Diabetes mellitus has become one of the major endocrine disorders in cats. Recently a tendency towards a higher incidence of this disease was reported by Prahl et al. (Reference Prahl, Guptill and Glickman1) and might be due to a rise in the frequency of the major predisposing factors such as obesity and physical inactivity(Reference Rand, Fleeman and Farrow2). However, the current dietary treatments for feline diabetes mellitus, especially the use of soluble fibres, originates from extrapolation of the results from human and canine studies. To our knowledge, the effect of soluble fibres on carbohydrate metabolism in both healthy and insulin-resistant cats (obese or diabetic cats) has not been studied.
Soluble fibres such as oligofructose and inulin have been shown to modulate glycaemia and insulinaemia, although effects may depend on nutritional (fasting v. postprandial) and pathological (diabetes mellitus, obesity) conditions(Reference Roberfroid and Delzenne3). In the literature, two hypotheses are proposed. At first, soluble fibres might impair digestion of macronutrients by delaying gastric emptying and/or by reducing small intestinal transit time(Reference Blaxter, Cripps and Gruffydd-Jones4–Reference Nelson6). Secondly, the production of SCFA in the hindgut is stimulated by offering soluble fibres as energy source for colonic microbial flora. After being absorbed, the SCFA, especially propionate, might modify the hepatic glucose metabolism by reducing hepatic gluconeogenesis and/or enhancing glycolysis; consequently, blood glucose concentrations will be decreased(Reference Roberfroid and Delzenne3). Hepatic gluconeogenesis might also be influenced indirectly by lowering the plasma fatty acid concentration, since increased plasma fatty acid availability may induce impaired insulin sensitivity by promoting fatty acid oxidation and inhibiting glucose uptake and oxidation but stimulating hepatic glucose production(Reference Delarue and Magnan7).
In other less strict carnivores than cats, such as dogs, few studies investigating the effect of soluble dietary fibre have been performed, resulting in a decreased postprandial glycaemia and/or insulinaemia(Reference Diez, Hornick and Baldwin8–Reference Hesta, Debraekeleer and Janssens12). Yet, to date, no data are available on the effect of prebiotics on the carbohydrate metabolism in more strict carnivorous species such as the cat.
Not only carbohydrate metabolism, but also acylcarnitine profile and selected characteristics of lipid (plasma cholesterol, TAG, NEFA concentrations) and protein metabolism (plasma urea, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and methylmalonylcarnitine concentration) of the true carnivorous cat were scrutinised in the present study. Acylcarnitine profile was studied as a reflection of metabolites available for the citric acid cycle(Reference Bremer13). Endocrine characteristics such as leptin and thyroid function were investigated as well, since these characteristics are identified to be related to obesity and insulin resistance. Leptin is distinguished to be strongly and positively correlated with adiposity in obese cats(Reference Appleton, Rand and Sunvold14) and insulin resistance is associated with increased leptin concentrations in both normal-weight and obese cats(Reference Appleton, Rand and Sunvold15). Given that thyroid hormones are involved in the regulation of metabolism, and regulate RMR, thermogenesis and lipolysis, thyroid function might be altered by developing obesity(Reference Ferguson, Caffall and Hoenig16). Hence, total fasting tri-iodothyronine (T3) and total thyroxine (T4) concentrations were also obtained in the present trial.
Seeing that very little is known about the metabolic effects of prebiotics in cats, the purpose of the present trial was to evaluate the effect of adding oligofructose and inulin to a basic diet on feline carbohydrate, lipid and protein metabolism as well as to determine the metabolic differences between healthy normal-weight and obese cats.
Materials and methods
Animals and housing
Sixteen domestic short-hair cats, six males and ten females, were included in the study. All male and female cats were neutered. All cats were adult and aged between 3·5 and 6 years. All cats were healthy apart from chronic obesity in eight cats and were not given any medication at the time of the study; none had prior medical problems. During the trial, the cats were housed in individual indoor cages. For 2 h/d, cats were allowed to play in their usual group cages. At that time, cats had no access to the food, but water was available ad libitum.
Diets and feeding
The control diet (C-diet) was a non-commercial extruded dry cat food, containing high concentrations of crude protein (46 % on DM basis), moderate amounts of crude fat (15 % DM) and low concentrations of carbohydrates (N-free extract 27 % DM), in order to trigger the cats towards insulin resistance (A Verbrugghe et al., unpublished results). The food also contained moderate concentrations of crude fibre (4·6 % DM) and crude ash (6·7 % DM) (Table 1) and was coated with palm oil on the outside of the kibble. No prebiotics and other soluble fibres were added. To make the prebiotic diet (P-diet), 2·5 % mixture of oligofructose and inulin (Beneo™ Synergy 1®; Beneo-Orafti, Beneo-Group, Tienen, Belgium) was added to the C-diet. This soluble fibre mixture is a co-spray dried 1:1 mixture of long-chain chicory inulin molecules, enriched with short-chain oligofructose obtained by partial enzymatic hydrolysis of chicory inulin and containing low concentrations of fructose, glucose and sucrose as well. The mean total number of fructose or glucose units (degree of polymerisation) was 25 for the inulin; mean degree of polymerisation of oligofructose was 4. The soluble fibre was not mixed with the ingredients, but was added to the palm oil coating. The proximate analysis of the diets is shown in Table 1. Total dietary fibre was determined by acid and enzymatic digestion using enzymes from a commercial test kit (Bioquant Total Dietary Fiber, Merck, USA), followed by correction for protein and ash and is also shown in Table 1.
Table 1 Composition of the control diet (C-diet) and prebiotic diet (P-diet; C-diet +2·5 % of a mixture of oligofructose and inulin)*
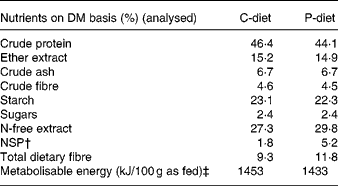
* Ingredients: greaves meal, wheat flour, chicken meal, wheat, bovine chicken fat, linseed, meat and bone meal, brewer's yeast, premium cat digest liquid, fish meal, premix, monosodium glutamate, salt, dl-methionine, iron oxide black, choline chloride 75 %.
† N-free extract − starch − sugars.
‡ Calculated: 15 × crude protein + 36 × ether extract+15 × (starch+sugars).
The amount of food calculated corresponded to the maintenance energy requirement (normal-weight cats 418 kJ/kg0·67; obese cats 544 kJ/kg0·4)(17) and was adapted in order to maintain a constant body weight. The food was available all day, except for the 2 h playtime. Cats were allowed free access to water at all times.
Experimental design
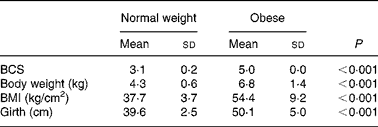
Prior to being entered into the study, the cats underwent a physical examination, a blood sample was drawn from the jugular vein after a 12 h fast for complete blood count and serum biochemistry and body weight, body condition score (BCS), BMI and girth circumference were recorded. The BCS was determined using a five-point body condition scoring system(Reference Scarlet, Donoghue and Saidla18). Non-obese cats with a score of 3/5 (mean body weight 4·3 kg; range 3·7–5·3 kg) and obese cats with a score of 5/5 (mean body weight 6·8 kg; range 5·3–9·8 kg) were used in the study. The BMI was calculated as described by Hoenig et al. (Reference Hoenig, Wilkins and Holson19). Girth circumference was measured directly behind the last rib(Reference Hoenig, Wilkins and Holson19). All measurements were performed under general anaesthesia by the same person to minimise variability (Table 2). For 4 weeks preceding the trial (adaptation period), all cats were fed the C-diet prior to being randomised to one of two groups, each containing four normal-weight and four obese cats. Each group of cats was assigned to each of two diets (C-diet and P-diet) in a random order at intervals of 4 weeks. This way, diets were examined in a crossover design. Absolute food intake was measured daily throughout the study and relative food intake (% of metabolisable energy intake relative to maintenance energy requirement) was calculated. Body weight was recorded twice weekly.
Table 2 Body condition score (BCS), body weight, BMI and girth circumference in eight normal-weight and seven obese neutered adult cats
(Mean values and standard deviations)
To determine the effect on glucose and insulin metabolism, an intravenous glucose tolerance test (IVGTT) was performed in each cat at the end of each testing period. Hence, a central venous catheter was placed into a jugular vein to allow glucose administration and blood sampling. At least 20 h prior to the IVGTT, cats were anaesthetised with buprenorphine (10 μg/kg intravenous; Temgesic®; Schering-Plough n.v., Heist-Op-Den-Berg, Belgium), followed by propofol (6–7 mg/kg to effect, intravenous; Propovet®; Abbott Lab, Leuven, Belgium), and a 20 G, 8 cm intravenous catheter (Leaderflex®, Vygon n.v., Brussels, Belgium) was placed in a jugular vein. Catheters were flushed twice daily with 1 ml heparinised saline (50 IU of heparin/ml in saline (0·9 % NaCl) solution) to maintain patency. Amoxycilline (15 mg/kg; Clamoxyl LA®; GlaxoSmithKline n.v., Genval, Belgium) was administered subcutaneously once at the time of catheter placement. The IVGTT was performed between 9.00 and 12.00 hours after a 12 h fast. Glucose (Glucose Sterop 500 mg/ml; Laboratoria Sterop n.v., Brussels, Belgium) was administered (0·5 g/kg), through the jugular vein catheter over 30–45 s, followed immediately by 1 ml normal saline to flush the catheter(Reference Appleton, Rand and Priest20). Blood samples were collected from the jugular catheter as described by Martin & Rand(Reference Martin and Rand21), prior to (0 min) and 2, 5, 10, 15, 30, 45, 60, 90 and 120 min after glucose administration(Reference Appleton, Rand and Priest20). At time zero, blood samples were collected in tubes containing lithium heparin for determination of AST, ALT, leptin, T3 and T4 concentrations, free carnitine and acylcarnitine profile. Serum tubes were used to determine fasting serum total cholesterol, TAG, NEFA, urea and creatinine concentrations, at time zero. At each time interval, blood samples were collected in tubes containing sodium fluoride for determination of plasma glucose concentrations and in serum tubes for determination of serum insulin concentrations. Plasma and serum were obtained by centrifugation and stored at − 20°C until assayed.
The experimental protocol was approved by the Ethical Committee of the Faculty of Veterinary Medicine, Ghent University, Belgium (EC 2007/013).
Analytical methods
Plasma glucose, serum total cholesterol, TAG, urea and creatinine concentrations were determined spectrophotometrically using the Roche/Hitachi Modular Analyzer (Roche Diagnostics GmbH, Mannheim, Germany). Likewise, activities of AST and ALT were analysed spectrophotometrically using the Roche/Hitachi Modular Analyzer with pyridoxal phosphate activation. Plasma NEFA concentrations were determined enzymatically using a commercially available method (NEFA; Randox Laboratories Ltd, Crumlin, UK) on RX Daytona (Randox). Serum insulin concentrations were measured using a commercially available immunoradiometric assay test kit (insulin immunoradiometric assay Ref 5251; Biosource Europe S.A., Nivelles, Belgium) as used by Slingerland et al. (Reference Slingerland, Robben and van Haeften22). Plasma leptin concentrations were determined using a commercially available RIA test kit (Multi-Species Leptin RIA Kit, catalogue number XL-85K; Linco Research Inc., St. Charles, MO, USA). This kit was developed to quantify leptin in plasma from several species and has been validated for use in cats(Reference Backus, Havel and Gingerich23). Fasting plasma T3 and T4 concentrations were determined using a specific RIA as described by Darras et al. (Reference Darras, Visser and Berghman24). Quantitative electrospray tandem-MS was used for free carnitine and acylcarnitine analysis, as described by Vreken et al. (Reference Vreken, van Lint and Bootsma25) and Rizzo et al. (Reference Rizzo, Boenzi and Wanders26).
The glucose disappearance coefficient (K gluc) and the half-life for glucose disappearance (glucose t 1/2) between 15 and 90 min after glucose administration were calculated as described by Link & Rand(Reference Link and Rand27). Area under the glucose curve (AUCgluc) and area under the insulin curve (AUCins) were calculated according to the trapezoidal method (baseline equal to zero). The basal insulin to glucose ratio and the ratio of area under the insulin to glucose curve (AUCins/AUCgluc) as well as the homeostasis model assessment (HOMA), the quantitative insulin sensitivity check index (QUICKI) and the Bennett index were calculated as described by Appleton et al. (Reference Appleton, Rand and Sunvold28).
Statistical analysis
Statistical analysis were performed using Superior Performing Software Systems version 16 (SPSS Inc., Chicago, IL, USA). For body weight, BCS, BMI and girth circumference at the beginning of the trial one-way ANOVA was performed. For plasma glucose and serum insulin concentrations during IVGTT, repeated-measures ANOVA was used, with BCS as between-subject factor and diet and time as within-subject factor. All remaining data, including the different glucose time-points during IVGTT, were statistically analysed by repeated-measures ANOVA with BCS as between-subject factor and diet as within-subject factor. Interactions between BCS and diet were also evaluated, but were not present. Statistical significance was accepted at P < 0·05. All data are expressed as means and standard deviations.
Results
All sixteen cats except one completed the trial. One obese cat died as a consequence of an unrelated cause during general anaesthesia. During the first sampling period, the IVGTT failed in four cats (one of each group) due to technical problems. In these cats only fasting blood samples could be obtained.
Effect of body condition
At the start of the trial, body weight, BCS, BMI and girth circumference (P < 0·001 for all) differed among normal-weight and obese cats (Table 2). During the trial, body weight remained stable in all cats (data not shown). The effect of BCS on feed intake, fasting metabolic and endocrine parameters, regardless of diet, is shown in Table 3. Obese cats ate more compared with normal-weight cats (P = 0·019), but no differences were noted in relative food intake. Fasting serum creatinine, NEFA and cholesterol concentrations were comparable between normal-weight and obese cats. However, fasting serum urea concentrations were lower (P = 0·011) and serum TAG concentrations tended (P = 0·094) to be higher in obese cats when compared to normal-weight cats. Plasma ALT activity was increased in obese cats (P = 0·043), but plasma AST activity did not change among obese and normal-weight cats. Fasting plasma leptin and T3 concentrations were also increased in obese cats in contrast to normal-weight cats (P = 0·003 and P = 0·026, respectively). Fasting plasma T4 concentrations did not differ in relation to BCS. The effect of BCS on glucose and insulin metabolism, regardless of diet, is shown in Table 4. Obese cats had higher fasting plasma glucose (P = 0·030), higher glucose concentration at any time during IVGTT, except for 5 and 120 min after glucose administration (also shown in Fig. 1; main effect: P = 0·013; time × BCS: P = 0·043), and higher AUCgluc (P = 0·022). The K gluc and glucose t 1/2 were not affected by body condition (Table 4). As shown in Fig. 2, body condition did not influence serum insulin concentrations at any time-point, as well as the height of the first and second insulin peak and the appearance time of the first insulin peak. Yet, the second insulin peak was delayed in obese cats when compared to normal-weight cats (P = 0·009). AUCins, fasting basal insulin to glucose ratio and AUCins/AUCgluc as well as HOMA, QUICKI and Bennett index were not affected by body condition (Table 4).
Table 3 Effect of body condition score on feed intake, fasting metabolic and endocrinologic characteristics, regardless of diet*
(Mean values and standard deviations)
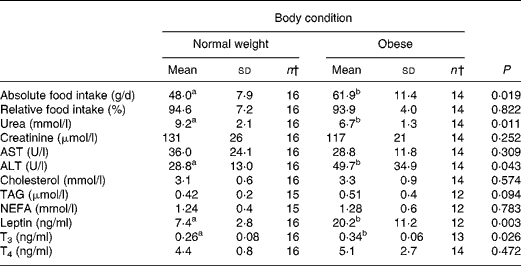
ALT, alanine aminotransferase; AST, aspartate aminotransferase; T3, tri-iodothyronine; T4, thyroxine.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* For details of procedures, see Materials and methods.
† n < 16 caused by missing values.
Table 4 Effect of body condition score on glucose and insulin metabolism, regardless of diet*
(Mean values and standard deviations)

AUCgluc, area under the curve for glucose; AUCins, area under the curve for insulin; fasting SI1, basal insulin to glucose ratio; glucose t 1/2, half-life for glucose disappearance between 15 and 90 min after glucose administration; HOMA, homeostasis model assessment; K gluc, glucose disappearance coefficient; QUICKI, quantitative insulin sensitivity check index.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* For details of procedures, see Materials and methods.
† n < 16 caused by missing values.
‡ Calculated according to Appleton et al. (Reference Appleton, Rand and Sunvold28).
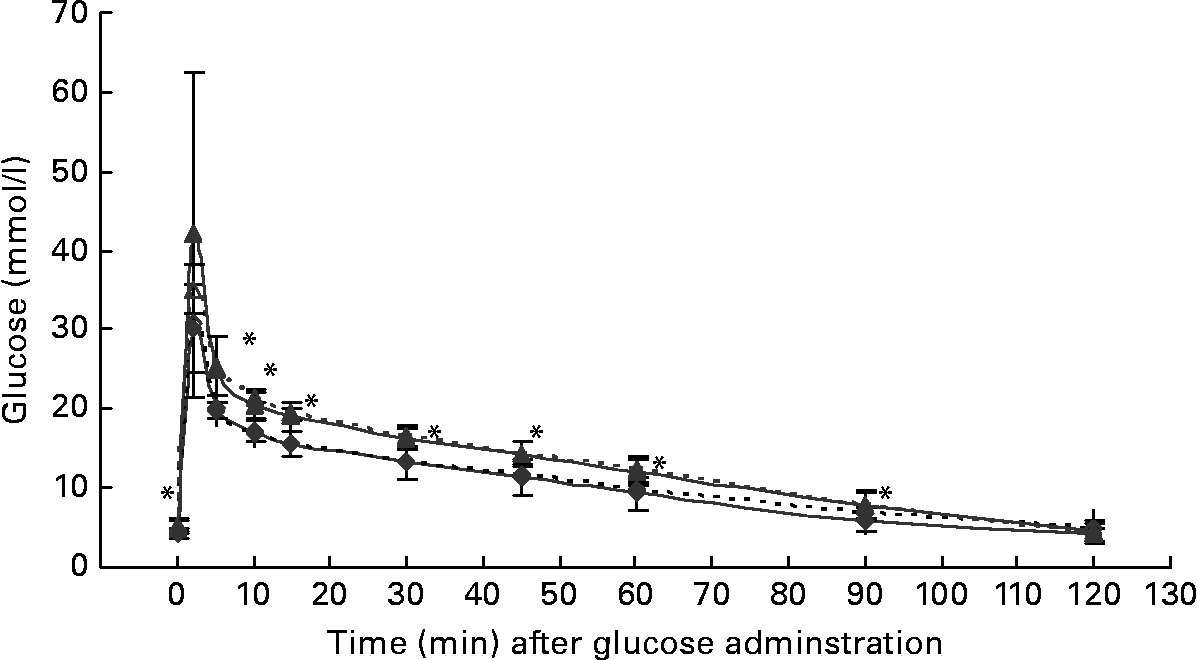
Fig. 1 Plasma glucose concentrations during the intravenous glucose tolerance test in healthy normal-weight (body condition score (BCS) 3/5) and obese (BCS 5/5) cats fed the control diet (C-diet) and prebiotic diet (P-diet; C-diet +2·5 % of a mixture of oligofructose and inulin). ♦, BCS 3, C-diet; ⋄, BCS 3, P-diet; ▲, BCS 5, C-diet; △, BCS 5, P-diet. Values are means with their standard deviations depicted by vertical bars. Mean values were significantly different at each different time-point between normal-weight and obese cats, regardless of dietary treatment: *P < 0·05.
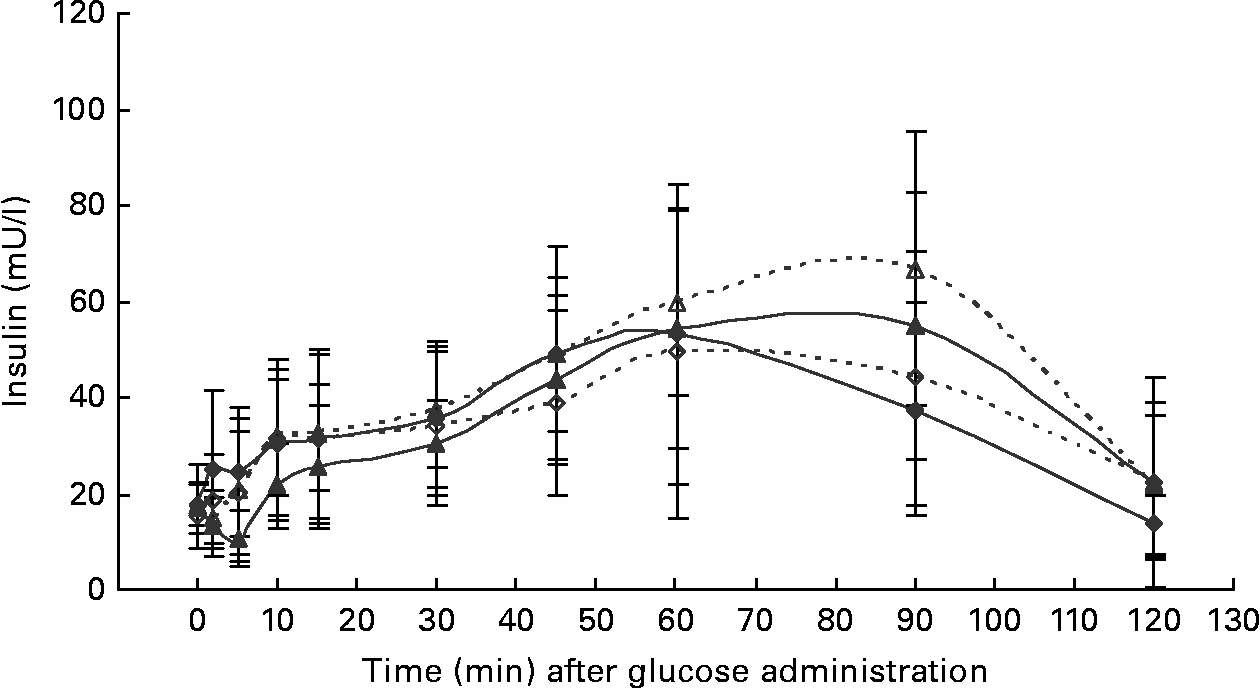
Fig. 2 Serum insulin concentrations during the intravenous glucose tolerance test in healthy normal-weight (BCS 3/5) and obese (BCS 5/5) cats fed the control diet (C-diet) and prebiotic diet (P-diet; C-diet +2·5 % of a mixture of oligofructose and inulin). ♦, BCS 3, C-diet; ⋄, BCS 3, P-diet; ▲, BCS 5, C-diet; △, BCS 5, P-diet. Values are means with their standard deviations depicted by vertical bars.
Effect of adding 2·5 % mixture of oligofructose and inulin to a control diet
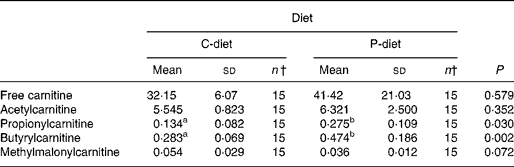
Both diets were well tolerated. During the trial, none of the cats refused to eat either of the diets and none showed signs of illness or maldigestion. Absolute and relative food intake showed no significant differences between diets. Adding 2·5 % mixture of oligofructose and inulin to the C-diet did not alter characteristics related to glucose and insulin metabolism in healthy normal-weight or obese cats. As shown in Fig. 1, fasting plasma glucose concentration and plasma glucose concentrations at each other time-point after glucose administration were comparable. Also the AUCgluc, K gluc and glucose t 1/2 remained unaffected. Similarly, fasting insulin, AUCins as well as height and appearance time of the insulin peaks were similar with both diets with slightly, though not significantly, higher release with fructan intervention (Fig. 2). All endocrine (leptin, T3, T4) and metabolic (cholesterol, NEFA, urea, creatinine, ALT) characteristics remained stable, except for serum TAG, which tended to be increased (P = 0·065) and plasma AST activity which was decreased (P = 0·025) when fed the P-diet compared to the C-diet. As shown in Table 5, plasma free carnitine concentrations did not differ among diets, as did plasma acetylcarnitine concentrations. Yet, propionylcarnitine (P = 0·03) and butyrylcarnitine (P = 0·002) were higher when fed the P-diet. Methylmalonylcarnitine tended to be decreased (P = 0·072) when fed the P-diet.
Table 5 Effect of adding 2·5 % of a mixture of oligofructose and inulin to the control diet on carnitine metabolism, regardless of body condition*
(Mean values and standard deviations)
C-diet, control diet; P-diet, prebiotic diet (C-diet +2·5 % of a mixture of oligofructose and inulin).
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* For details of procedures and diets, see the Materials and methods section and Table 1.
† n < 16 caused by missing values.
Discussion
In the current study, obese cats confirmed a higher risk for insulin resistance and impaired glucose tolerance than normal-weight cats. Under the test conditions, differences in main blood glucose control characteristics were not observed. Obese cats, taking part in the present trial, had no significantly higher fasting serum insulin concentrations, but the increased AUCgluc, the higher fasting plasma glucose concentration and the later appearance of the second insulin peak during IVGTT may have resulted in an impaired glucose tolerance and a higher insulin resistance in obese cats regardless of diet. The present findings were also concluded from previous studies in cats(Reference Nelson, Himsel and Feldman29, Reference Hoenig, Thomaseth and Brandao30). The significantly higher fasting plasma leptin concentrations in obese cats might also predict the occurrence of insulin resistance, since leptin is postulated to mediate some of the metabolic consequences of obesity. In cats, Appleton et al. (Reference Appleton, Rand and Sunvold15) have demonstrated a strong positively relationship between leptin and insulin resistance. The significantly higher fasting plasma T3 concentrations in obese cats during the present trial corresponds to the significant and positive correlation of T3 with body weight, girth circumference and BMI as observed by Ferguson et al. (Reference Ferguson, Caffall and Hoenig16). T4 also correlated positively with all indices of obesity and with leptin, but not with NEFA(Reference Ferguson, Caffall and Hoenig16). Still, fasting T4 concentrations remained unchanged in the present trial. Ferguson et al. (Reference Ferguson, Caffall and Hoenig16) also demonstrated increased leptin and NEFA after weight gain and proposed an obesity-induced relative state of thyroid hormone resistance, either caused by leptin or by increased NEFA concentrations, or both. In the present trial, fasting leptin concentrations were indeed elevated in obese cats, but no significant differences were noted for serum NEFA concentrations. This supports that the relative state of thyroid hormone resistance might be most probably explained by the rise in fasting plasma leptin concentrations. In overweight man, hyperleptinaemia was also observed to be strongly correlated with elevated serum ALT activity(Reference Ruhl and Everhart31), most probably due to the high association between elevated liver enzymes, most notably high serum ALT activity and obesity (percentage body fat(Reference Vozarova, Stefan and Lindsay32); obesity class(Reference Marchesini, Avagnina and Barantani33)) and insulin resistance (measured by hyperinsulinaemic euglycaemic clamp technique(Reference Vozarova, Stefan and Lindsay32) or HOMA(Reference Marchesini, Avagnina and Barantani33)) as observed by Vozarova et al. (Reference Vozarova, Stefan and Lindsay32) and Marchesini et al. (Reference Marchesini, Avagnina and Barantani33). Therefore, the occurrence of insulin resistance might explain the rise in plasma ALT activity observed in obese cats. In addition, serum TAG also tended to rise in obese cats, in accordance with the results found by Hoenig et al. (Reference Hoenig, Wilkins and Holson19), who studied the effect of obesity on lipid profiles in neutered cats. Hoenig et al. (Reference Hoenig, Wilkins and Holson19) also noted a significant rise in plasma total cholesterol concentrations in obese cats; yet, this could not be demonstrated from the present trial. At last, fasting serum urea concentrations were reduced in obese cats compared to normal-weight cats. In rats made obese by feeding a cafeteria-diet, similar results were demonstrated. According to Barber et al. (Reference Barber, Vina and Vina34), a decreased serum urea concentration in obese rats might be due to a decrease in the activities of all enzymes of the urea cycle and a lower rate of synthesis of urea from precursors in hepatocytes. To date, no data are available on the impact of feline obesity on urea concentrations and the activity of urea cycle enzymes, yet in obese cats suffering from severe hepatic lipidosis it is observed that blood urea nitrogen might be subnormal caused by chronic anorexia or presuming impaired urea cycle function(Reference Center35). However, during the present trial cats were not anorectic, did not lose any weight and did not show any other clinical signs reflecting hepatic lipidosis, which probably indicates reduced serum urea concentrations due to compromised urea cycle function as observed in obese rats.
Adding 2·5 % mixture of oligofructose and inulin to a basic diet did not alter the investigated standard characteristics for glucose and insulin metabolism, or other metabolic and endocrine characteristics, in healthy normal-weight and obese cats; except for serum TAG concentrations, the activity of AST and several acylcarnitines. Serum TAG concentrations tended to be increased in cats supplemented with oligofructose and inulin. The present finding contrasts with the results from earlier trials in rodents(Reference Levrat, Rémésy and Demigné36–Reference Busserolles, Gueux and Rock40) and dogs(Reference Diez, Hornick and Baldwin8) which revealed lower TAG concentrations following soluble fibre supplementation. Since body weight remained unchanged during the trial, an increase in body weight could not explain the present observation. Nevertheless, serum TAG concentrations remained within the references range. When compared to other trials conducted in rats(Reference Agheli, Kabir and Berni-Canani39–Reference Kok, Roberfroid and Robert41) and dogs(Reference Diez, Hornick and Baldwin8–Reference Massimino, McBurney and Field10, Reference Hesta, Debraekeleer and Janssens12), the amount of soluble fibre used in the present trial (0·5 g/kg body weight) seems rather low. Nevertheless, in human studies(Reference Luo, Rizkalla and Alamowitch42–Reference Luo, Van Yperselle and Rizkalla44) doses of oligofructose were similar to the dose used in the present trial. In healthy human subjects ingesting 20 g fructooligosaccharide/d (0·3 g/kg body weight) for 4 weeks, fructooligosaccharides did not modify fasting glucose and insulin concentrations, but did lower basal hepatic glucose production(Reference Luo, Rizkalla and Alamowitch42). According to Yamashita et al. (Reference Yamashita, Kawai and Itakura45), 8 g fructooligosaccharide/d (0·1 g/kg body weight) for 2 weeks resulted in a reduced fasting glycaemia in type 2 diabetic patients. For the results of the current study, species differences including insulin sensitivity must be taken into account. For higher doses, Hesta et al. (Reference Hesta, Janssens and Debraekeleer46) showed that faeces became formless and apparent digestibility of protein and fat were reduced when cats ingested more then 3 % oligofructose or inulin, suggesting a limitation of more than 3 % oligofructose or inulin for practical use in cats. Concerning the type of soluble fibre, Diez et al. (Reference Diez, Hornick and Baldwin8) observed significantly decreased postprandial glucose concentrations after incorporation of both fructooligosaccharides and sugar beet fibre in healthy dogs, in spite of non-significant effects on fasting glucose concentration or postprandial glucose curve after supplementation of inulin or sugar-beet fibre(Reference Diez, Hornick and Baldwin9). In the present trial the diet was supplemented with a mixture of both oligofructose and inulin, containing a higher proportion of inulin which has a higher degree of polymerisation compared to oligofructose. This suggests a slower fermentation in the relative short large bowel of the cat. Another reason for the absence of effect on standard characteristics for glucose and insulin metabolism might be the control diet. At first, it has been demonstrated in man(Reference Jenkins, Wolever and Bacon47) and dogs(Reference Nelson, Ihle and Lewis48) that supplementing soluble as well as insoluble fibre to a basic diet only results in a better glucose and insulin response when the diet contains more than 40 % of the metabolisable energy as carbohydrates, whereas our basic diet only contained 26 % of metabolisable energy as carbohydrates. Secondly, insulin resistance might have been triggered better by using a diet containing high amounts of fat, as demonstrated in man and rodents(Reference Storlien, Baur and Kriketos49, Reference Lichtenstein and Swab50) as well as in cats(Reference Thiess, Becskei and Tomsa51), instead of a high-protein, low-carbohydrate diet as used in the present trial. Nevertheless, a previous trial showed an increased insulin resistance in healthy non-obese cats fed a low-carbohydrate diet when compared to a low-fat and a low-protein diet (A Verbrugghe et al., unpublished results). Mechanisms responsible for enhancing glucose tolerance and insulin sensitivity due to soluble fibre as described in other species might be less pronounced in cats.
Despite the absence of a direct effect on glucose tolerance and insulin sensitivity, an effect on colonic fermentation and the production of SCFA could be demonstrated by acylcarnitine analysis. Higher production of propionate and butyrate and metabolisation of these SCFA were observed by the higher propionyl- and butyrylcarnitine concentrations in cats when fed the P-diet. Acetylcarnitine was not altered among diets. Acetyl-CoA is not only generated from acetate, but also from amino acids and other fatty acids. Moreover, propionate might inhibit the rise in acetylcarnitine concentrations caused by butyrate, as demonstrated by Brass & Beyerinck(Reference Brass and Beyerinck52).
In the present trial, oligofructose and inulin might have contributed to the citric acid cycle through propionate. Theoretically, propionate, being a gluconeogenic SCFA, can be converted to glucose via succinyl-CoA and oxaloacetate(Reference Wolever, Brighenti and Royall53). In ruminants(Reference Judson, Anderson and Luick54, Reference Yost, Young and Schmidt55) and horses(Reference Simmons and Ford56), propionate is known to be the primary gluconeogenic substrate. In strictly carnivorous species, such as cats, no data are available yet. It is also known from studies in man as well as experimental animals that propionate decreases gluconeogenesis from pyruvate. This occurs directly through inhibition of pyruvate carboxylase via its specific intermediaries, methylmalonyl CoA and propionyl CoA and indirectly through depletion of acetyl CoA, a specific allosteric activator of this enzyme(Reference Blair, Cook and Lardy57, Reference Anderson and Bridges58). The present trial revealed increased propionylcarnitine concentrations, suggesting inhibited gluconeogenesis from pyruvate, resulting in sparing amino acids. Methylmalonylcarnitine tended to decrease among diets, which supports the hypothesis of reduced amino acid catabolism, since methylmalonylcarnitine is known to be a metabolite of valine, methionine and isoleucine catabolism. Moreover, the significantly decreased plasma AST activity in cats supplemented with oligofructose and inulin indicates inhibited gluconeogenesis from aspartate.
In conclusion, impaired glucose tolerance and increased insulin resistance were observed in obese cats compared to normal-weight cats, regardless of diet. Adding 2·5 % of a mixture of oligofructose and inulin to a basic diet did not affect glucose tolerance in healthy normal-weight or in obese cats. However, modulation of glucose metabolism by enhancing gluconeogenesis from propionate and therefore inhibition of amino acid catabolism can be suggested, and can be beneficial in the treatment of feline insulin resistance and diabetes. Yet, further research investigating the postprandial effects of prebiotics on acylcarnitine profile can be of interest.
Acknowledgements
This study is within the scope of the postgraduate study of A.V. and is funded by the Institute for Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen). Expenses for the laboratory analyses were covered by the Beneo-Orafti, Beneo-Group (Tienen, Belgium), who also provided the oligofructose and inulin mixture (Beneo™ Synergy 1®). The pet food was provided by Versele-Laga (Deinze, Belgium). A. V. was responsible for the study design, study performance, characteristic analysis, data analysis and manuscript drafting. K. G. was responsible for catheter placement and contributed to manuscript drafting. B. W. and J. B. supervised the plasma analyses and also contributed to the manuscript drafting. M. H., S. D. and G. P. J. J., all three promoters of A. V. contributed to the development of the study design, data analysis and manuscript drafting. At last, we gratefully acknowledge Herman De Rycke for performing the food analyses, Inge Vaesen for the plasma analyses, Rebekka Hollebosch and Steven Galle for taking care of the experimental animals, and Stephanie Van Weyenberg and Georgios Papadopoulos for technical assistance. It is declared by the corresponding author that no conflict of interest exists for this paper.









