Congenital diaphragmatic hernia (CDH), which is characterised by incomplete formation of the diaphragm during embryogenesis, occurs in approximately one in 2500 live births(Reference Greer1). Despite the development of neonatal intensive care, roughly one in four infants born with CDH in Japan die due to pulmonary hypoplasia and/or pulmonary hypertension, which are the major complications of CDH(Reference Nagata, Usui and Kanamori2). And even if they survive the life-threatening stage of CDH, survivors typically experience pulmonary, cardiovascular, gastrointestinal, neurodevelopmental or musculoskeletal morbidities during the long-term follow-up period(Reference Peetsold, Heij and Kneepkens3). In addition, patients’ (i.e. children’s) long-term quality of life after treatment of congenital anomalies such as CDH was related to the quality of life of their mothers(Reference Kubota, Yamakawa and Yamamoto4). Therefore, we should explore the possibility of primary prevention, to reduce as much as possible the number of infants with CDH, in addition to focusing on reducing CDH-related morbidity and mortality.
The pathogenesis of CDH is largely unknown; however, vitamin A is known to play a role in diaphragm development(Reference Klaassens, de Klein and Tibboel5). Since vitamin A is an essential nutrient for embryonic growth and development, circulating vitamin A obtained from the mother’s diet is transferred to their fetus via the placenta(Reference Spiegler, Kim and Wassef6). In the 1940s, experiments in rats showed vitamin A deficiency during pregnancy resulted in a high incidence of CDH in their offspring(Reference Andersen7). Vitamin A deficiency-induced CDH appears to share a common pathogenic mechanism with nitrofen-induced CDH(Reference Clugston, Klattig and Englert8), which is known as an animal model of CDH(Reference Kluth, Kangah and Reich9). Null mutant of receptors of retinoic acid, which is active form of vitamin A, led to diaphragmatic defects in mice(Reference Mendelsohn, Lohnes and Decimo10). In light of evidence from experimental studies, a number of epidemiological studies have investigated the association between maternal dietary intake of vitamin A and CDH. In the USA, low vitamin A intake increased the risk of CDH among women who used vitamin supplements periconceptionally(Reference Yang, Shaw and Carmichael11). Also, in the Netherlands, adequate-weight mothers who delivered infants with CDH showed a lower vitamin A intake than control mothers(Reference Beurskens, Schrijver and Tibboel12). However, these were both case–control studies. Therefore, evidence from a cohort study, which has the advantage that exposures are assessed before the outcome occurrence, would undoubtedly be useful for exploring the causal association.
We thus explored whether maternal intake of vitamin A was associated with CDH occurrence. Our hypothesis was that low vitamin A intake was a risk factor for CDH.
Methods
Study participants
The concept and design of the Japan Environment and Children’s Study (JECS) have been previously detailed(Reference Kawamoto, Nitta and Murata13). Briefly, we recruited women as early in pregnancy as possible, in fifteen Regional Centres located throughout Japan, and registered 103 099 pregnancies from 2011 through 2014(Reference Michikawa, Nitta and Nakayama14–Reference Morisaki, Nagata and Yasuo16). The respective distributions of maternal and infant characteristics in the JECS were comparable to those in the national survey(Reference Michikawa, Nitta and Nakayama14). The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving participants were approved by the Japan Ministry of the Environment’s Institutional Review Board on Epidemiological Studies (No. 100406001) and the Ethics Committees of all participating institutions. Written informed consent was obtained from all participants.
Among the 103 099 pregnancies, we restricted the study participants to 95 170 unique mothers (the first JECS pregnancy) with subsequent delivery record. After excluding 5512 participants who had twin or triplet pregnancies (n 947), had miscarriages or stillbirths (n 1427), did not respond to the FFQ during the first trimester, reported extreme energy intake (lower and upper first percentile) (n 3134) or had missing information on maternal age at delivery (n 4), the remaining 89 658 mothers who delivered singleton live births were included in the present analysis (online Supplementary Fig. S1).
Assessment of vitamin A intake
Two surveys consisting of self-administered questionnaires, including FFQ, were conducted; the first during the first trimester (median fill-in week of gestation = 15) and the second during the second/third trimester (27th week of gestation). We adopted the long-FFQ developed for the Japan Public Health Centre-based prospective study for the next generation. This FFQ was validated by comparing intake from a 12-d weighted food record for Japanese adults aged 40–74 years(Reference Yokoyama, Takachi and Ishihara17). In the first FFQ, we asked about the usual dietary intake in the preceding year, and in the second, the usual intake after awareness of pregnancy. In the present study, we assumed the intake estimates based on the first FFQ to be a marker of dietary intake in early pregnancy, because our target exposure period for diaphragmatic development was roughly 4–8 weeks of gestation(Reference Keijzer and Puri18). We used data from the second FFQ as a marker of dietary intake in mid-late pregnancy.
The nutrients of our target in the present study were total vitamin A (expressed sum of retinol and provitamin A carotenoids as retinol activity equivalents (RAE)), retinol and provitamin A carotenoids (α- and β-carotenes and β-cryptoxanthin). In Japan, the main source of vitamin A intake is provitamin A carotenoids that come from vegetables, particularly green and yellow vegetables(19). Hence, we included the respective intake of total vegetables and green and yellow vegetables in our targets. The daily vegetable intake (g/d) was calculated by multiplying the intake frequency by the standard-equivalent portion size, for thirty-eight vegetable items, including nineteen items of green and yellow vegetables. In the FFQ, the choices of portion size for each food item were small (50 % less than standard), medium (equal to standard) and large (50 % more than standard); and the choices of frequency were <1 time/month, 1–3 times/month, 1–2 times/week, 3–4 times/week, 5–6 times/week, 1 time/d, 2–3 times/d, 4–6 times/d and ≥7 times/d. The daily intake of each nutrient from each food item was then estimated with reference to the Standard Tables of Food Composition in Japan 2010(20). The energy-adjusted intake of vitamin A and other nutrients above were calculated using the residual model(Reference Willett, Howe and Kushi21). Information on supplemental use of multi-vitamins, including vitamin A, from foetation to 12 weeks of gestation was obtained via face-to-face interviews. Due to no information about the amount and frequency of supplemental use, we did not calculate the nutrient intake from such supplements.
Identification of congenital diaphragmatic hernia cases
The occurrence of CDH was identified from the medical records. In accordance with the JECS in-house standard operating procedures, physicians, midwives/nurses and/or research co-ordinators transcribed medical information from the medical records to the JECS transcription forms three times: first during the first trimester, second after delivery and finally during the first month health check-up after delivery. The forms after delivery and at a month after delivery contained a list of sixty-one congenital anomalies, including CDH (10th edition of the International Classification of Diseases: Q79.0)(Reference Mezawa, Tomotaki and Yamamoto-Hanada22). When CDH was reported either at delivery or at the end of the first month, we defined it as an occurrence of CDH in the present study. Additionally, isolated CDH was defined as cases of CDH with no other major congenital anomalies, because the occurrence of multiple anomalies appears to depend on genetic factors rather than exogenous factors. In the present study, such major anomalies included anencephaly, spina bifida, encephalocele, microphthalmia, cleft palate, cleft lip (with or without cleft palate), congenital heart diseases (not including patent ductus arteriosus), gastroschisis, omphalocele, oesophageal atresia, small intestinal atresia, anorectal malformations, hypospadias, reduction defects of the upper and/or lower limbs and chromosomal anomalies (Down’s syndrome, 18 trisomy and 13 trisomy)(23,Reference Parker, Mai and Canfield24) . Information on the type and side of the diaphragmatic hernia was not collected.
Statistical analysis
We performed an exploratory analysis on an observational study, based on a large sample size, therefore we did not perform a power calculation.
We calculated the number of CDH cases per 10 000 live births among each strata of maternal and infant characteristics. The following characteristics were summarised (Table 1): maternal age at delivery, smoking habits, alcohol consumption, pre-pregnancy BMI, current history of diabetes mellitus (DM) or gestational DM (GDM), infertility treatment, educational background, household income, occupation in early pregnancy, use of multi-vitamin supplement in early pregnancy, routine use of folic acid supplement, morning sickness, week of pregnancy at delivery, parity and infant sex.
Table 1. Baseline characteristics of mothers who delivered infants with congenital diaphragmatic hernia (CDH), Japan Environment and Children’s Study (2011–2014)
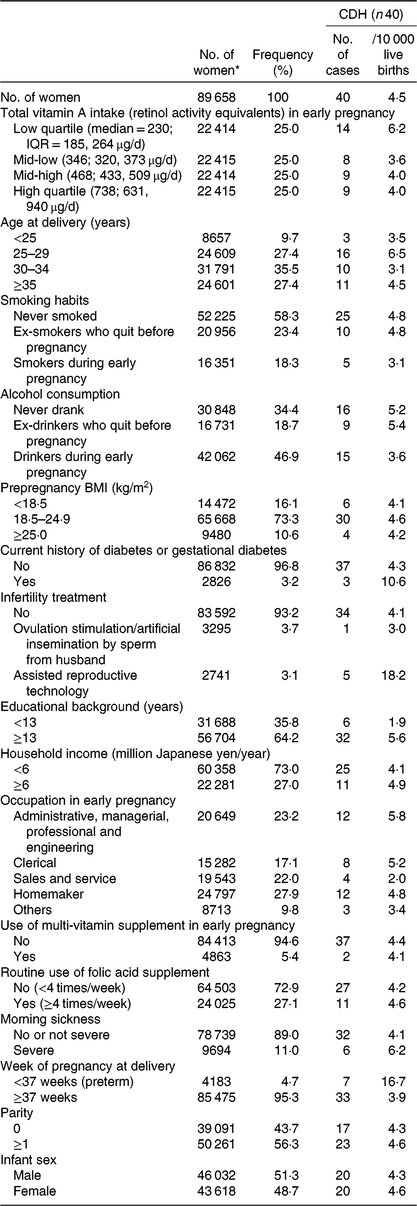
IQR, interquartile range.
* Subgroup totals do not equal the overall number because of missing data.
We performed a logistic regression analysis to estimate the OR and 95 % CI for vitamin A and other intakes in early pregnancy with CDH occurrence. We initially adjusted for maternal age at delivery. Then we selected potential a priori confounders, including smoking habits, alcohol consumption, prepregnancy BMI and DM/GDM, based on past evidence(Reference Balayla and Abenhaim25–Reference McAteer, Hecht and De Roos27) and adjusted for these in addition to other factors associated with CDH in the present study. Participants with missing values for these confounders were excluded from the adjusted analysis. According to the findings from the earlier study(Reference Beurskens, Schrijver and Tibboel12), we examined this association among mothers with adequate weight (18·5 ≤ prepregnancy BMI < 25·0 kg/m2). To verify the robustness of the observed association, further sensitivity analyses were conducted as follows: (1) further adjusting for socioeconomic status, including educational background, household income and occupation in early pregnancy; (2) excluding users of multi-vitamin supplements in early pregnancy or mothers with severe morning sickness to avoid the misclassification of vitamin A intake; (3) restricting to isolated CDH cases, because complicated CDH might be different from isolated CDH in aetiology and (4) further adjusting for dietary intake of folate and vitamin C, which are similar to dietary sources of vitamin A, and use of folic acid supplement, or for total vitamin A intake in mid-late pregnancy, to explore the possibility of the independent association between vitamin A intake in early pregnancy and CDH.
We also explored the association of vitamin A and other intake in mid-late pregnancy with CDH occurrence. In this case, we included 88 642 mothers who had valid data from the second FFQ and delivered their infants after more than 28 weeks of gestation. The present study used the data set jecs-ag-20160424, which was released in June 2016, and revised in October 2016, along with the supplementary data set jecs-ag-20160424-sp1. All analyses were conducted using Stata 14 (StataCorp LP).
Results
Of the 89 658 mothers (mean age at delivery 31·2 (sd 5·0) years), the mean intake of total vitamin A in early pregnancy was 475 (SD 344) μg RAE/d. The correlations between the intake of total vitamin A and the other investigated nutrients are shown in online Supplementary Table S1. We documented forty cases of CDH (4·5/10 000 live births), including twenty-eight isolated cases and twelve complicated cases, in the present study population. As shown in Table 1, the frequency of CDH in the low quartile of total vitamin A intake was 6·2/10 000, somewhat higher than in other three quartiles. A relatively high frequency of CDH tended to be observed among mothers who had suffered from DM/GDM, those who had utilised assisted reproductive technology for this specific pregnancy and those who delivered preterm infants.
We also explored the association between the respective quartiles of total vitamin A intake in early pregnancy and CDH occurrence (online Supplementary Table S2). After adjustment for maternal age, smoking habits, alcohol consumption, prepregnancy BMI, DM/GDM and infertility treatment, the respective CDH ORs for the low to high quartiles were 1·0 (reference), 0·6 (95 % CI 0·2, 1·3), 0·6 (0·3, 1·4) and 0·6 (0·3, 1·5), revealing similarly decreased values in all except the low quartile. Therefore, we combined the three categories (mid-low, mid-high and high) into a single ‘high’ category. The baseline characteristics of 89 658 mothers, for the two levels of vitamin A intake, are shown in online Supplementary Table S3. When using the low category (bottom quartile, median intake = 230 μg/d) for total vitamin A intake as a reference, the adjusted OR for the high category (median = 468 μg/d) was 0·6 (95 % CI 0·3, 1·2); and risk reduction was also observed with intake of α- and β-carotenes, total vegetables and green and yellow vegetables (Table 2).
Table 2. Risk for congenital diaphragmatic hernia (CDH), for vitamin A and other intake in early pregnancy, Japan Environment and Children’s Study (2011–2014) (Odds ratios and 95 % confidence intervals)
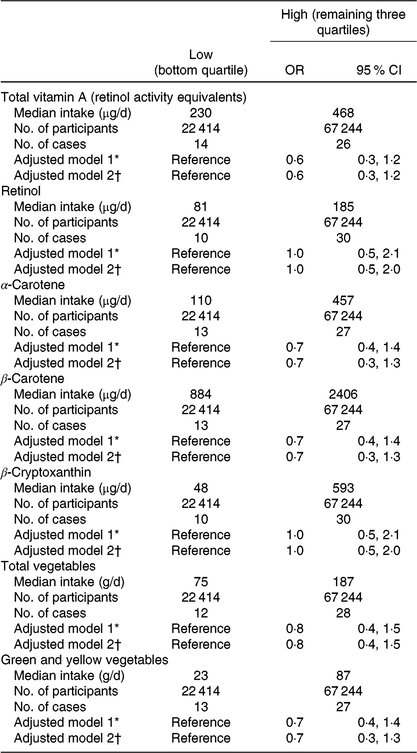
* Adjusted for maternal age at delivery.
† Adjusted for maternal age at delivery, smoking habits, alcohol consumption, prepregnancy BMI, current history of diabetes or gestational diabetes and infertility treatment. Participants with missing values for these factors were excluded, which left 89 481 in adjusted model 2.
Among adequate-weight mothers, total vitamin A intake was inversely associated with CDH (adjusted OR for the high- v. low-intake category = 0·5, 95 % CI 0·2, 1·0) (Table 3). No association was observed in the case of any other intake; however, the OR point estimates were below unity for the high-intake category of α- and β-carotenes, total vegetables and green and yellow vegetables. We investigated the robustness of this association and found a similar pattern of decreased risk of CDH in all analyses (Table 4).
Table 3. Association between vitamin A and other intake in early pregnancy and congenital diaphragmatic hernia (CDH) among mothers with adequate weight (18·5 ≤ prepregnancy BMI < 25·0 kg/m2) (Odds ratios and 95 % confidence intervals)
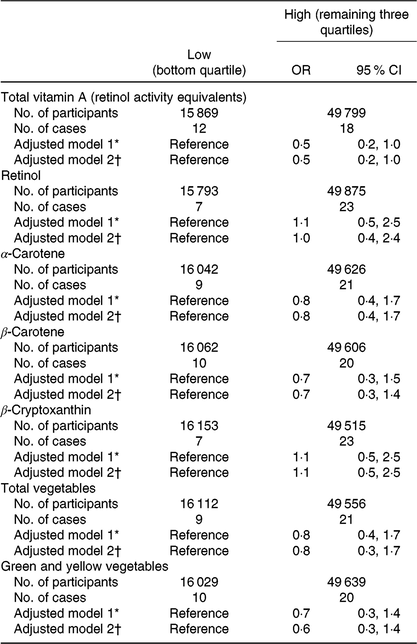
* Adjusted for maternal age at delivery.
† Adjusted for maternal age at delivery, smoking habits, alcohol consumption, current history of diabetes or gestational diabetes and infertility treatment. Participants with missing values for these factors were excluded, which left 65 568 in adjusted model 2.
Table 4. Sensitivity analyses of the association between total vitamin A intake in early pregnancy and congenital diaphragmatic hernia (CDH) among mothers with adequate weight (18·5 ≤ prepregnancy BMI < 25·0 kg/m2) (Odds ratios and 95 % confidence intervals)
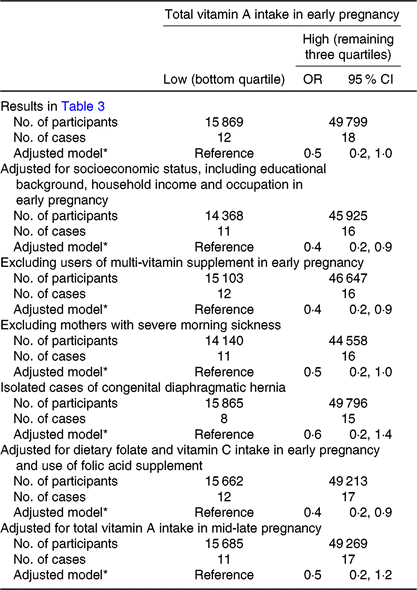
* Adjusted for maternal age at delivery, smoking habits, alcohol consumption, current history of diabetes or gestational diabetes and infertility treatment.
When we focused on dietary intakes in mid-late pregnancy, we observed no association between total vitamin A intake and CDH (online Supplementary Table S4). The Spearman’s coefficient between total vitamin A intake in early and mid-late pregnancy was 0·50. Only an inverse association between α-carotene intake and CDH was found. When we restricted to mothers with adequate-weight, vitamin A tended to show an inverse association, but the CI included unity (adjusted OR for the high- v. low-intake category = 0·7, 95 % CI 0·3, 1·6) (online Supplementary Table S5).
Discussion
To the authors’ knowledge, this is the first prospective study to report that adequate-weight mothers with relatively low total vitamin A intake, which reflected dietary intake in the period of diaphragmatic development, had an elevated risk of giving birth to infants with CDH. A similar pattern of inverse association was also observed for α- and β-carotenes and both total vegetables and green and yellow vegetables, but not for retinol. This result is understandable, because vitamin A intake in Japan is mainly a result of vegetable intake, including α- and β-carotenes(19). Also, total vitamin A intake in mid-late pregnancy tended to be inversely related to CDH. It seems that this reflected the moderate correlation between vitamin A intake in early and mid-late pregnancy.
The JECS involves a large-scale birth cohort, and CDH occurrence in the present study was in line with past reports (5·1/10 000 live births in Japan, 2012)(28); however, the number of CDH cases was small, which might lead to imprecision in the 95 % CI for the measure of effect. Weighing against the possibility that this was a chance finding, however, the study analysed information on various factors provided by the JECS and attempted to verify the robustness of the association. Through adjustment for these factors, and the sensitivity analyses, we carefully controlled the confounding effects on the reported association. To prevent the misclassification of vitamin A intake, we excluded users of multi-vitamin supplements or mothers with severe morning sickness. To explore whether vitamin A was associated with CDH regardless of vegetable-related nutrients, we additionally adjusted for folate and vitamin C intake. To consider the possibility of aetiological difference, we also analysed only isolated cases of CDH. In all the analyses, the OR point estimates for the association showed the same pattern and did not vary substantially in magnitude.
Subsequently, we examined the consistency of our findings with previous reports. Two case–control studies assessed the association between vitamin A intake and CDH. In a Rotterdam study, vitamin A intake was inversely associated with CDH among mothers with a pre-pregnancy BMI of 18·5–24·9 kg/m2 (Reference Beurskens, Schrijver and Tibboel12). This association was replicated in our study, and was reasonable, because exposure misclassification due to underreporting of energy intake might occur among under- or overweight women(Reference McGowan and McAuliffe29,Reference Okubo and Sasaki30) . The study reported that the elevated risk of CDH was observed below the Dutch daily recommended vitamin A intake of 800 μg/d. Although, in Japan, the recommended vitamin A intake for women is from 650 to 700 μg/d, depending on age strata(31), as of 2013 the majority of women in the general population did not achieve this recommended intake(19), and the mean intake in the JECS population was 475 μg/d. In the present study, we found that fairly low vitamin A intake increased the risk of CDH. Another study in the USA, the National Birth Defects Prevention Study (NBDPS), revealed that lower intake of total vitamin A (below the 10th percentile) among vitamin users resulted in an increased risk of CDH, but no association was observed among non-vitamin users(Reference Yang, Shaw and Carmichael11). In the NBDPS, the percentage of vitamin users was approximately 75 %, while that of multi-vitamin users was 5·5 % in the present study. Therefore, it is not surprising that an inverse association was observed among non-users of multi-vitamins. Pregnant women should avoid excessive intake of vitamin A, which is a fat-soluble vitamin(Reference Spiegler, Kim and Wassef6), and adequate intake of vitamin A from dietary sources, without supplement dependence, may be enough to prevent CDH. Overall, our results in the prospective study did not differ essentially from those of the earlier, case–control studies that were vulnerable to recall bias.
Additionally, we considered biological plausibility. The retinoic acid signalling pathway is known to play an important role in diaphragmatic morphogenesis. Retinoic acid is likely to regulate the gene expressions, such as Wilms’ tumour 1 (wt1) and chicken ovalbumin upstream promotor transcription factor II (COUP-TF II), that are related to the development of the diaphragm(Reference Klaassens, de Klein and Tibboel5,Reference Keijzer and Puri18) . In one study, female rats fed a diet deficient in vitamin A delivered offspring with CDH(Reference Andersen7); and in another study, CDH model rats exposed to nitrofen showed a reduced occurrence of CDH with administration of vitamin A(Reference Babiuk, Thebaud and Greer32). Nitrofen appears to lead to diaphragm defects by inhibiting the release of retinal dehydrogenase-2, which is an enzyme related to the production of retinoic acid(Reference Mey, Babiuk and Clugston33). In the present study, an elevated risk of CDH was observed in the fairly low vitamin A intake group. The level of vitamin A in the fetus, which is necessary for embryonic development, depends on transportation from the maternal bloodstream via the placenta, but the vitamin is also essential for the health of the mothers(Reference Spiegler, Kim and Wassef6). Thus, when vitamin A intake is significantly below the recommended value, the amount of fetal vitamin A transferred from the mothers may decrease due to the maintenance of maternal vitamin A homeostasis. One study revealed that the retinol levels in the cord blood of infants with CDH were lower than those in control infants, but the retinol levels of CDH case and control mothers were similar(Reference Beurskens, Tibboel and Lindemans34). Although our study focused on vitamin A, the NBDPS reported that choline, methionine and cysteine, which are involved in DNA methylation pathway, were associated with CDH(Reference Yang, Shaw and Carmichael11). Also, there was evidence that concentrations of homocysteine, another related factor of DNA methylation, in cord blood were not different between CDH and control cases(Reference Beurskens, de Jonge and Schoonderwaldt35). Additional studies are required to explore the contribution of DNA methylation to the diaphragm development. In the present study, the supportive evidence (consistency and biological plausibility), as well as the robustness of the association, suggested that the observed association between vitamin A and CDH was not explicable simply on the basis of chance.
One limitation of the study was that the FFQ used was not validated specifically for pregnant women. In addition, participants were not directly asked about their dietary habits during the diaphragm development period. These were likely to lead to a non-differential misclassification based on measurement errors in the assessment of vitamin A intake, leading us to underestimate the association between vitamin A and CDH. Another limitation was lack of information on serum vitamin A levels, so further studies using them would provide additional evidence linking vitamin A to CDH. The lack of information on the type and side of the hernia was also a limitation. However, approximately 80 % of CDH cases have involved Bochdalek hernias(Reference Greer1), and there is little evidence thus far that the associated factors for CDH differ with hernia type or side. Finally, we restricted to mothers who had live births and thus did not include stillbirths. However, since vitamin A supplementation has not been associated with stillbirth(Reference West, Christian and Labrique36), we believe that the association we observed was not likely to be distorted by this.
In conclusion, low dietary intake of vitamin A in early pregnancy was associated with the occurrence of CDH among adequate-weight mothers in Japan. Although we acknowledge the limitation represented the small number of outcomes, the results would appear to support our hypothesis that low vitamin A intake is a risk factor for CDH.
Acknowledgements
We would like to express our gratitude to all of the Japan Environment and Children’s Study participants and participating Co-operating health care providers.
The Japan Environment and Children’s Study was funded by the Ministry of the Environment, Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the Ministry of the Environment, Japan.
T. M., S. Y. and H. N. designed the present study; T. M., S. Y., M. S. and H. N. contributed to the data analysis; T. M., S. F. N., T. I., E. S., T. K. and H. N. contributed to the data collection; T. M. wrote the initial draft of the manuscript; T. K., T. K. and H. N. provided study supervision. All authors contributed to the interpretation of data, provided critical revisions of the manuscript and approved submission of the final manuscript.
None of the authors has any conflicts of interest to declare.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114519002204
Appendix
Members of the Japan Environment and Children’s Study (JECS) as of 2018 (principal investigator, Toshihiro Kawamoto): Yukihiro Ohya (National Centre for Child Health and Development, Tokyo, Japan), Reiko Kishi (Hokkaido University, Sapporo, Japan), Nobuo Yaegashi (Tohoku University, Sendai, Japan), Koichi Hashimoto (Fukushima Medical University, Fukushima, Japan), Chisato Mori (Chiba University, Chiba, Japan), Shuichi Ito (Yokohama City University, Yokohama, Japan), Zentaro Yamagata (University of Yamanashi, Chuo, Japan), Hidekuni Inadera (University of Toyama, Toyama, Japan), Michihiro Kamijima (Nagoya City University, Nagoya, Japan), Takeo Nakayama (Kyoto University, Kyoto, Japan), Hiroyasu Iso (Osaka University, Suita, Japan), Masayuki Shima (Hyogo College of Medicine, Nishinomiya, Japan), Yasuaki Hirooka (Tottori University, Yonago, Japan), Narufumi Suganuma (Kochi University, Nankoku, Japan), Koichi Kusuhara (University of Occupational and Environmental Health, Kitakyushu, Japan) and Takahiko Katoh (Kumamoto University, Kumamoto, Japan).







