There is a growing worldwide epidemic of obesity. It affects men and women, young and old. For example, under the current US military's recommended enlistment standard for BMI (defined as weight in kilograms divided by the square of height in meters) 40 % of young women and 25 % of young men in the USA are not eligible due to being overweight(Reference Sackett, and Mavor1). Women of reproductive age have been especially susceptible. In 1999–2002, 62 % of US women aged 20 years or older were overweight (defined as having a BMI > 25 kg/m2) and one-third were obese (BMI ≥ 30 kg/m2)(Reference Hedley, Ogden, Johnson, Carroll, Curtin and Flegal2, Reference Moore3). Obesity in adolescence also is an increasing concern; 15 % of girls aged 12–19 years were overweight (defined as having a BMI ≥ the 95th percentile for age according to the Centers for Disease Control growth charts)(Reference Hedley, Ogden, Johnson, Carroll, Curtin and Flegal2).
What is amazing, and frightening, is how quickly this change in human body weight is occurring. Within a few generations the bell-curve of human-weight distribution has shifted and become skewed toward greater weight. The median-weight individual of today would have been considered to be heavier than average only a short time ago and there are more extremely obese individuals. This trend would appear to be continuing(Reference Hedley, Ogden, Johnson, Carroll, Curtin and Flegal2). The rapidity with which the incidence of obesity has increased worldwide suggests that genetic change on a population level is an unlikely cause, although assortative mating, the increased probability that individuals are more likely to marry other individuals with similar BMI, could play some role, both genetic and environmental(Reference Hebebrand, Wulftange, Goerg, Ziegler, Hinney, Barth, Mayer and Remschmidt4). Technological change, culture and socio-economic factors certainly play important roles in the change in human adiposity. However, whatever underlying genetic and biological factors that are contributing to significant numbers of individuals to be obesity-prone in the modern environment probably have been extant in our species for a considerable time.
Although men and women are both susceptible to obesity, the incidence and health consequences differ between the sexes. Men and women differ in the patterns of fat deposition, fat mobilisation, utilisation of fat as a metabolic fuel, and the consequences of both excess and insufficient fat stores. Many of these differences may reflect evolved adaptive differences that stem from the differences in male and female reproductive costs. Reproduction is more nutritionally expensive for women than it is for men. The costs of gestation and lactation dwarf male reproductive effort. This asymmetry in reproductive cost is reflected in the asymmetry in fat storage and in the utilisation of fat as fuel.
In the present review we examine differences in fat storage and metabolism between men and women and the ways in which those differences might underlie the differences in incidence and types of obesity experienced by men and women. This topic has been recently reviewed by othersReference Woods, Gotoh and Clegg(5Reference Williams6Reference Mittendorfer7. The novel aspect of our paper is that we approach these topics from the perspective of evolutionary biology. We hypothesise that many of the characteristics that predispose individuals to weight gain derive from adaptive forces in our past. Other characteristics may have been selectively neutral, due to the infrequency with which the obesity phenotype was expressed in the past, and thus may have accumulated in our lineage via genetic drift. We propose that modern obesity can be explained as evolutionary adaptive (or neutral) responses that in the modern environment result in maladaptive physiological responses. We further propose that many of the differences between men and women in the propensity to obesity and the associated health consequences are reflections of the different adaptive pressures that have shaped male and female biology.
Sex differences in adiposity
Women and men differ in the proportion of body fat and in how that fat is distributed. These differences begin early in life, and are further strengthened during puberty. These differences stem from metabolic and hormonal differences between the sexes, and contribute to differences between women and men in health risks attributable to obesity.
Women have greater adipose stores than men, even after correcting for BMI. This is true for all races and all cultures. Indeed, the mean percentage of body fat for normal-weight women (BMI 18–25 kg/m2) is similar to the percentage body fat of men who are classified as obese (BMI >30 kg/m2) (Fig. 1) (Reference Nielson, Guo, Johnson, Hensrud and Jensen8). This sex difference in adiposity is present at birth. Female babies have more subcutaneous fat than do male babies for all gestational ages(Reference Rodríguez, Samper, Olivares, Ventura, Moreno and Pérez-González9). Prepubertal girls have more fat in their legs and pelvis than do prepubertal boys(Reference He, Horlick, Thornton, Wang, Pierson, Heshka and Gallagher10).
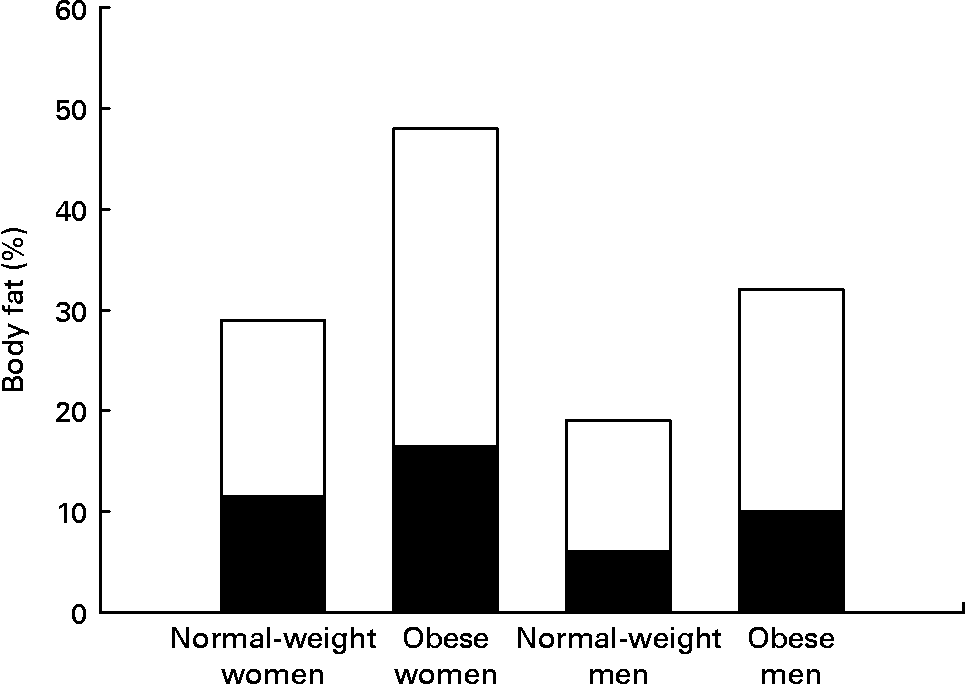
Fig. 1 Women have both higher total percentage body fat and a greater proportion of fat in legs than do men at all BMI values. Normal-weight men and women, BMI < 25 kg/m2; obese men and women, BMI > 30 kg/m2. (■), Leg fat; (□), other fat. Data from Nielson et al.(Reference Nielson, Guo, Johnson, Hensrud and Jensen8).
Body fat is distributed differently between men and women (Figs. 1 and 2). Women have greater adipose stores in thighs and buttocks(Reference Nielson, Guo, Johnson, Hensrud and Jensen8); men tend to be more likely to have significant amounts of abdominal fat, and to be more susceptible to abdominal adiposity(Reference Nielson, Guo, Johnson, Hensrud and Jensen8). Women have larger stores of subcutaneous fat; men are more likely to have visceral fat(Reference Lemieux, Prud'homme, Bouchard, Tremblay and Deprés11). All of this is a matter of degree. Obese women will have large amounts of visceral fat (Fig. 2); obese men will have large amounts of subcutaneous fat on their legs (Fig. 1).

Fig. 2 Women have a greater proportion of their abdominal fat in subcutaneous depots compared with men; men have significantly more visceral fat at all values of BMI. Obese men and women, BMI > 30 kg/m2. (■), Visceral fat area; (□), abdominal subcutaneous fat area. Data from Nielson et al.(Reference Nielson, Guo, Johnson, Hensrud and Jensen8).
Waist circumference is a significant risk factor for the co-morbidities of obesity. Waist circumference in men and women is significantly associated with abdominal subcutaneous and visceral fat; however, the relationships differ significantly between the sexes. The regression lines of waist circumference against subcutaneous abdominal fat for men and women are parallel; however, women have on average 1·8 kg more subcutaneous abdominal fat than men for any given waist circumference(Reference Kuk, Lee, Heymsfield and Ross12). In contrast, the slope of the regression line of waist circumference against visceral fat is significantly greater for men than for women(Reference Kuk, Lee, Heymsfield and Ross12). Age and menopausal status also have significant effects on the relationships between waist circumference and visceral fat. Older men and women have significantly higher regression slopes than do their younger counterparts. The slopes of the regression lines for men are greater than for women standardised to any age; however, the standardised slope for 40-year-old women is the same as the standardised slope for 25-year-old men. The slope for menopausal women is greater than the slope for premenopausal women, and approaches the male pattern(Reference Kuk, Lee, Heymsfield and Ross12).
Central v. peripheral obesity
‘Not all fat is alike’(Reference Arner13). Central or abdominal obesity, excess adipose tissue in the abdominal area, is associated with higher risks of co-morbid disease states, such as type 2 diabetes, hypertension, dyslipidaemia and CVD in both men and women(Reference Racette, Hagberg, Evans, Holloszy and Weiss14Reference Goodpaster, Krishnaswami, Harris, Katsiaras, Kritchevsky, Simonsick, Nevitt, Holvoet and Newman15Reference Van Pelt, Evans, Schechtman, Ehsani and Kohrt16Reference Karelis, St-Pierre, Conus, Rabasa-Lhoret and Poehlman17. For example, abdominal obesity was found to be the strongest predictor of insulin resistance among men and women aged over 50 yearsReference Racette, Hagberg, Evans, Holloszy and Weiss(14). Lower body adiposity is associated with a less unhealthy metabolic profile. Overweight and obese women and obese men who had a higher proportion of fat in subcutaneous thigh adipose tissue were significantly less likely to display symptoms of the metabolic syndrome(Reference Goodpaster, Krishnaswami, Harris, Katsiaras, Kritchevsky, Simonsick, Nevitt, Holvoet and Newman15). Obese individuals with mostly peripheral fat, distributed in subcutaneous depots in the glutealfemoral region, are at lower risk of the common co-morbidities of obesity than are obese individuals with a large proportion of their fat in intra-abdominal depots(Reference Van Pelt, Evans, Schechtman, Ehsani and Kohrt16).
Although the accumulation of subcutaneous fat in the lower body might represent a healthier regulation of fat stores compared with abdominal fat, excess adipose tissue is still associated with poor health outcomes. Metabolically healthy obese individuals may be less at risk than other obese individuals, but they still appear to be more at risk than the general population(Reference Karelis, St-Pierre, Conus, Rabasa-Lhoret and Poehlman17).
Abdominal fat mainly consists of visceral and subcutaneous adipose tissue; the proportions of fat between these depots differ between men and women, and also differ among racial and ethnic groups. The metabolic and health consequences appear to differ as well. Visceral fat is associated with a greater likelihood of adverse health conditions(Reference Racette, Hagberg, Evans, Holloszy and Weiss14, Reference Karelis, St-Pierre, Conus, Rabasa-Lhoret and Poehlman17), although excess subcutaneous abdominal fat has been implicated in poor glucose regulation(Reference Garg18, Reference Jensen19).
Visceral fat is found within the peritoneal cavity. Many authors have suggested that visceral adipose tissue differs from subcutaneous fat in ways that increase the health risks of obesity. Excess visceral fat is a significant risk factor for the metabolic and health complications of obesity(Reference Racette, Hagberg, Evans, Holloszy and Weiss14, Reference Karelis, St-Pierre, Conus, Rabasa-Lhoret and Poehlman17, Reference Fujioka, Matsuzawa, Tokunaga and Tarui20). About 20 % of obese men and women have metabolically healthy profiles. These individuals generally have significantly smaller proportion of adipose tissue as visceral fat(Reference Karelis, St-Pierre, Conus, Rabasa-Lhoret and Poehlman17). There are also men and women who exhibit the opposite phenotype: normal in weight but exhibiting a metabolically ‘obese’ profile. These individuals have a higher fat mass than would be predicted from their BMI, but also a higher proportion of adipose tissue as visceral fat(Reference Karelis, St-Pierre, Conus, Rabasa-Lhoret and Poehlman17). A higher proportion of fat as visceral adipose tissue was a significant risk factor for the metabolic syndrome (insulin resistance, dyslipidaemia and hypertension) in older men and women, even among those of normal weight(Reference Goodpaster, Krishnaswami, Harris, Katsiaras, Kritchevsky, Simonsick, Nevitt, Holvoet and Newman15).
There are two main, non-exclusive hypotheses why visceral fat has more unhealthy consequences. One suggests that adipokine (for example, leptin, IL-1, IL-6, TNF-α, or adiponectin) secretion by visceral fat differs from subcutaneous fat, and that these differences underlie the different risks to health(Reference Karelis, St-Pierre, Conus, Rabasa-Lhoret and Poehlman17). Although the secretion of some adipokines has been shown to differ between visceral and subcutaneous fat (for example, less leptin from visceral fat) there are few data to assess the health consequences. The other hypothesis is based on the fact that NEFA released by much (but not all) visceral fat go directly into the portal vein. Thus large amounts of visceral fat will result in the liver being exposed to a greater concentration of NEFA than would be predicted from systemic NEFA availability. The contribution of visceral adipose tissue to hepatic NEFA delivery increases with the amount of visceral fat in both men and women(Reference Nielson, Guo, Johnson, Hensrud and Jensen8). Liver fat has been shown to be associated with poor glucose control and higher concentrations of NEFA(Reference Seppälä-Lindroos, Vehkavaara, Hakkinen, Goto, Westerbacka, Sovijarvi, Halavaara and Yki-Jarvinen21). Visceral fat is suggested to play a significant role in hepatic insulin resistance(Reference Bergman, Kim, Catalano, Hsu, Chiu, Kabir, Hucking and Ader22); however, some have questioned its importance for overall systemic insulin resistance, noting that visceral adipose tissue contributes a small proportion of total systemic NEFA. These authors point to abdominal subcutaneous fat as the major source of circulating NEFA(Reference Garg18, Reference Jensen19, Reference Koutsari and Jensen23).
Interestingly, not only do men on average have a greater proportion of fat as visceral fat, it would appear that turnover of visceral fat is higher in men compared with women. Men have consistently been shown to have greater rates of both fatty acid release (lipolysis) and fatty acid uptake (lipogenesis) in visceral fat compared with women(Reference Williams6). Adrenergic stimulation increases splanchnic fatty acid release in men but not in women(Reference Jensen, Cryer, Johnson and Murray24). Thus, not only are men more susceptible to excess visceral fat, the effects of visceral fat on health may differ between the sexes as well.
Visceral fat is associated with dysregulation of cortisol production and metabolism. Cushing's syndrome, in which there is adrenal hypersecretion of cortisol, is associated with increased visceral fat. Conversely, women with visceral obesity (but not suffering from Cushing's syndrome) are more sensitive to a corticotropin-releasing hormone challenge than are normal-weight women or obese women with excess glutealfemoral fat as opposed to visceral obesity(Reference Pasquali, Cantobelli, Casimirri, Capelli, Bortoluzzi, Flamia, Labate and Barbara25). Urinary excretion of cortisol and its metabolites is increased in women with excessive visceral adipose tissue(Reference Pasquali, Cantobelli, Casimirri, Capelli, Bortoluzzi, Flamia, Labate and Barbara25).
There appear to be racial differences in the susceptibility to acquiring visceral fat. Asians have higher percentage body fat for any given BMI than do Caucasians or individuals of sub-Sahara African descent(Reference Deurenberg, Deurenberg-Yap and Guricci26), with a greater proportion of fat in visceral adipose tissue(Reference Park, Allison, Heymsfield and Gallagher27, Reference Yajnik28). Obese postmenopausal African-American women have less visceral fat for any given BMI than do postmenopausal Caucasian women, but a higher proportion of subcutaneous abdominal fat(Reference Conway, Yanovski, Avila and Hubbard29, Reference Tittelbach, Berman, Nicklas, Ryan and Goldberg30). Young African-American men and women have less visceral adipose tissue on average than do their Caucasian counterparts, despite African-American women generally having higher total fat(Reference Cossrow and Falkner31). Interestingly, African-Americans and Caucasians differ in their susceptibility to different aspects of the metabolic syndrome, with Caucasians more likely to express dyslipidaemia (for example, unfavourable cholesterol pattern and high TAG) while African-Americans appear more susceptible to dysregulation of glucose metabolism(Reference Cossrow and Falkner31).
Fat metabolism
Fat metabolism in women and men differs in a number of ways consistent with the differences in body fat percentage and distribution between men and women. Women appear to be metabolically inclined to store fat more so than are men. Interestingly, women also appear to utilise fat as an energy substrate during periods of sustained exertion more so than do men.
At rest, women shunt more circulating NEFA into re-esterification pathways than do men(Reference Nielsen, Guo, Albu, Klein, O'Brien and Jensen32). Women have higher VLDL-TAG production rates than men, but similar circulating concentrations(Reference Mittendorfer7). This is further evidence that women have higher rates of re-esterfication and thus reuptake of NEFA into adipose tissue than do men. In the basal condition, women are physiologically adapted to store fat more so than are men.
The rates of fatty acid uptake and release depend on the type of adipose tissue as well as differing between men and women, and this is reflected in the differing patterns of fat deposition between men and women. Women have higher rates of fat uptake into leg fat depots than do men(Reference Votruba and Jensen33). Rates of fatty acid release from abdominal adipose tissue are higher in women than men, but they are lower from gluteal or femoral adipose tissue(Reference Williams6). After feeding, fatty acid uptake is higher in abdominal adipose tissue relative to gluteal or femoral in both men and women. However, in women the majority of fatty acid uptake in abdominal adipose tissue is into subcutaneous fat, while in men a larger proportion goes into visceral fat(Reference Williams6). These findings are consistent with women being more likely to store fat subcutaneously and preferentially in the gluteal and femoral regions compared with men.
Women have higher rates of fat oxidation than men during sustained bouts of increased energy expenditure, such as endurance training. Men are more likely to up regulate glucose and amino acid metabolism during sustained exercise bouts(Reference Lamont, McCullough and Kalhan34, Reference Lamont35). The difference is associated with oestrogen. Giving exogenous oestrogen to males decreases carbohydrate and amino acid metabolism during exercise, and increases fat oxidation(Reference Hamadeh, Devries and Tarnopolsky36). Thus it would appear that women are more physiologically geared to use fat as a metabolic fuel under conditions of sustained increased demand, while men rely more on glucose and protein metabolism.
Effects of sex hormones on fat deposition and metabolism
The gonadal hormones affect adipose tissue metabolism, and appear to play significant roles in the resulting distribution and consequences of stored fat. Testosterone acts to increase lipolysis, inhibit lipoprotein lipase activity, and decrease TAG accumulation in adipose tissue. Lowering circulating testosterone levels in healthy young men increases total adipose tissue, with the largest percentage increase occurring in subcutaneous adipose tissue; raising circulating testosterone decreases total adipose tissue(Reference Woodhouse, Gupta, Bhasin, Singh, Ross, Phillips and Bhasin37). Oestrogens play multiple roles in the regulation of adipose tissue, in both men and women. Oestradiol has direct effects on adipose tissue, and also acts centrally to affect food intake and energy expenditure. Androgens appear to block proliferation and differentiation of preadipocytes(Reference Singh, Artaza, Taylor, Braga, Yuan, Gonzalez-Cadavid and Bhasin38). Oestradiol enhances proliferation of preadipocytes from both men and women in vitro(Reference Anderson, McTernan, Barnett and Kumar39). The effect was greater in preadipocytes from females compared with those from males.
Oestradiol favours the deposition of subcutaneous fat; lack of oestrogen in women leads both to weight gain, and a larger proportion of fat gain in visceral fat. Menopausal women have higher visceral fat mass than do premenopausal women for the equivalent percentage body fat(Reference Tchernof, Desmeules, Richard, Laberge, Daris, Mailloux, Rheaume and Dupont40). Oestradiol-treated postmenopausal women have lower lipoprotein lipase activity(Reference Pedersen, Kristensen, Hermann, Katzenellenbogen and Richelsen41).
Adipose tissues express both androgen and oestrogen receptors. Visceral fat has higher levels of androgen and oestrogen receptors than does subcutaneous fat, and this is true for both men and women(Reference Rodriguez-Cuenca, Monjo, Proenza and Roca42). Both the α and β oestrogen receptors are found in adipose tissue(Reference Pedersen, Kristensen, Hermann, Katzenellenbogen and Richelsen41). In subcutaneous fat, oestradiol acts through the α receptor to up regulate α2A-adrenergic receptors which results in decreased lipolysis. In contrast, oestradiol does not appear to affect the concentration of α2A-adrenergic receptors in adipocytes from visceral fat(Reference Pedersen, Kristensen, Hermann, Katzenellenbogen and Richelsen41). Subcutaneous adipocytes from premenopausal women have higher α2A-adrenergic receptor density and lower lipolytic activity in response to adrenaline than do subcutaneous adipocytes from men(Reference Richelsen43).
Adipose tissue as an endocrine organ
Adipose tissue is far more metabolically active than was once believed(Reference Kershaw and Flier44). Adipose tissue serves as an endocrine organ, producing leptin and many other regulatory peptides (Table 1). Adipose tissue is a source of steroids, either stored or metabolically converted from precursors. For example, oestrone is converted to oestradiol and androstendione is converted to testosterone in adipose tissue (Table 1). Indeed, most if not all circulating oestradiol in postmenopausal women comes from their adipose tissue(Reference Kershaw and Flier44). Interestingly, in rats adipose tissue from males had higher concentrations of testosterone, but did not differ in oestrogen concentration compared with adipose tissue from females(Reference Rodriguez-Cuenca, Monjo, Proenza and Roca42). Thus adipose tissue appears to regulate the local oestrogen environment somewhat independently from the gonads.
Table 1 A partial list of fat-derived peptides and steroid hormone-converting enzymes

Adipose tissue expresses 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1), which converts cortisone to cortisol and 5α-reductase enzymes which convert cortisol to 5α-tetrahydrocortisol. Thus adipose tissue regulates the local concentrations of glucocorticoids, and contributes to metabolic clearance of glucocorticoids(Reference Andrew, Phillips and Walker45, Reference Rask, Walker, Söderber, Livingstone, Eliasson, Johnson, Andrew and Olsson46).
Obesity is associated with both increased adrenal glucocorticoid production and higher glucocorticoid metabolic clearance, which appears to result in normal plasma concentrations. In obese individuals, 11β-HSD1 activity is reduced in liver and the inactivation of cortisol by 5α-reductase is enhanced(Reference Andrew, Phillips and Walker45Reference Rask, Walker, Söderber, Livingstone, Eliasson, Johnson, Andrew and Olsson46Reference Stewart, Boulton, Kumar, Clark and Shakleton47. However, 11β-HSD1 activity is enhanced in adipose tissue of both obese men and womenReference Rask, Walker, Söderber, Livingstone, Eliasson, Johnson, Andrew and Olsson(46, Reference Rask, Olsson, Söderber, Andrew, Livingstone, Johnson and Walker48). Thus, obese individuals have increased hepatic inactivation of cortisol, which is generally balanced by increased regeneration of cortisol in adipose tissue. Production of cortisol from cortisone via 11β-HSD1 can make a significant contribution to both local and circulating cortisol concentrations(Reference Rask, Walker, Söderber, Livingstone, Eliasson, Johnson, Andrew and Olsson46). The effect appears stronger in women compared with men(Reference Rask, Walker, Söderber, Livingstone, Eliasson, Johnson, Andrew and Olsson46), possibly due to the higher fat mass in women for a given BMI.
Leptin and insulin
To date, the only circulating hormones that meet the criteria to be an adiposity signal are leptin and insulin. Basal circulating concentrations of both insulin and leptin are in proportion to fat mass. Both are transported across the blood–brain barrier, and act centrally to regulate appetite, reduce food intake, and possibly increase energy metabolism(Reference Woods, Gotoh and Clegg5).
Leptin and insulin differ in important ways; circulating levels of leptin and insulin appear to reflect different fat depots. Leptin concentration is more reflective of subcutaneous fat, and insulin is more reflective of visceral fat. Because of the differences between men and women in the proportion of visceral to subcutaneous fat, in general leptin is better correlated with total adipose mass in women and insulin is more highly correlated to total adipose mass in men(Reference Woods, Gotoh and Clegg5).
Normal-weight men and women differ in the responses to central insulin and leptin. Men are more sensitive to central insulin, and women are more sensitive to central leptin. Intranasal administration of insulin led to weight loss, and specifically fat loss, in men; it resulted in weight gain, primarily extracellular water, in women. Intranasal insulin reduced feelings of hunger in men but not in women(Reference Hallschmid, Benedict, Schultes, Fem, Born and Kern49). The same results have been obtained in rats. Male rats are more sensitive to central insulin, female rats to central leptin(Reference Clegg, Riedy, Smith, Benoit and Woods50).
These differences appear to stem from effects of the gonadal hormones. Male rats given exogenous oestrogen are more sensitive to the effects of central leptin than are control males(Reference Clegg, Brown, Woods and Benoit51). Oestrogen appears to blunt the effects of central insulin; intact male and ovariectomised female rats reduced food intake after central administration of insulin. Intact female rats and male rats given exogenous oestrogen did not. Interestingly, castrated male rats without exogenous oestrogen also showed no effect of central insulin on food intake, implying that testosterone also affects central insulin signalling(Reference Clegg, Brown, Woods and Benoit51).
Increased fatness, whether measured by BMI, waist:hip ratio, waist circumference, or actual measures of body fat, is associated with a reduction in peripheral insulin sensitivity. Men and women differ in this regard. Despite having a greater amount of body fat than do men, insulin sensitivity in women appears to be less affected by the amount of body fat. Increases in body fat among women are associated with smaller decreases in insulin sensitivity compared with men(Reference Sierra-Johnson, Johnson, Bailey and Turner52). Visceral fat and subcutaneous fat differ in their responses to insulin, both metabolically and in the synthesis and secretion of adipokines(Reference Einstein, Atzmon, Yang, Ma, Rincon, Rudin, Muzumdar and Barzilai53). Excess visceral fat is associated with insulin resistance(Reference Racette, Hagberg, Evans, Holloszy and Weiss14, Reference Karelis, St-Pierre, Conus, Rabasa-Lhoret and Poehlman17). Thus the fat distribution differences between men and women have metabolic, endocrine and health consequences.
Serum leptin concentration displays some persistent sex differences that begin even before birth. Circulating serum leptin is higher in pregnancies where the fetus is a girl(Reference Alexe, Syridou and Petridou54). Women have higher leptin levels than do men, even at birth, and this difference persists throughout life. These differences do not simply reflect the differences in total adipose tissue between men and women (Fig. 3); women have higher circulating leptin for any given amount of fat mass(Reference Ostlund, Yang, Klein and Gingerich55Reference Rosenbaum, Nicolson, Hirsch, Heymsfield, Gallagher, Chu and Leibel56Reference Kennedy, Gettys, Watson, Wallace, Ganaway, Pan and Garvey57Reference Saad, Damani, Gingerich, Riad-Gabriel, Khan, Boyadjian, Jinagouda, El-Tawil, Rude and Kamdar58. In vitro spontaneous secretion of leptin was greater in adipose tissue samples from women compared with samples from men. Oestradiol and glucocorticoids induced leptin secretion in the adipose tissue samples from women, but not in those from menReference Casabiell, Piñeiro, Peino, Lage, Camiña, Gallego, Vallejo, Dieguez and Casanueva(59).
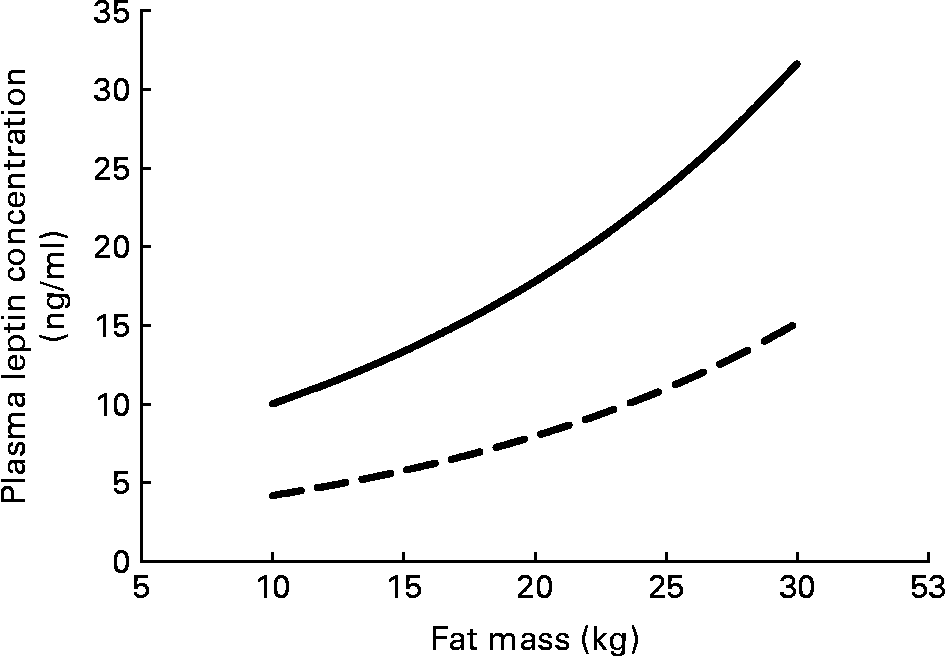
Fig. 3 Plasma leptin concentration increases exponentially with fat mass; women (—) have higher plasma leptin concentrations than do men (– –) for any fat mass. The equations for the curves are from Saad et al.(Reference Saad, Damani, Gingerich, Riad-Gabriel, Khan, Boyadjian, Jinagouda, El-Tawil, Rude and Kamdar58).
These differences appear to reflect a difference between men and women in the importance of fat v. carbohydrate and protein in metabolism. Women appear to be more adapted to use and respond to fat.
Fat, leptin and reproduction
Fat is intimately tied to reproduction through leptin. Leptin has significant effects on many aspects of reproduction. The leptin-deficient obese mice were also infertile, both males and females. Adding back leptin reversed the infertility(Reference Chehab, Lim and Lu60). The reproductive functions of leptin include an association with the onset of puberty, a role in fertility for males and females, a role in ovarian folliculogenesis, and in implantation of the fertilised ovum. Leptin is expressed by the placenta, the umbilical cord, and other fetal membranes as well as by fetal adipose tissue(Reference Ashworth, Hoggard, Thomas, Mercer, Wallace and Lea61, Reference Leperq, Challier, Guerre-Millo, Cauzac, Vidal and Haugel-de Mouzon62). Leptin receptors are widespread in fetal tissues, and leptin is suggested to play a role in fetal development(Reference Henson and Castracane63). Spermatozoa secrete leptin(Reference Aquila, Gentile, Middea, Catalano, Morelli, Pezzi and Andò64). Leptin appears to have many functions beyond any potential ‘lipostatic’ function.
Leptin is important in regulating the transition through puberty. Giving leptin to mice resulted in their attaining sexual maturity at a significantly earlier age(Reference Chehab, Lim and Lu60). The age of menarche has shown a consistent decline over time in the USA(Reference McDowell, Brody and Hughs65), paralleling the increase in overweight and obesity among adolescent girls. Girls with higher BMI from early life on average begin menstruating at an earlier age(Reference Lee, Appugliese, Kaciroti, Corwyn, Bradley and Lumeng66). It is reasonable to hypothesise that the on-average higher BMI of today's young girls are associated with higher on-average levels of circulating leptin, and that this is one possible mechanism behind the decrease in the age at menarche.
Because leptin is strongly associated with a measure of maternal nutritional status (fat mass), it is a plausible candidate for being an important metabolic signal for the maintenance and duration of pregnancy. Low leptin levels are associated with pregnancy loss in humans. Placental leptin synthesis may be abnormally high in pregnancies complicated by conditions such as diabetes mellitus and pre-eclampsia(Reference Hauguel-de Mouzon, Lepercq and Catalano67). Although the evidence does not indicate that leptin is a primary signal for either puberty or pregnancy, the evidence does imply that it may function as one, among many, metabolic signals that maternal condition is satisfactory for reproduction.
Placental weight is correlated with placental leptin mRNA(Reference Jakimiuk, Skalba, Huterski, Haczynski and Magoffin68). Cord serum leptin is correlated with placental leptin mRNA, maternal serum leptin, and with fetal mass(Reference Jakimiuk, Skalba, Huterski, Haczynski and Magoffin68). In humans, maternal serum leptin concentration is highest at mid-gestation, and then declines(Reference Henson and Castracane69). Pregnancy is considered to be a state of hyperleptinaemia with leptin resistance; i.e. high maternal leptin does not decrease food intake. Maternal circulating leptin levels drop precipitously at parturition(Reference Alexe, Syridou and Petridou54, Reference Hauguel-de Mouzon, Lepercq and Catalano67), as do neonatal concentrations(Reference Henson and Castracane63), providing further evidence that placental leptin contributes to circulating levels in both mother and fetus.
Leptin is associated with insulin, insulin-like growth factor, and growth hormone, but appears to be an independent predictor of fetal size in humans. Large-for-gestational-age fetuses have higher than normal leptin, small-for-gestational-age fetuses have lower leptin. In twin pregnancies, the larger twin has higher circulating leptin(Reference Sooranna, Ward and Bajoria70). In humans, cord-blood leptin is associated with both length and head circumference of neonates. Evidence supports the hypothesis that fetal leptin is of both fetal adipose tissue and of placental origin(Reference Hauguel-de Mouzon, Lepercq and Catalano67). Leptin is suspected of having endocrine, autocrine and paracrine effects in placental and fetal tissues. Leptin receptors are found in placenta. Human data are lacking, but in rodents leptin receptors are found in many if not most fetal tissues (for example, besides adipocytes also in hair follicles, cartilage, bone, lung, pancreatic islets cells, kidney, testes, and so forth). Leptin receptors are found in the baboon fetal lung tissue, and markedly increase at the end of gestation(Reference Henson, Swan, Edwards, Hoyle, Purcell and Castracane71). It is hypothesised that leptin has important functions in regulating fetal growth and development(Reference Henson and Castracane63).
Adaptation or genetic drift?
The propensity to obesity among groups of individuals in the modern world reflects a complex interaction among genetics, environment, culture and socio-economics. This complexity in part explains the rather low success at identifying genetic underpinnings of the obesity epidemic. In addition, however, the large number of metabolic pathways that could be involved in predisposing individuals to gain weight suggests that even on a genetic level there will be a large number of candidate genes. There are many paths to weight gain.
Although a genetic propensity to obesity could be thought maladaptive in the modern, developed world with easy and reliable access to plentiful food, it is unclear what, if any, adaptive consequences polymorphisms that affected the development, regulation, and metabolism of fat stores would have had in our past. The advantages of storing energy obtained from episodic conditions of plentiful food probably outweighed the long-term health consequences associated with the rare possibility of becoming obese. In the past there was an asymmetry in selective advantage such that genes that predisposed an individual to fatness were more likely to survive than lean genes. It is only under the modern milieu that these thrifty gene variants result in less than optimal health.
Many authors have suggested that obesity results from a mismatch between our evolved, adaptive responses to past conditions in which obtaining food required extensive physical effort and food scarcity was common with the modern condition of easy access to plentiful, energy-dense foods. Many of the arguments have focused on survival during famines as an evolutionary force behind what are now obesity-prone traits among humans(Reference Speakman72).
We do not dispute these arguments, though in many cases they appear somewhat weak, and have been criticised. On close examination, famine in our past may not have provided a sufficiently strong selective force to favour an obesity-prone genotype(Reference Speakman72, Reference Speakman, Fantuzzi and Mazzone73). However, evolutionary success depends on reproductive success, which includes more than survival. We argue that the effects of even milder (and probably quite common) conditions of food insecurity in our past would have had significant consequences on female fertility and reproductive success, and led to an adaptive advantage for genes that enabled females to store body fat in readily metabolisable depots.
Fat and reproduction are intimately linked in women. Leptin, the molecule of ‘fat homeostasis’, has direct effects on female fertility and fetal growth and development(Reference Henson and Castracane69). Women with low body fat (or low leptin for any reason) have decreased fertility. This does not appear to be the case for men. Fertility in men is largely unaffected by BMI of 15–26 kg/m2, but declines with further increases of BMI (Fig. 4) (Reference Sallmén, Sandler, Hoppin, Blair and Baird74). Thus men and women differ in the reproductive consequences of low body fat.
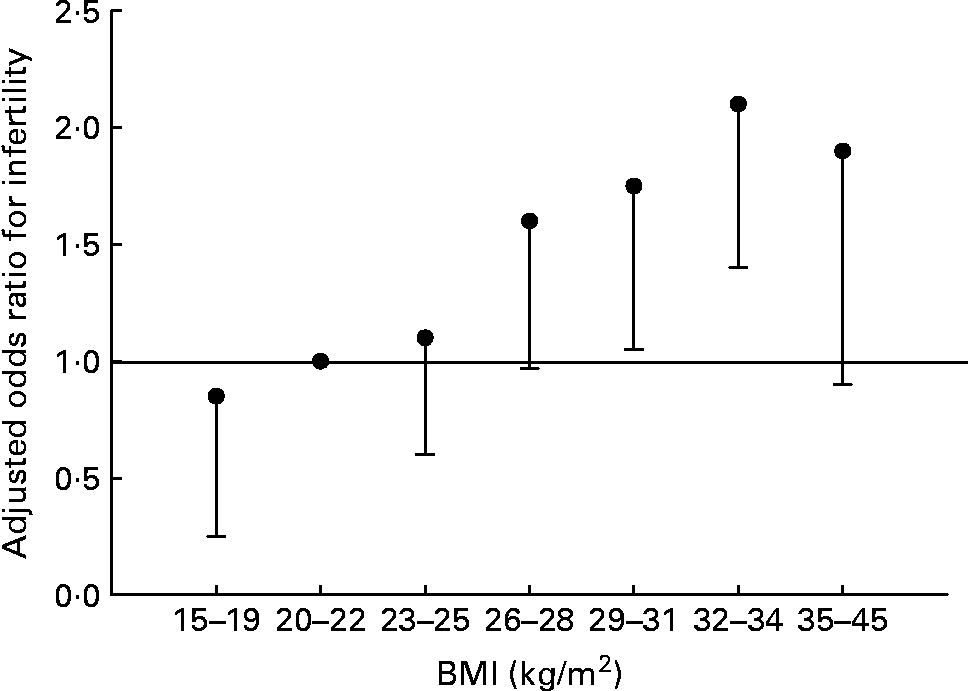
Fig. 4 Risk of male infertility relative to a BMI of 20–22 kg/m2, adjusted for age, smoking, alcohol use, and solvent and pesticide exposure. Values are OR, with the lower 95 % CI represented by the vertical bars. There is no statistical difference for male infertility for all BMI < 26 kg/m2. Data from Sallmén et al.(Reference Sallmén, Sandler, Hoppin, Blair and Baird74).
The association between fatness and reproductive success in women may start at birth. Circulating leptin levels are higher in female compared with male infants, and the levels are correlated with infant adiposity(Reference Alexe, Syridou and Petridou54). A high ponderal index at birth (birth weight divided by the cube of birth length) in female infants is associated with both higher oestradiol levels(Reference Jasienska, Ziomkiewicz, Lipson, Thune and Ellison75) and resistance to oestradiol suppression by activity as adults(Reference Jasienska, Thune and Ellison76). Thus a measure of fatness at birth is associated with ovarian function as an adult. These data suggest that in our past women producing lean, low-leptin, female babies would have been selectively at a disadvantage.
However, much of the genetic variability among individuals in the propensity to gain weight probably did not arise through adaptation. There appears to be substantial variation among individuals in the propensity to deposit fat and where that fat gets deposited. Some of this variation is associated with geographic regions of origin, and hence possibly reflects long-standing genetic differences. For example, individuals from the Indian subcontinent appear to have higher adiposity at any particular BMI than do Caucasians or sub-Saharan Africans, and a greater propensity to central adiposity(Reference Yajnik28). African-American women tend to have less visceral fat but more subcutaneous fat than do Caucasian women(Reference Tittelbach, Berman, Nicklas, Ryan and Goldberg30). However, African-American women tend to have higher total fat(Reference Cossrow and Falkner31). Caucasian women appear to have a greater capacity to switch metabolism to fat oxidation when given a high-fat meal than do African-American women(Reference Berk, Kovera, Boozer, Pi-Sunyer and Albu77), which may in part explain the higher susceptibility to obesity among African-American women eating a Western diet.
These differences may or may not reflect adaptive changes. Indeed, we hypothesise that many polymorphisms among human beings that make them susceptible to obesity and to the negative health consequences of excess weight in the modern milieu may have been selectively invisible in our past. In our past, external constraints (food availability, predation pressure, competition between and within species) on the amount of body fat individuals could attain were at least as important as internal constraints (mechanisms of energy homeostasis and ‘lipostatic’ mechanisms). As a species, we probably favoured a ‘thrifty’ genotype and phenotype in our past just to maintain a BMI of 18 kg/m2 or higher. External constraints made attaining a BMI above 25 kg/m2 very unlikely. Thus subtle variation in the propensity to store fat in different depots may not have had much if any adaptive significance.
Speakman(Reference Speakman72) has produced a model that shows how random, genetic drift acting on multiple gene targets, coupled with the proposed asymmetry between the dangers of becoming lean v. fat in our past when obesity was rare, could produce genetic subpopulations with ‘set points’ for higher BMI than could have actually been achieved. In other words, individuals that in our past could not achieve their physiologically determined BMI due to external factors (for example, lower food abundance and greater energy expenditure) are now expressing that previously invisible genetic potential. He hypothesises that changes in behaviour (tool use, fire, social behaviour) decreased our ancestors' risk of predation and led to a relaxation of selection on the upper limit to body fat. This created an asymmetry whereby selection against extremely low BMI was still in force while selection against higher BMI was relaxed. Of course the extremely high BMI seen today were invisible to selection because they could not be obtained. Thus, some of the genetic differences among humans that predispose some to gain weight in the modern era may have had no evolutionary significance in our past. The number of genes involved, and the number of variants, is probably very large.
Although obesity was not adaptive, the physiology, metabolism and behaviour that can lead to obesity in today's world may still have conferred adaptive advantages. The female pattern of adiposity, with predominantly lower body, subcutaneous adipose stores, appears to be a healthier pattern than the male pattern of more visceral fat. It is associated with fewer co-morbidities. The liver will be exposed to lower concentrations of NEFA than for equivalent amounts of visceral fat. The metabolic costs of storing fat are lower, and the advantages for reproduction, at least in the past, significant. The costs of female reproduction would provide a potent adaptive force driving adipose tissue metabolism in women. In addition, the ability to produce ‘fatter’ female infants and children (within the context of our past, and not at the level of fatness today) may have had reproductive benefits in terms of earlier age at menarche and more resilient ovarian function for these offspring as adults (increased total reproductive life span).
Again, we are not arguing that obesity in women was adaptive. Indeed, maternal obesity is associated with a number of reproductive problems, including decreased fertility, increased early pregnancy loss, increased risk of birth dystocia, and increased risk of birth defects(Reference Cogswell, Perry, Schieve and Dietz78Reference Yu, Teoh and Robinson79Reference Catalano and Ehrenberg80. Maternal obesity is also associated with an increased risk of later adult obesity in the offspring, possibly due to fetal programming of physiologyReference Catalano and Ehrenberg(80). This leads to the spectre of obese mothers passing on to their daughters characteristics that will increase the likelihood that those daughters will be obese, and who will subsequently have obesity-prone offspring in turn.
Obesity does not confer a reproductive fitness advantage to either men or women. However, a sexual dimorphism in adiposity is understandable given the potential benefits to sustaining reproduction (for example, fertility, lactation, age at menarche) in women, and a lack of such adaptive pressures in men. Low BMI does not appear to reduce male fertility (Fig. 4). However, excess body fat in men is associated with decreased fatty acid availability and oxidation during endurance exercise; this would not have been an advantage to our hunter–gatherer forefathers.
Men are more susceptible to central adiposity. Central adipose tissue deposits are more resistant to mobilisation. There would appear to be little adaptive advantage to storing visceral fat. We suggest that the pattern of central obesity, more commonly seen in men, and associated with greater co-morbidity, reflects the genetic drift hypothesis of human susceptibility to obesity. Under conditions common in our past few individuals would have been able to remain in positive energy balance long enough for significant visceral adipose tissue to accumulate.
The differing fat storage patterns between men and women and the metabolic differences in how they meet sustained energy demands reflect their asymmetrical costs of reproduction. In the past, fat was more important to the reproductive success of women. We propose that the female pattern of excess adiposity in the lower extremities in obesity reflects an exaggeration of an adaptation for female reproductive success. The modern environment allows the adaptive pattern to go beyond its evolved function, and into pathology.
Acknowledgements
Partial funding was provided by US PHS grant R01 DK077639 to M. L. P. (co-principal investigator).







