservation.
Acknowledgements
This is a contribution from Project Seahorse. We thank colleagues at Project Seahorse, the members of the IUCN Species Survival Commission Seahorse, Pipefish and Seadragon Specialist Group, and the IUCN Red List Unit for their insights and assistance. Lily Stanton sourced photographs, created figures and reviewed this research. Richard Smith (OceanRealmImages.com) and iNaturalist users provided photographs. David Harasti, Nuno Monteiro, Nick Dulvy and an anonymous reviewer critiqued the text. We thank the many volunteers and students at The University of British Columbia who helped gather and collate the information to drive these assessments: Daphne Austin, Lindsay Aylesworth, Claire Cameron, Cody Carlisle, Tíng-Chūn Kūo, Sarina Clay-Smith, Katelyn Dick, Hannah Fiegenbaum, Kyle Gillespie, Courtney Graham, Olivia Jamieson, Julia Lawson, Clayton Manning, Liria Nair, Thea Rachinski, Tanvi Vaidyanathan and Xiong Xhang. We thank the IUCN Red List Committee and Synchronicity Earth for financial support. Project Seahorse is supported by Guylian Chocolates (Belgium) in a partnership for marine conservation.
Author contributions
Study design, data collection: RAP, ACJV; review and publication of assessments, data analysis, writing: all authors.
Conflicts of interest
None.
Ethical standards
This research abided by the Oryx guidelines on ethical standards. There were no human subjects, experimentation with animals, or collection of specimens associated with this work.







