Case report
Seven years ago, a 57-year-old woman with a family history of Huntington's disease presented with depression, changes in personality, apathy, anxiety, poor memory and clumsiness. Her genetics confirmed Huntington's disease, with a CAG repeat number of 17/46. Over the subsequent 4 years, her condition was marked by psychiatric symptoms and mild chorea, which responded well to tetrabenazine 25 mg once daily, reaching 25 mg twice daily by 2014. Her mood was controlled with citalopram followed by sertraline. Her main complaints were bouts of introversion and tearfulness, but she remained socially engaged with friends. By early 2015, her swallowing had ostensibly become problematic. However, a speech and language assessment and a gastroscopy determined that her swallowing was normal. By spring 2016, she was starting to fall often, with worsening athetoid movements and increased somnolence. These changes led to a switch in medication to olanzapine at 15 mg per day and an increase in her dose of sertraline to 100 mg a day.
By summer of 2017, her choreoathetoid movements had worsened and were accompanied by weight loss and a deterioration in her mood. She required a short in-patient stay for fluids via a nasogastric tube. A psychiatric review diagnosed her with a depressive psychosis with retarded affect, mood-congruent delusions and auditory command hallucinations. She believed she did not deserve to eat and drink, and the command hallucinations ordered her not to do so. She had extracampine hallucinations, sensing people at the end of her garden. In light of her clinical state of emaciation and the requirement for support, a trial of bilateral electroconvulsive therapy (ECT) was suggested for her depressive psychosis. A twice-weekly regimen was started, with the aim of optimising seizure length to between 30 and 60 s. In winter of 2017, she received an initial 12 courses of biweekly bilateral ECT treatments using between 75 and 150 mC. Her initial response was favourable and she became euthymic, with remission of her psychomotor retardation, but her psychotic symptoms persisted in the form of command hallucinations despite developing insight. Her delusion of guilt also resolved and, by the tenth dose of ECT, she started to eat and drink again. The initial course of treatment was complete by early February 2018 and, with the exception of her hallucinations, her psychiatric symptoms ceased and insight was retained.
However, the persistence of psychotic symptoms in the absence of a significant mood component led to an additional 12 sessions of bilateral ECT (administered biweekly with 150–225 mC; maintenance treatment consisting of bilateral 150–300 mC treatment biweekly continued into late 2019) with the aim of eradicating the hallucinations. She then developed a respiratory tract infection, which led to a relapse in her psychiatric symptoms with agitation, hallucinations, and marked choreoathetosis and dyskinesia that led to the reintroduction of tetrabenazine. Despite this setback, she did improve following the last 12 doses of ECT. This course was completed in late spring 2019. She was kept on maintenance ECT once per week, with partial remission of the auditory hallucinations, which had reduced in frequency and no longer distressed her. There was a subsequent increase in the frequency of the hallucinations, with preservation of insight, and a corresponding reduction in ECT to fortnightly. She has remained stable on this regimen to date. Her involuntary movements have also improved over that time, although she remains on tetrabenazine, haloperidol and sertraline.
Method
A literature search of PubMed for ‘Electroconvulsive therapy and Huntington's’ and ‘ECT and Huntington's’ led to the discovery of 20 papers, 18 of which were in English. Six publications were based on in vitro studies and animal studies, leaving 12 publications for review. A further three papers that were not listed in the PubMed search were sourced from references in papers from earlier years. All publications were either single-case reports or retrospective case series; there were no randomised controlled trials or prospective studies.
Consent for publication
Signed informed consent was obtained from the patient described in our case report.
Results
Table 1 shows a breakdown by demographic and disease characteristics of the 37 patients described in 15 studies. Tables 2–5 summarise the predominant psychiatric complaints, the effects of ECT on the number of medications used during ECT treatment, the CAG copy number, ECT treatment and seizure duration. Supplementary Table 1 (available online at https://doi.org/10.1192/bjb.2020.51) summarises individual patient details from the literature, including our aforementioned case.
Table 1 Summary of patient characteristics and ECT treatment

N/A, not available.
Table 2 Breakdown of main presenting symptoms
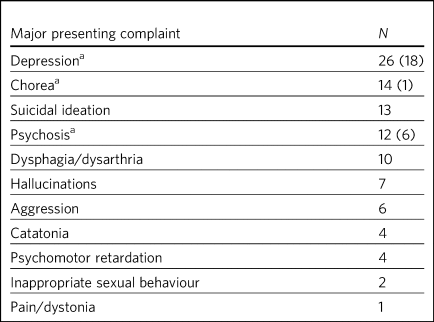
a. In total (sole reason); see text for details.
Table 3 Treatment before, during and after ECT
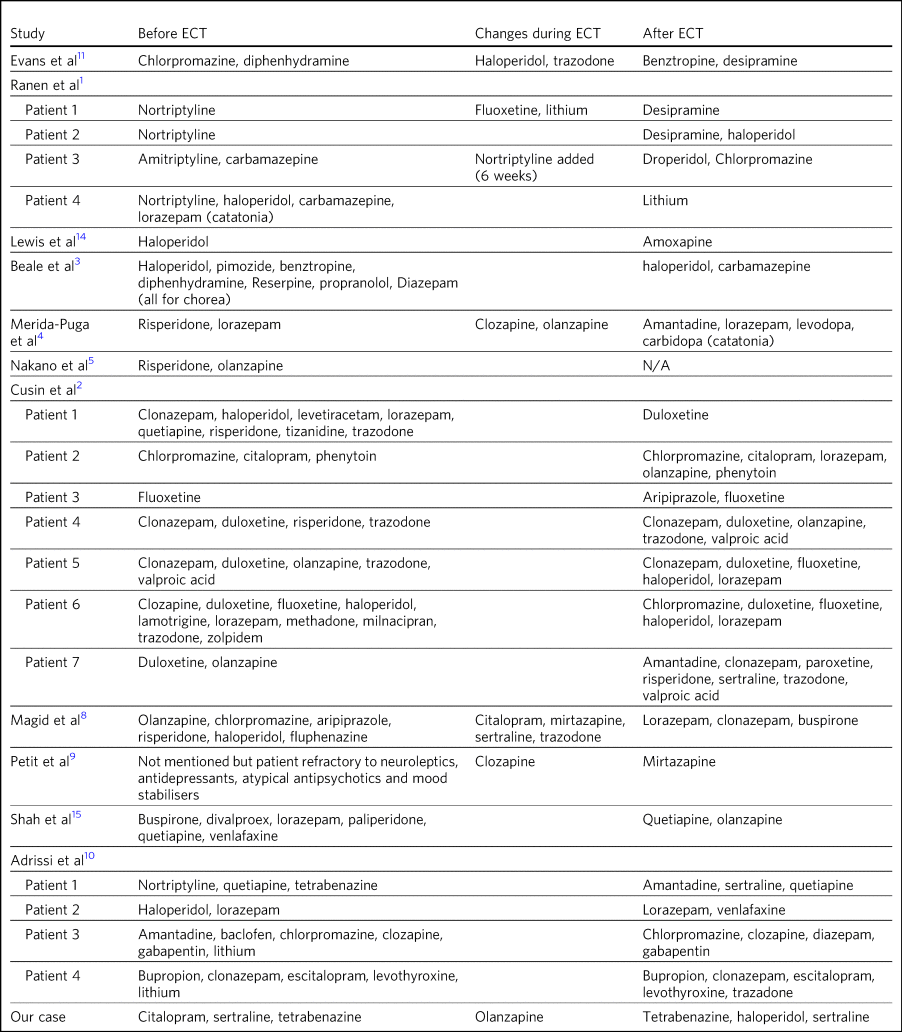
N/A, not available.
Table 4 Number of trinucleotide repeats, age of diagnosis, ECT treatments and length of psychiatric symptoms prior to ECT
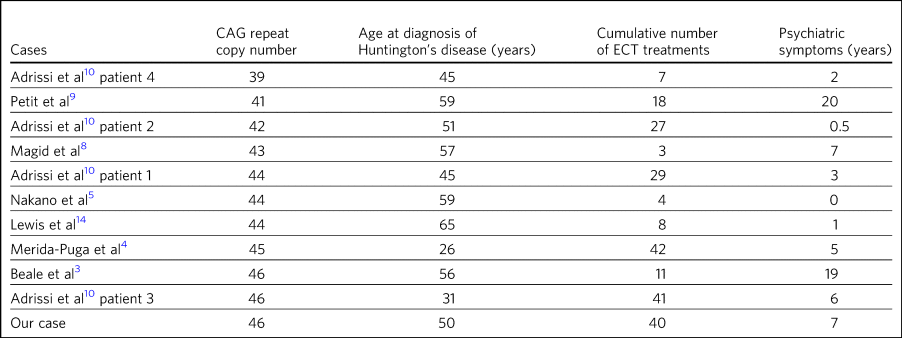
Table 5 ECT seizure length in seconds and treatment characteristics
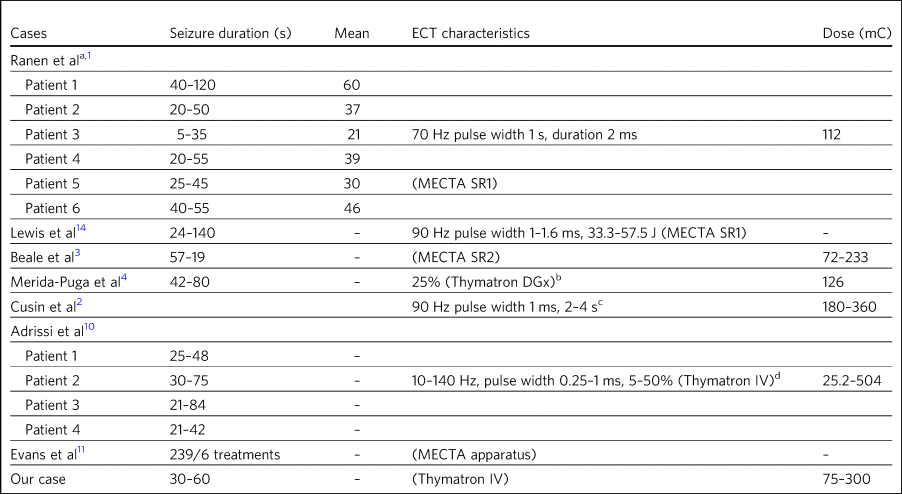
a. Double stimulus was administered in Ranen et al’s patients, but no further details were provided.
b. Assumed to be the USA version with 0.9 A and charge of 504 mC. The lowest percentage given to Merida-Puga et al’s patient was calculated according to the half-life method. While no figure was given for the lowest percentage, 25% was the maximum stated.
c. Text does not state machine used or charge or current characteristics but states 'stimulus intensities comparable to patients without HD'. 0.8 A is presumed for the purpose of calculation.
d. As per specification sheet from the manufacturer, the maximum output is 504 mC with the range calculated based on quoted percentage delivered. This machine is capable of double stimulus, but this was not mentioned in Adrissi et al's paper.
The time to ECT treatment after the diagnosis of Huntington's disease was between 0 and 17 years, with a median of 3 years and mean of 4.42 years. However, the sixth patient of Ranen et al. had previously had ECT for depression before she was diagnosed.Reference Ranen, Peyser and Folstein1 The age of psychiatric symptoms at presentation for ECT varied from 0 to 22 years prior, with a median of 2.5 years and a mean of 5.73 years.
It should be noted that the diagnostic confirmation of Huntington's was solely clinical prior to 1993; however, despite the availability of genetic testing after 1993 (Huntington disease Collaborative Research Group), diagnosis only preceded presentation in this group of patients after 2013 (Cusin et al's first patientReference Cusin, Franco, Fernandez-Robles, DuBois and Welch2). Prior to 2013, patients first presented on average 7.4 years before the diagnosis of Huntington's was made. After 2013, all patients with psychiatric complaints who went on to have ECT presented an average of 6.6 years after the diagnosis of Huntington's had been established. Six patients in total were diagnosed at the time of presentation, although five of them were diagnosed prior to 2013. For example, Beale et al's patient had choreiform movements (and no psychiatric manifestations) from the age of 35, but was not diagnosed until the age of 56 in 1995.Reference Beale, Kellner, Gurecki and Pritchett3 However, this apparent lag after the introduction of genetic testing is likely to reflect the 16-year gap between Beale et al's paper in 1995 and those of Merida-Puga et al and Nakano et al in 2011 and 2013, respectively.Reference Beale, Kellner, Gurecki and Pritchett3–Reference Nakano, Ono, Yamaguchi, Sugimoto, Yamaguchi and Morimoto5 Merida-Puga et al's patient was diagnosed following postpartum psychosis and a family history suggestive of Huntington's in her father; she went on to have ECT for catatonia. Nakano et al's patient was diagnosed owing to his brother's recent diagnosis with Huntington's at another hospital, having already received ECT for treatment-refractory psychosis.
Reason for referral for ECT
Table 2 shows a breakdown of the main characteristics of the clinical presentations; 48.6% of patients were referred for depression alone without psychotic features, and psychosis was the main cause of referral in 16%. Many of the referrals for depression alone were from some of the earliest reports, which lacked a clear description of the referral characteristics.Reference Brothers and Meadows6,Reference Folstein, Abbott, Chase, Jensen and Folstein7 The remainder of patients had numerous coexisting factors such as paranoia, delusions and other manifestations of psychosis, including hallucinationsReference Ranen, Peyser and Folstein1,Reference Cusin, Franco, Fernandez-Robles, DuBois and Welch2,Reference Merida-Puga, Ramirez-Bermudez, Aguilar-Venegas, Fricchione and Espinola-Nadurille4,Reference Nakano, Ono, Yamaguchi, Sugimoto, Yamaguchi and Morimoto5,Reference Magid, Trevino, Reid, Jalalat, Husain and Kahn8–Reference Evans, Pedersen and Tancer11 .
Coexistent motor symptoms were present in 20 patientsReference Ranen, Peyser and Folstein1–Reference Beale, Kellner, Gurecki and Pritchett3,Reference Nakano, Ono, Yamaguchi, Sugimoto, Yamaguchi and Morimoto5,Reference Petit, Hozer, Youssov, Lavaud, Hardy and Mouaffak9,Reference Adrissi, Nadkarni, Gausche and Bega10,Reference Benson and Blumer12–Reference Shah, Alluri and Sharma15 (including our case), with chorea being the most commonly used term. Involuntary movement and impaired gait were also terms used without further clarification (e.g. Cusin et al uses both terms for different patients within the same seriesReference Cusin, Franco, Fernandez-Robles, DuBois and Welch2). Psychomotor retardation was reported in four patientsReference Ranen, Peyser and Folstein1,Reference Cusin, Franco, Fernandez-Robles, DuBois and Welch2,Reference Adrissi, Nadkarni, Gausche and Bega10 (including our case), with one patient being described as having neurovegetative symptoms (Cusin et al,Reference Cusin, Franco, Fernandez-Robles, DuBois and Welch2 patient 3), and coexistent dysarthria/dysphagia in two patients. Catatonia was mentioned in four patientsReference Ranen, Peyser and Folstein1,Reference Cusin, Franco, Fernandez-Robles, DuBois and Welch2,Reference Merida-Puga, Ramirez-Bermudez, Aguilar-Venegas, Fricchione and Espinola-Nadurille4 (fourth and fifth patient of series,Reference Ranen, Peyser and Folstein1 postpartumReference Merida-Puga, Ramirez-Bermudez, Aguilar-Venegas, Fricchione and Espinola-Nadurille4 and the second patient of seriesReference Cusin, Franco, Fernandez-Robles, DuBois and Welch2) but was the primary focus of treatment only in Merida-Puga et alReference Merida-Puga, Ramirez-Bermudez, Aguilar-Venegas, Fricchione and Espinola-Nadurille4; see Supplementary Table 1.
Effects on psychiatric symptoms
The primary reason for prescribing ECT in all but seven patients (six for psychosis and one for chorea) was depression (Table 2 and Supplementary Table 1). There were universally favourable outcomes in the use of ECT for this purpose. It was reported to be successful in most cases, and the patients who were reported to relapse did so on shorter courses of ECT.Reference Benson and Blumer12,Reference Heathfield13 However, these were early reports, and there have been numerous subsequent reports of relatively short courses with no relapse. For example, Ranen et al's third patient, who had depression, psychosis and catatonia, only needed five ECT treatments to achieve symptom resolution.
Psychosis responded to ECT in all six patientsReference Cusin, Franco, Fernandez-Robles, DuBois and Welch2,Reference Merida-Puga, Ramirez-Bermudez, Aguilar-Venegas, Fricchione and Espinola-Nadurille4,Reference Magid, Trevino, Reid, Jalalat, Husain and Kahn8,Reference Evans, Pedersen and Tancer11 who were reported to have it as the primary presentation, which included our case. When coexistent disease such as depression was considered, 12 patients in total had prominent psychosis, and all responded to varying degrees, including our case.Reference Ranen, Peyser and Folstein1,Reference Nakano, Ono, Yamaguchi, Sugimoto, Yamaguchi and Morimoto5,Reference Petit, Hozer, Youssov, Lavaud, Hardy and Mouaffak9,Reference Adrissi, Nadkarni, Gausche and Bega10 Merida-Puga et al reported refractory psychosis thought to be due to use of depot dopamine antagonists, although the focus of their treatment was the refractory catatonia.Reference Merida-Puga, Ramirez-Bermudez, Aguilar-Venegas, Fricchione and Espinola-Nadurille4
Hallucinations, either auditory (three cases including our case),Reference Ranen, Peyser and Folstein1,Reference Evans, Pedersen and Tancer11,Reference Heathfield13 visual (two cases)Reference Cusin, Franco, Fernandez-Robles, DuBois and Welch2,Reference Adrissi, Nadkarni, Gausche and Bega10 or both visual and auditory (two cases),Reference Merida-Puga, Ramirez-Bermudez, Aguilar-Venegas, Fricchione and Espinola-Nadurille4,Reference Magid, Trevino, Reid, Jalalat, Husain and Kahn8 also responded well to treatment. The exception was Ranen et al's fourth patient, where this was unclear, although the hallucinations probably improved with the patient's other symptoms.
There were four cases of reported psychomotor retardation, including our patientReference Ranen, Peyser and Folstein1,Reference Adrissi, Nadkarni, Gausche and Bega10,Reference Benson and Blumer12 (in addition, speech retardation was reported in Nakano et al); all were described as improved following ECT without further clarification. Benson and Blumer's first patient had a ‘temporary recovery’.
Catatonia (second and fourth patients of Ranen et al; second patient of Cusin et alReference Ranen, Peyser and Folstein1,Reference Cusin, Franco, Fernandez-Robles, DuBois and Welch2,Reference Merida-Puga, Ramirez-Bermudez, Aguilar-Venegas, Fricchione and Espinola-Nadurille4 ) was described in four patients and improved in all. This improvement often paralleled the response to ECT of depression, suicidality and psychosis. Two of these cases were resolved by relatively short courses of ECT (five treatments for Ranen et al's fourth patient – though their condition was, surprisingly, described as refractory – and seven treatments for Cusin et al's second patient). However, the presence of catatonia was refractory in the remaining two patients. Both Ranen et al's second patient (who relapsed repeatedly) and Merida-Puga et al's patientReference Merida-Puga, Ramirez-Bermudez, Aguilar-Venegas, Fricchione and Espinola-Nadurille4 (who required withdrawal from long-acting antipsychotics) needed extended ECT courses (4 years for Ranen et al's patient but just over 3 months of an in-patient stay for the latter) to resolve the catatonia, with 35 and 42 ECT treatments, respectively. Merida-Puga et al's patient had a Busch–Francis catatonia score of 26 (total severity) on first admission, falling to 4 after a second hospital admission and discharge.
Our patient required repeated doses of ECT following a relapse of psychosis over 2 years. Eventually, she showed a partial response, with improvements in her mobility and psychomotor retardation. She started to gain weight, although her delusions persisted. Improved gait was reported in six cases, including Lewis et al and the first, fourth, fifth and sixth patients of Cusin et al, who had bilateral frontotemporal and right unilateral (RUL) placement, respectively.Reference Cusin, Franco, Fernandez-Robles, DuBois and Welch2,Reference Lewis, DeQuardo and Tandon14
Disorders of eating and/or speech were mentioned for a total of ten patients. Dysphagia was mentioned in two cases (Cusin et al'sReference Cusin, Franco, Fernandez-Robles, DuBois and Welch2 second patient and ours) and dysarthria in another two (Cusin et al's third and sixth patients). Both dysarthria and dysphagia were reported for two patients (Cusin et al's fourth and fifth patients). For all six of these patients, their symptoms were described as resolving or dramatically improved. Refusal to eat was described in two patients (Ranen et al's second patient and Magid et al's patientReference Ranen, Peyser and Folstein1,Reference Magid, Trevino, Reid, Jalalat, Husain and Kahn8 ), while poor appetite was described in another two (Ranen et al's sixth patient and Adrissi et al's secondReference Adrissi, Nadkarni, Gausche and Bega10). Both of Ranen et al's patients were described as improved, but there was no further clarification regarding outcome for the remaining two.
Aggression,Reference Ranen, Peyser and Folstein1,Reference Cusin, Franco, Fernandez-Robles, DuBois and Welch2,Reference Adrissi, Nadkarni, Gausche and Bega10–Reference Benson and Blumer12,Reference Shah, Alluri and Sharma15 inappropriate sexual behaviourReference Cusin, Franco, Fernandez-Robles, DuBois and Welch2,Reference Shah, Alluri and Sharma15 and agitationReference Shah, Alluri and Sharma15 were also mentioned and described as improved.
Only two reports documented improvement using psychiatric rating scales, with the BPRS-E (Brief Psychiatric Rating Scale, Expanded) score dropping from 88 to 38 after 12 ECT sessions in Petit el al's patient, and BPRS dropping from 139 to 68 in Nakano et al's patient (the PANS (Positive and Negative Syndrome Scale) score fell from 139 to 68 in the latter).Reference Nakano, Ono, Yamaguchi, Sugimoto, Yamaguchi and Morimoto5,Reference Petit, Hozer, Youssov, Lavaud, Hardy and Mouaffak9 Both Beale et al and Lewis et al documented improvement in the Hamilton rating scale for depression from 36 pre-treatment to 13 post-treatment in Beale et al and 36 to 10 post-treatment in Lewis et al.Reference Beale, Kellner, Gurecki and Pritchett3,Reference Lewis, DeQuardo and Tandon14
Effects on motor symptoms
Chorea was mentioned in 14 cases,Reference Ranen, Peyser and Folstein1–Reference Beale, Kellner, Gurecki and Pritchett3,Reference Nakano, Ono, Yamaguchi, Sugimoto, Yamaguchi and Morimoto5,Reference Petit, Hozer, Youssov, Lavaud, Hardy and Mouaffak9–Reference Evans, Pedersen and Tancer11,Reference Heathfield13–Reference Shah, Alluri and Sharma15 including our case (three patients of Cusin et al and two of Adrissi et alReference Cusin, Franco, Fernandez-Robles, DuBois and Welch2,Reference Adrissi, Nadkarni, Gausche and Bega10 ). Improvement was documented in three cases (Beale et al, Petit et al and Shah et al), although five were described as showing no change (Ranen et al, Lewis et al, Nakano et al, Cusin et al's fifth patient and Adrissi et al's first patient). In two cases, chorea was described as worse (Adrissi et al's second patient and Evans et al). In the remaining four patients, no details were given, despite this symptom initially having been described as present.
Only one patient was treated with ECT specifically for chorea. This patient demonstrated improvement initially and, despite worsening, their chorea never returned to the original level and was sustained at the improved level for a year.Reference Beale, Kellner, Gurecki and Pritchett3
Our patient showed some response with respect to the choreoathetoid movements, which had become unresponsive to tetrabenazine. The medication had been withdrawn given her depression and fears of worsening those symptoms. Olanzapine, however, did not lead to any improvement in her chorea or psychiatric symptoms. Following a favourable response of the chorea to ECT, a low dose of tetrabenazine was reintroduced with good effect and had no further influence on her mood. Her gait and mobility also improved.
The use of rating scales for motor function was mentioned in only three cases. The Unified Huntington's Disease Rating Scale (UHDRS) motor score was recorded before and after only for Adrissi et al's second patient (27/31 out of a total of 124; their first case had an initial score of 49 with no follow-up score), while Petit et al's patient's UHDRS score decreased from 47 to 37 after 12 treatments and then rose to 57 after 1 year.Reference Petit, Hozer, Youssov, Lavaud, Hardy and Mouaffak9,Reference Adrissi, Nadkarni, Gausche and Bega10 For most cases, there was no mention of any response, which is not surprising because this was the focus of the treatment in only one of the studies. Surprisingly, despite admitting their patient specifically for the treatment of chorea (there were no psychiatric manifestations), Beale et al used no rating scales to document improvement.Reference Beale, Kellner, Gurecki and Pritchett3
Effects on cognition
Many of the case reports mentioned problems with cognition, but few documented it with formal scores either before or after treatment. Scores were recorded before and after treatment by Nakano et al (Mini-Mental State Examination (MMSE) 27/26), Lewis et al (MMSE 23/24) and Ranen et al (second and fourth patients; MMSE 20/30 rose to 26 by discharge in the former and was 20/30 rising to 24–26/30 (administered twice) in the latterReference Ranen, Peyser and Folstein1,Reference Nakano, Ono, Yamaguchi, Sugimoto, Yamaguchi and Morimoto5,Reference Lewis, DeQuardo and Tandon14 ). Cusin et al used the Montreal Cognitive Assessment (MoCA) scale for their patients and described the scores as improved, although they did not publish the values.Reference Cusin, Franco, Fernandez-Robles, DuBois and Welch2
Ranen et al's third patient showed a drop in MMSE from 26/30 to 18/28, with an episode of delirium after his eighth ECT treatment. No further scores were recorded, but the patient was described as ‘not completely recovered cognitively’.Reference Ranen, Peyser and Folstein1 Adrissi et al's second patient had an initial MoCA of 17/20, but no further score was documented.Reference Adrissi, Nadkarni, Gausche and Bega10
Effect on medication used
Table 3 documents the treatment at admission; changes, if any, that occurred during the course of treatment for the psychiatric manifestations of disease; and discharge medication for those patients where it was recorded.
In those patients who required a number of drugs to treat the psychiatric manifestations of Huntington's disease, implying difficulty in management, there was not necessarily a requirement for more ECT doses or prolonged ECT treatment. However, in patients with pharmacological treatment resistance and the requirement for many drugs to manage symptoms, there does appear to be scope for significant rationalisation of pharmacological therapy when ECT is used adjunctively. Beale et al's patient and Cusin et al's first and sixth patients all presented between 10.5 and 19 years after diagnosis but responded well to limited ECT treatments and were discharged on much less medication.Reference Cusin, Franco, Fernandez-Robles, DuBois and Welch2,Reference Beale, Kellner, Gurecki and Pritchett3 Only Adrissi et al's third patient, Ranen et al's second patient and ours required extended ECT. It is not clear why there was resistance to conventional treatment in these cases.Reference Ranen, Peyser and Folstein1,Reference Adrissi, Nadkarni, Gausche and Bega10
However, this was not a consistent outcome; for example, Cusin et al's seventh patient was discharged on more medication after ECT than prior, and Adrissi et al's third and fourth patients were discharged on a comparable number of drugs to those given on admission.Reference Cusin, Franco, Fernandez-Robles, DuBois and Welch2,Reference Adrissi, Nadkarni, Gausche and Bega10
Effect of CAG copy number on disease or treatment
The earliest reference to CAG copy number, and therefore genetic confirmation of the diagnosis, comes from Lewis et al in 1994 – in keeping with testing, which became available after the discovery of the trinucleotide repeat a year earlier by the Huntington Disease Collaborative Research Group. Copy numbers of trinucleotide repeats have no effect on the severity of the disease, but the age of presentation is inversely correlated with increasing copy number.Reference Vassos, Panas, Kladi and Vassilopoulos16
Table 4 shows copy number, number of ECT treatments and length of psychiatric symptoms for cases where this information was documented. Although those requiring more cumulative ECT treatments may appear to be clustered with those with higher repeat copy numbers, Petit et al's and Adrissi et al's second and first patients represent evidence to the contrary.Reference Petit, Hozer, Youssov, Lavaud, Hardy and Mouaffak9,Reference Adrissi, Nadkarni, Gausche and Bega10 Given that there was only a difference of seven CAG repeats among the 11 patients, there appears to be no significance to this. In keeping with this, the youngest patient in the review, who at 20 years old was likely to have had genetic testing, although the results of this were not documented, responded well to ECT, with his symptoms described as resolved after only seven treatments (Cusin et al's second patientReference Cusin, Franco, Fernandez-Robles, DuBois and Welch2).
ECT treatment course
Treatment courses varied between three and 42 treatments in total, with a median of eight. Relapse was mentioned in seven cases (Cusin et al's fifth and sixth patients).Reference Cusin, Franco, Fernandez-Robles, DuBois and Welch2,Reference Merida-Puga, Ramirez-Bermudez, Aguilar-Venegas, Fricchione and Espinola-Nadurille4,Reference Benson and Blumer12,Reference Heathfield13,Reference Shah, Alluri and Sharma15 For Heathfield's patient (who had three treatments) and Benson and Blumer's patients, relapse was described in general terms, with no description of the treatment course in the latter's series.Reference Benson and Blumer12,Reference Heathfield13 In the remaining patients, it is not clear why they relapsed, except for having received relatively short courses of between five and nine ECT treatments. Merida-Puga et al's patient had treatment-resistant catatonia exacerbated by dopamine antagonists prescribed for her psychosis; this led to relapses and an extended in-patient stay.
Most patients had treatment for up to 1 year (22 patients), although our patient has been undergoing continuing maintenance treatment at increasing intervals for more than 2 years to date. Ranen et al's second patient required treatment for 4 years and Petit et al's patient for more than 1 year.Reference Ranen, Peyser and Folstein1,Reference Petit, Hozer, Youssov, Lavaud, Hardy and Mouaffak9 Those requiring extended treatment presumably did so because of continued symptomsReference Ranen, Peyser and Folstein1,Reference Adrissi, Nadkarni, Gausche and Bega10 (see below).
Patients who had an extended course, arbitrarily taken to be more than 20 treatments (seven patients including oursReference Ranen, Peyser and Folstein1,Reference Merida-Puga, Ramirez-Bermudez, Aguilar-Venegas, Fricchione and Espinola-Nadurille4,Reference Petit, Hozer, Youssov, Lavaud, Hardy and Mouaffak9,Reference Adrissi, Nadkarni, Gausche and Bega10 ), did not have different characteristics from patients with similar symptoms but much less cumulative ECT treatment. Characteristics such as the nature of symptoms, length of time from diagnosis, length of time of psychiatric symptoms, age and sex did not appear to affect the number of ECT treatments. However, refractory catatonia complicating psychosis may have been a factor in extended treatment, with Ranen et al's second patient and Merida-Puga et al's patientReference Merida-Puga, Ramirez-Bermudez, Aguilar-Venegas, Fricchione and Espinola-Nadurille4 requiring 35 and 42 treatments, respectively.
The four patients who had ECT more than 10 years after diagnosisReference Ranen, Peyser and Folstein1,Reference Cusin, Franco, Fernandez-Robles, DuBois and Welch2 (Ranen et al's sixth, Cusin et al's first, fifth and seventh patients) also showed excellent responses to ECT with between 8 and 13 treatments.
Length of seizure in seconds
Table 5 shows the seizure duration in the cases where it was recorded.Reference Ranen, Peyser and Folstein1,Reference Beale, Kellner, Gurecki and Pritchett3,Reference Merida-Puga, Ramirez-Bermudez, Aguilar-Venegas, Fricchione and Espinola-Nadurille4,Reference Adrissi, Nadkarni, Gausche and Bega10,Reference Evans, Pedersen and Tancer11,Reference Lewis, DeQuardo and Tandon14 Only Beale et al mentioned a reduction in seizure time from 57 s at the beginning of treatment to 19 s by the end. This was for the sole case in which ECT was administered for chorea.Reference Beale, Kellner, Gurecki and Pritchett3 Our patient required ongoing treatment with increasing doses of ECT, although control of delusions was eventually achieved with seizure lengths between 30 and 60 s.
Dose of ECT treatment
It was not always possible to discern the dose of treatment given, as doses were rarely documented and were not necessarily consistent with other reports.Reference Ranen, Peyser and Folstein1–Reference Merida-Puga, Ramirez-Bermudez, Aguilar-Venegas, Fricchione and Espinola-Nadurille4,Reference Adrissi, Nadkarni, Gausche and Bega10,Reference Evans, Pedersen and Tancer11,Reference Lewis, DeQuardo and Tandon14 For example, Beale et al's patient was stated as needing 72 mC initially, rising to 233 mC, with a corresponding drop in seizure duration from 57 s initially to 19 s at the end.Reference Beale, Kellner, Gurecki and Pritchett3 When recorded, the frequency and pulse width varied. Alternatively, descriptions of percentage of maximum charge, where stated, together with the machine used (and its specifications), allowed for calculation of this figure where it was not explicitly stated (Table 5).
There did not appear to be any factors to explain why five patients, including our patient, required higher cumulative ECT treatments (Ranen et al's second patient, Adrissi et al's first, second and third patients).Reference Ranen, Peyser and Folstein1,Reference Adrissi, Nadkarni, Gausche and Bega10 More generally, most patients were started on relatively low doses of ECT and titrated as treatment progressed, though this was not always documented. Only Adrissi et al's third patient was started on maximum charge dose of 100% (504 mC) due to refractory psychosis. This was later cut down to 50% (252 mC) owing to a bout of delerium that was subsequently felt to be due to medication. The presence of catatonia in Merida-Puga et al and Ranen et al's second patient may be considered a proxy for severity, but this was not the case for the other two catatonic patients (see above). Likewise, short treatment courses were reported in the earlier studies to be more likely to lead to relapse, but this was not borne out by later studies (see above). Getting the dose and the induced seizure length right appears to take more time in the out-patient setting, as may be expected. The three intense in-patient stays for our patient, Merida-Puga et al's patient and Adrissi et al's third patient (3 months in the latter two cases) allowed this to be achieved more rapidly, but the cumulative doses were all high.Reference Merida-Puga, Ramirez-Bermudez, Aguilar-Venegas, Fricchione and Espinola-Nadurille4,Reference Adrissi, Nadkarni, Gausche and Bega10 Overall, there appear to be no overt factors that predict who is likely to respond quickly or otherwise, although most patients will not require extended treatment based on this review.
Lead positioning
The predominant positions were RUL in 12 patients, one RUL unilateral and bilateral (unspecified), four bilateral (one bilateral frontotemporal, three bilateral) and two bitemporal (Supplementary Table 1).
Conclusion
The diagnosis of Huntington's disease is usually preceded by psychiatric symptoms in cases where family history is absent, sometimes by years.Reference Julien, Thompson, Wild, Yardumian, Snowden and Turner17 However, as we found in this review, patients are now likely to have an established diagnosis of Huntington's by the time they are considered for ECT. Although a CAG repeat number greater than 36 in the Huntington gene confirms the disease, an increased number of repeats is negatively correlated with age at presentation rather than severity of disease.Reference Vassos, Panas, Kladi and Vassilopoulos16
Psychiatric manifestations of Huntington's disease vary according to the stage of the disease; apathy, for example, is found in 50% of patients by stage four on the UHDRS. Also present are obsessive–compulsive behaviour, irritability and aggression. Depression also increases over time, with only psychosis remaining consistent throughout at 11%.Reference Dujin, Craufurd, Hubers, Giltay, Bonelli and Rickards18
The prevalence of depression is about 50%, compared with anxiety at 17–61%, irritability at 35–73%, obsessive–compulsive disorders at 7–50% and psychosis at 3–11%. The prevalence of hypersexuality is between 2.1 and 30% and is slightly lower in women, whereas the prevalence of hyposexuality is higher at 63% in men and 75% in women.Reference Paoli, Botturi, Ciammola, Silani, Prunas and Lucchiari19 The use of ECT, however, is recommended only for depression on the basis of two of the above series.Reference Ranen, Peyser and Folstein1,Reference Lewis, DeQuardo and Tandon14,Reference Bachoud-Levi, Ferreira, Massart, Youssov, Rosser and Busse20
There appeared to be little in the way of any effect on cognition with the use of ECT, although this was not a primary concern in this patient group. In the few reports where cognition was mentioned, the patients responded well and appeared to retain comparable pre-treatment scores over time, although the numbers were too small for us to draw any firm conclusions. However, Nakano et al's patient, despite comparable pre- and post-treatment MMSE scores (and significantly improved PANSS and BPRS scores), had greatly decreased 99 mTc uptake in the basal ganglia, cingulate gyrus and thalamus on SPECT after 21 ECT treatments compared to pre-treatment SPECT.Reference Nakano, Ono, Yamaguchi, Sugimoto, Yamaguchi and Morimoto5
There is little consistent evidence regarding the use of ECT for the motor manifestations of the disease. Chorea, a hyperkinetic movement disorder, shows variable response. In the studies considered in this review, chorea often responded to ECT (including for our patient), but this finding was not always documented and thus it cannot be extrapolated to a recommendation given the small number of patients. However, psychomotor retardation and catatonia, which are manifestations of psychiatric disease, all responded favourably, although the presence of catatonia may require more protracted ECT treatment. The reported swallowing issues and weight loss in our patient and others responded well. A situation that appeared terminal in our case has been managed effectively for the patient and her family with good control, for over 2 years following the first dose of ECT.
All the studies considered in the current review were either single-case reports or retrospective case series. There were no comparison or prospective studies. The most striking aspect of this literature was how the choice of ECT as a treatment came to be prescribed. In nearly all cases, ECT was a last resort when all else had failed, with the exception of one case in which it was used for the specific management of chorea.Reference Beale, Kellner, Gurecki and Pritchett3 Consequently, strict psychiatric criteria were lacking prior to the decision to start ECT. The main concern in using ECT was related to controlling the manifestations of psychosis or depression/suicidality, with concerns regarding other manifestations, such as outward aggression, in only a few cases. Clinical success was documented by a general clinical sense of improvement, serving as an indication of efficacy, with no clearly defined end points. Similarly, the cognitive and motor scoring of patients was haphazard and inconsistent, with only limited numbers of patients having clear objective scoring on any scales – motor, cognitive or psychiatric performance – either before or after ECT was administered in order to monitor patient responses.
It is clear that the preliminary though limited evidence from this review supports the use of ECT for relieving depressive symptoms. However, this is often considered as a last resort when all other interventions have failed. The current review suggests that additional consideration be given to the use of ECT as an adjunct in conventional treatment-resistant cases of depression, as well as for wider psychiatric manifestations of the disease, especially where depressive and psychotic symptoms coexist. These wider psychiatric manifestations and complications, which include psychomotor retardation and hallucinations, appear to respond well to the use of ECT. Where there are wider psychiatric manifestations of Huntington's disease, ECT may not only control these more effectively but could also lead to rationalisation of polypharmacy. Chorea may be less likely to respond to ECT, and so its use for this cannot be recommended based on the current review. Further investigative work with clear criteria and monitoring may lead to ECT being considered earlier and more often for patients with difficult-to-manage psychiatric manifestations of Huntington's disease.
About the authors
Walied Mowafi is a Consultant Neurologist at the Department of Neurology, Calderdale Royal Hospital, West Yorkshire, UK. Jon Millard is a Consultant Psychiatrist at South West Yorkshire Partnership NHS Foundation Trust, Wakefield, UK.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjb.2020.51
Declaration of interest
None.
ICMJE forms are in the supplementary material, available online at https://doi.org/10.1192/bjb.2020.51.









eLetters
No eLetters have been published for this article.