Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Roberts, S. K.
Berger, F.
and
Brownlee, C.
1993.
The Role of Ca2+ in Signal Transduction Following Fertilization in Fucus Serratus
.
Journal of Experimental Biology,
Vol. 184,
Issue. 1,
p.
197.
Cheek, Timothy R.
McGuinness, Orla M.
Vincent, Caroline
Moreton, Roger B.
Berridge, Michael J.
and
Johnson, Martin H.
1993.
Fertilisation and thimerosal stimulate similar calcium spiking patterns in mouse oocytes but by separate mechanisms.
Development,
Vol. 119,
Issue. 1,
p.
179.
Swann, Karl
1993.
The soluble sperm oscillogen hypothesis.
Zygote,
Vol. 1,
Issue. 4,
p.
273.
Gillot, Isabelle
and
Whitaker, Michael
1993.
Imaging Calcium Waves in Eggs and Embryos.
Journal of Experimental Biology,
Vol. 184,
Issue. 1,
p.
213.
Galione, Antony
McDougall, Alex
Busa, William B.
Willmott, Nick
Gillot, Isabelle
and
Whitaker, Michael
1993.
Redundant Mechanisms of Calcium-Induced Calcium Release Underlying Calcium Waves During Fertilization of Sea Urchin Eggs.
Science,
Vol. 261,
Issue. 5119,
p.
348.
Roberts, S. K.
Gillot, I.
and
Brownlee, C.
1994.
Cytoplasmic calcium and Fucus egg activation.
Development,
Vol. 120,
Issue. 1,
p.
155.
Lambert, Charles C.
Gonzales, Genalyn P.
and
Miller, Kimberly M.
1994.
Independent Initiation of Calcium Dependent Glycosidase Release and Cortical Contractions during the Activation of Ascidian Eggs.
Development, Growth & Differentiation,
Vol. 36,
Issue. 2,
p.
133.
Berridge, Michael J.
and
Dupont, Geneviève
1994.
Spatial and temporal signalling by calcium.
Current Opinion in Cell Biology,
Vol. 6,
Issue. 2,
p.
267.
McDougall, Alex
Sardet, Christian
and
Lambert, Charles. C
1995.
Different calcium-dependent pathways control fertilisation-triggered glycoside release and the cortical contraction in ascidian eggs.
Zygote,
Vol. 3,
Issue. 3,
p.
251.
Shen, Sheldon S.
1995.
Vol. 30,
Issue. ,
p.
63.
Tesarik, Jan
Sousa, Mario
and
Mendoza, Carmen
1995.
Sperm‐induced calcium oscillations of human oocytes show distinct features in oocyte center and periphery.
Molecular Reproduction and Development,
Vol. 41,
Issue. 2,
p.
259.
Carroll, David J.
Ramarao, Chodavarapu S.
Mehlmann, Lisa M.
Roche, Serge
Terasaki, Mark
and
Jaffe, Laurinda A.
1997.
Calcium Release at Fertilization in Starfish Eggs Is Mediated by Phospholipase Cγ .
The Journal of Cell Biology,
Vol. 138,
Issue. 6,
p.
1303.
Stephano, José Luis
and
Gould, Meredith C.
1997.
The Intracellular Calcium Increase at Fertilization inUrechis caupoOocytes: Activation without Waves.
Developmental Biology,
Vol. 191,
Issue. 1,
p.
53.
Lee, Shyh-Jye
Madden, Patrick J.
and
Shen, Sheldon S.
1998.
U73122 Blocked the cGMP-Induced Calcium Release in Sea Urchin Eggs.
Experimental Cell Research,
Vol. 242,
Issue. 1,
p.
328.
Carey, Marc B.
and
Matsumoto, Steven G.
1999.
Spontaneous Calcium Transients Are Required for Neuronal Differentiation of Murine Neural Crest.
Developmental Biology,
Vol. 215,
Issue. 2,
p.
298.
Carroll, David J.
Albay, Diana T.
Terasaki, Mark
Jaffe, Laurinda A.
and
Foltz, Kathy R.
1999.
Identification of PLCγ-Dependent and -Independent Events during Fertilization of Sea Urchin Eggs.
Developmental Biology,
Vol. 206,
Issue. 2,
p.
232.
Shearer, Joanne
Nadai, Céline De
Emily-Fenouil, Françoise
Gache, Christian
Whitaker, Michael
and
Ciapa, Brigitte
1999.
Role of phospholipase Cγ at fertilization and during mitosis in sea urchin eggs and embryos.
Development,
Vol. 126,
Issue. 10,
p.
2273.
Stricker, Stephen A.
1999.
Comparative Biology of Calcium Signaling during Fertilization and Egg Activation in Animals.
Developmental Biology,
Vol. 211,
Issue. 2,
p.
157.
Lim, Dmitri
Kyozuka, Keiichiro
Gragnaniello, Giovanni
Carafoli, Ernesto
and
Santella, Luigia
2001.
NAADP+initiates the Ca2+response during fertilization of starfish oocytes.
The FASEB Journal,
Vol. 15,
Issue. 12,
p.
2257.
Morgan, Anthony J.
and
Galione, Antony
2002.
Cyclic ADP-Ribose and NAADP.
p.
167.
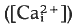 that begins at the point of sperm entry and crosses the egg in about 20 s. Thimerosal is a sulphydryl reagent that sensitises calcium release from intracellular stores in a variety of cell types. Treatment of unfertilised eggs with thimerosal causes a slow increase
that begins at the point of sperm entry and crosses the egg in about 20 s. Thimerosal is a sulphydryl reagent that sensitises calcium release from intracellular stores in a variety of cell types. Treatment of unfertilised eggs with thimerosal causes a slow increase 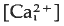 that results eventually in a large, spontaneous calcium transient and egg activation. At shorter times after thimerosal treatment, egg activation and the calcium transient can be triggered by calcium influx through voltage-gated calcium channels, a form of calcium-induced/calcium release (CICR). Thimerosal treatment also reduces the latency of the fertilisation calcium response and increases the velocity of the fertilisation wave. These results indicate that thimerosal can unmask CICR in sea urchin eggs and suggest that the ryanodine receptor channel based CICR may contribute to explosive calcium release during the fertilisation wave.
that results eventually in a large, spontaneous calcium transient and egg activation. At shorter times after thimerosal treatment, egg activation and the calcium transient can be triggered by calcium influx through voltage-gated calcium channels, a form of calcium-induced/calcium release (CICR). Thimerosal treatment also reduces the latency of the fertilisation calcium response and increases the velocity of the fertilisation wave. These results indicate that thimerosal can unmask CICR in sea urchin eggs and suggest that the ryanodine receptor channel based CICR may contribute to explosive calcium release during the fertilisation wave.