The current situation in fertility clinics worldwide
Forty-two years have passed since Drs. Robert Edwards and Patrick Steptoe in the UK reported the first successful birth of a child following in vitro fertilization (IVF) (Steptoe and Edwards, Reference Steptoe and Edwards1978). Furthermore, 28 years have passed since the birth of the first child conceived using intracytoplasmic sperm injection (ICSI) technology (Palermo et al., Reference Palermo, Joris, Devroey and Van Steirteghem1992). Importantly, Louise Brown, the first baby born as a result of IVF, subsequently gave birth herself to a healthy child following an unassisted conception and an uneventful pregnancy. This event showed that IVF had no adverse effect on the reproductive capacity of offspring born using assisted reproductive technology (ART).
Recent advances in ART have allowed many infertile couples to have children. However, due to societal changes such as delays both in marriage and in starting a family, the number of patients attending fertility clinics is increasing and the number of births to older parents worldwide is also increasing. Globally, the number of patients treated using ART is increasing every year (Calhaz-Jorge et al., Reference Calhaz-Jorge, De Geyter, Kupka, de Mouzon, Erb, Mocanu, Motrenko, Scaravelli, Wyns and Goossens2017; Irahara et al., Reference Irahara, Kuwahara, Iwasa, Ishikawa, Ishihara, Kugu, Sawa, Banno and Saito2017; De Geyter et al., Reference De Geyter, Calhaz-Jorge, Kupka, Wyns, Mocanu, Motrenko, Scaravelli, Smeenk, Vidakovic and Goossens2018; Sunderam et al., Reference Sunderam, Kissin, Zhang, Jewett, Boulet, Warner, Kroelinger and Barfield2020). In Europe, the number of ICSI cases is more than double the number of IVF treatments (Calhaz-Jorge et al., Reference Calhaz-Jorge, De Geyter, Kupka, de Mouzon, Erb, Mocanu, Motrenko, Scaravelli, Wyns and Goossens2017; De Geyter et al., Reference De Geyter, Calhaz-Jorge, Kupka, Wyns, Mocanu, Motrenko, Scaravelli, Smeenk, Vidakovic and Goossens2018). Even in Japan, the number of ICSI cases is also increasing annually (Irahara et al., Reference Irahara, Kuwahara, Iwasa, Ishikawa, Ishihara, Kugu, Sawa, Banno and Saito2017).
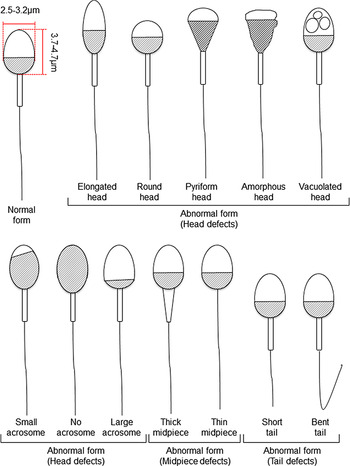
The fact that up to half the cases of infertility in humans are due to male infertility (Irvine, Reference Irvine1998) is less commonly recognized. Most investigations into the causes of infertility have been carried out on females and, until recently, comparatively little attention has been paid to males. Concomitant with the increase in maternal age of pregnancy, paternal age has also risen. This rise has led to the recent investigation of the relationships between paternal age and the quality of spermatozoa, embryogenesis, and clinical outcomes (Belloc et al., Reference Belloc, Benkhalifa, Junca, Dumont, Bacrie and Menezo2009; Alshahrani et al., Reference Alshahrani, Agarwal, Assidi, Abuzenadah, Durairajanayagam, Ayaz, Sharma and Sabanegh2014; Sagi-Dain et al., Reference Sagi-Dain, Sagi and Dirnfeld2015; Sharma et al., Reference Sharma, Agarwal, Rohra, Assidi, Abu-Elmagd and Turki2015; Wu et al., Reference Wu, Kang, Zheng, Liu, Huang and Liu2016; Chapuis et al., Reference Chapuis, Gala, Ferrieres-Hoa, Mullet, Bringer-Deutsch, Vintejoux, Torre and Hamamah2017; Bartolacci et al., Reference Bartolacci, Pagliardini, Makieva, Salonia, Papaleo and Vigano2018; Kaarouch et al., Reference Kaarouch, Bouamoud, Madkour, Louanjli, Saadani, Assou, Aboulmaouahib, Amzazi, Copin, Benkhalifa and Sefrioui2018). These studies have shown that sperm parameters diminish with paternal ageing. Moreover, embryo quality and blastocyst formation rates also worsen with paternal ageing (Frattarelli et al., Reference Frattarelli, Miller, Miller, Elkind-Hirsch and Scott2008; Luna et al., Reference Luna, Finkler, Barritt, Bar-Chama, Sandler, Copperman and Grunfeld2009; Wu et al., Reference Wu, Kang, Zheng, Liu, Huang and Liu2016), although clinical and neonatal outcomes do not appear to be affected (Frattarelli et al., Reference Frattarelli, Miller, Miller, Elkind-Hirsch and Scott2008; Luna et al., Reference Luna, Finkler, Barritt, Bar-Chama, Sandler, Copperman and Grunfeld2009; Sagi-Dain et al., Reference Sagi-Dain, Sagi and Dirnfeld2015; Wu et al., Reference Wu, Kang, Zheng, Liu, Huang and Liu2016). Conversely, it has also been reported that both severe male factor infertility and the use of ICSI itself slightly increase the risk of mental retardation and autism in offspring (Sandin et al., Reference Sandin, Nygren, Iliadou, Hultman and Reichenberg2013; Kissin et al., Reference Kissin, Zhang, Boulet, Fountain, Bearman, Schieve, Yeargin-Allsopp and Jamieson2015; Rumbold et al., Reference Rumbold, Sevoyan, Oswald, Fernandez, Davies and Moore2019). Nevertheless, it is important to obtain good quality embryos and blastocysts for use in ART. To this end, ICSI is generally performed following sperm selection according to World Health Organization (WHO) criteria (Table 1) (World Health Organization, 2010). Established sperm selection methods are subjective and based on morphological features such as the shape and size of the sperm head, midpiece, and tail and by the presence or absence of vacuoles within the sperm head, and size of acrosome (Figure 1). However, as sperm selection based on sperm head size is subjective and made by the embryologists who perform the ICSI protocol, it is possible that this subjective selection process may affect the clinical outcome. Therefore, we felt it would be timely to review the current understanding of the relationship between sperm head morphology and fertilization from the standpoint of human ART and ICSI. In this review, we also consider the relationship between sperm head size and chromatin proteins. Finally, we summarize studies on sperm head shape and fertility and propose potential improvements for ART in infertility treatment.
Table 1. Sperm selection methods for ICSI in human ART to date
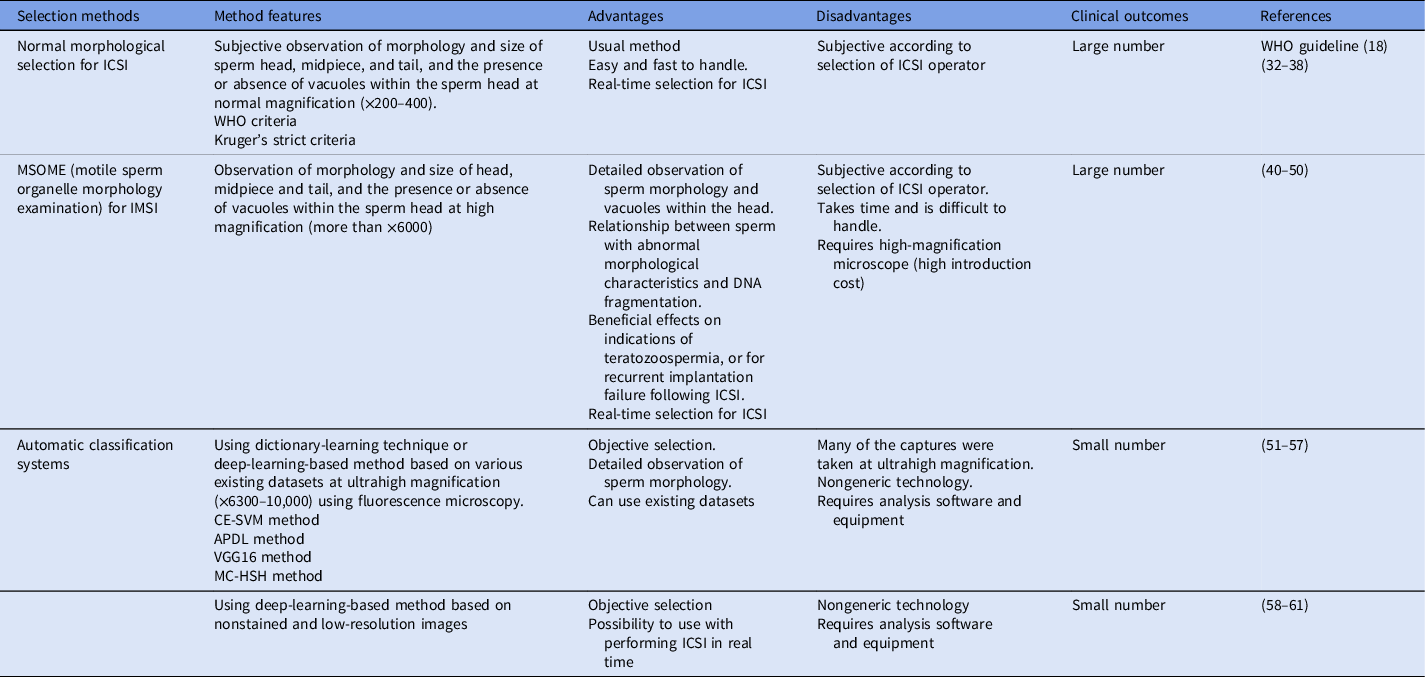
Note: APDL, adaptive patch-based dictionary learning; CE-SVM, cascade ensemble of support vector machines; ICSI, intracytoplasmic sperm injection; IMSI, intracytoplasmic morphologically selected sperm injection; MC-HSH, morphological classification of human sperm heads; VGG16, the convolutional neural network model; WHO, World Health Organization.
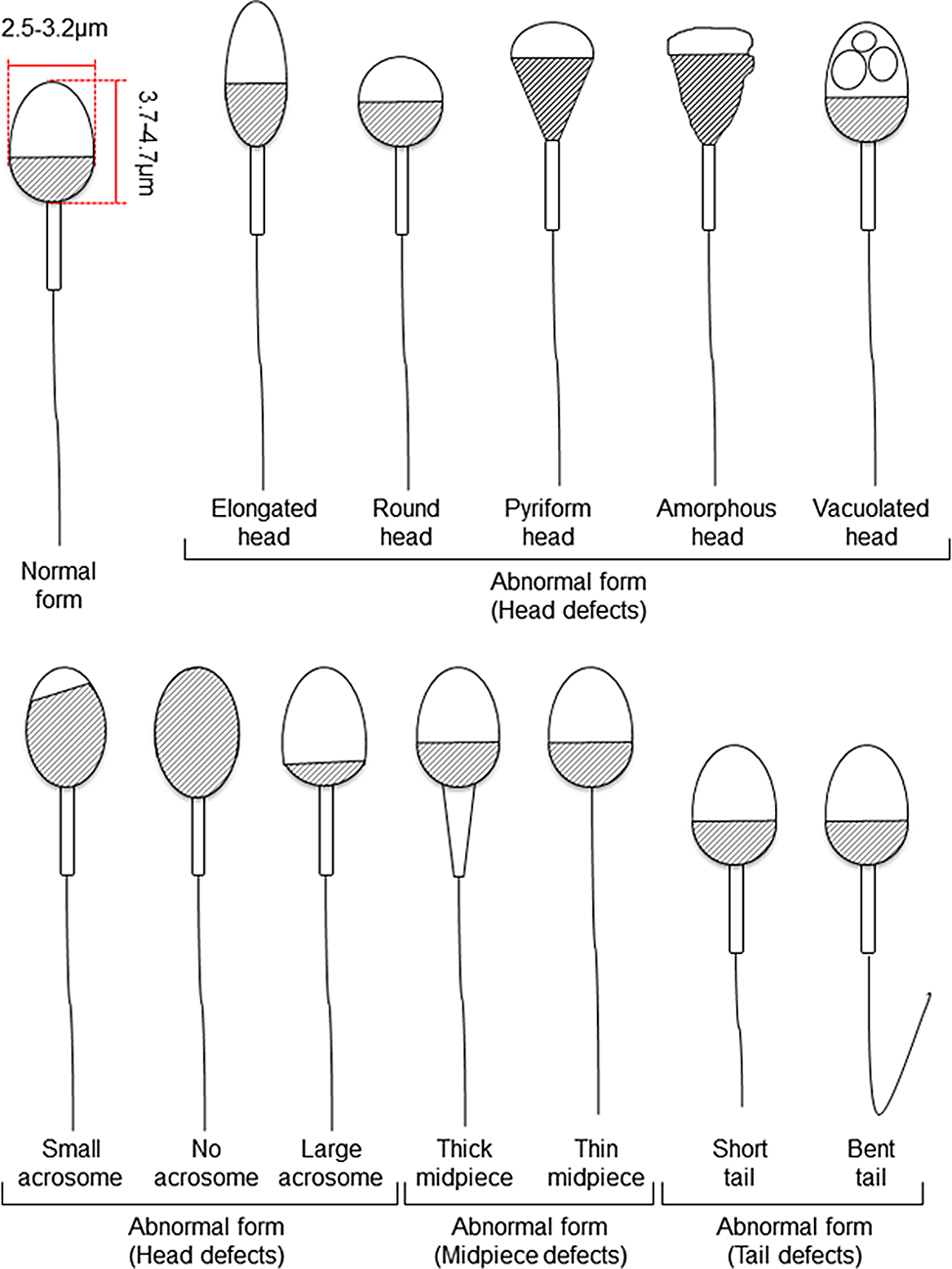
Figure 1. Schematic diagram of human sperm showing normal and abnormal forms based on Kruger’s strict criteria (Kruger et al., Reference Kruger, Acosta, Simmons, Swanson, Matta and Oehninger1988) and WHO criteria (World Health Organization, 2010).
The relationship between sperm shape and chromatin proteins
Sperm cells are produced in the seminiferous tubules of the testes. During spermatogenesis, spermatogonial stem cells give rise to differentiating spermatogonia that undergo dramatic morphological change to eventually become fertilization-competent mature spermatozoa (Clermont, Reference Clermont1972; Griswold, Reference Griswold2016).
Spermatogenesis has two major steps, spermatocytogenesis and spermiogenesis (Griswold, Reference Griswold2016). Spermatocytogenesis is the process of cell division from mitosis of spermatogonia to the production of haploid round spermatids by meiosis of spermatocytes. Conversely, spermiogenesis is the process by which round spermatids transform into sperm via elongated spermatids. During spermiogenesis, the nuclei of the spermatids become condensed and a midpiece is developed along with a tail for motility. At the end of this differentiation process, the cells have formed streamlined spermatozoa. The gametes undergo further modifications during their movement from the caput to the cauda epididymis (Bedford and Calvin, Reference Bedford and Calvin1974), to produce functional sperm capable of fertilization.
Mashiko et al. (Reference Mashiko, Ikawa and Fujimoto2017) monitored the changes in the heads of mouse spermatozoa as the cells transited from the caput to the cauda epididymis, and in the uterus post coitus until they reached the perivitelline space of oocytes in the oviduct, just before fertilization (Mashiko et al., Reference Mashiko, Ikawa and Fujimoto2017). They found that during the maturation of spermatozoa (following transit from the caput epididymis to the corpus and then to the cauda), sperm heads became thinner and the ratio of length to width (the so-called aspect ratio) of the sperm head became larger. Notably, after ejaculated sperm entered the uterus and swam through the oviduct to the oocytes, there were no significant changes in the aspect ratio. Intriguingly, the authors found that the spermatozoa that penetrated the zona pellucida had statistically thinner heads than those in the cauda epididymis. It is not known why spermatozoa with thinner heads appear to have a higher survival, but it may be that the thinner head is advantageous for passing between cumulus cells to reach the egg. As noted below, embryologists at clinics empirically choose spermatozoa with thinner heads for ICSI.
Eukaryotic genomic DNA is wrapped around basic proteins called histones and is tightly folded for compact storage within the cell nucleus. During spermatogenesis, many chromatin-related proteins are produced that remodel chromatin structure in spermatogonia and spermatocytes, enabling meiotic recombination in the latter cell type (Govin et al., Reference Govin, Caron, Lestrat, Rousseaux and Khochbin2004; Zhao et al., Reference Zhao, Shirley, Hayashi, Marcon, Mohapatra, Suganuma, Behringer, Boissonneault, Yanagimachi and Meistrich2004; Ueda et al., Reference Ueda, Harada, Urahama, Machida, Maehara, Hada, Makino, Nogami, Horikoshi, Osakabe, Taguchi, Tanaka, Tachiwana, Yao, Yamada, Iwamoto, Isotani, Ikawa, Tachibana, Okada, Kimura, Ohkawa, Kurumizaka and Yamagata2017). Moreover, in a process called histone-to-protamine transition, histones are replaced with strong basic proteins named protamines (Govin et al., Reference Govin, Caron, Lestrat, Rousseaux and Khochbin2004). This change alters the circular or ellipsoid shape of the nucleus to become the characteristic shape of spermatozoa (the actual sperm head shape varies among species). Interestingly, not all histones are replaced by protamines, and the amount of histone replaced varies among species. For example, in mice, 92–99% of histones in the cell nucleus are replaced by protamines (Brykczynska et al., Reference Brykczynska, Hisano, Erkek, Ramos, Oakeley, Roloff, Beisel, Schubeler, Stadler and Peters2010; Jung et al., Reference Jung, Sauria, Lyu, Cheema, Ausio, Taylor and Corces2017; Yamaguchi et al., Reference Yamaguchi, Hada, Fukuda, Inoue, Makino, Katou, Shirahige and Okada2018), while in humans, approximately 10–15% of histones remain in mature spermatozoa (Hammoud et al., Reference Hammoud, Nix, Zhang, Purwar, Carrell and Cairns2009). It is presumed that the remaining histones, together with other chromatin-related proteins, affect the morphology and size of the sperm head. Male mammals produce very large numbers of spermatozoa during their lifetimes. We speculate that the relative amounts of histones and chromatin-related proteins in each sperm head may be highly heterogeneous and therefore cause the variability observed in shape and size of each sperm head. Application of recently developed single-cell technologies may provide insight into the validity of this speculation (Shapiro et al., Reference Shapiro, Biezuner and Linnarsson2013; Potter, Reference Potter2018).
The relationship between sperm head morphology and fertilization rate in humans
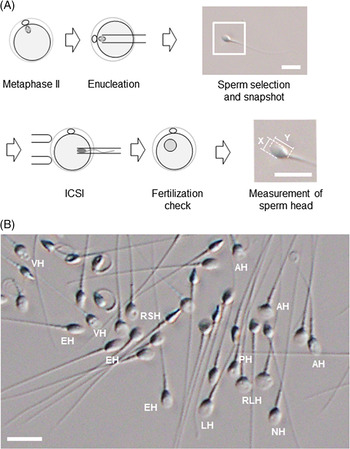
For human ARTs, the morphology, size, and acrosome of sperm heads are important criteria for sperm selection. Kruger et al. (Reference Kruger, Acosta, Simmons, Swanson, Matta and Oehninger1988) published strict criteria for morphological evaluation of human sperm (Figure 1 and Table 1). Under these criteria, good sperm have an elliptical or barrel-shaped head, a straight midpiece that is neither swollen nor thickened, a straight tail, and an acrosome occupying c. 40–70% of the sperm head (Kruger et al., Reference Kruger, Acosta, Simmons, Swanson, Matta and Oehninger1988). Furthermore, normal sperm have a head length of c. 4.1 μm (range 3.7–4.7 μm), width of 2.8 μm (range 2.5–3.2 μm), and an aspect ratio of 1.5 (range 1.3–1.8) according to WHO guidelines (World Health Organization, 2010). Since the birth of the first ICSI baby in the world, described by Palermo et al. (Reference Palermo, Joris, Devroey and Van Steirteghem1992), many reports on sperm morphology have been published. Currently, most embryologists who perform ICSI subjectively select sperm with a good-looking head shape and good motility among many other motile sperm. Sperm that fulfil the above criteria are selected for ICSI using an inverted microscope at ×200–400 magnification (Mansour et al., Reference Mansour, Aboulghar, Serour, Amin and Ramzi1995; Nagy et al., Reference Nagy, Liu, Joris, Verheyen, Tournaye, Camus, Derde, Devroey and Van Steirteghem1995; Svalander et al., Reference Svalander, Jakobsson, Forsberg, Bengtsson and Wikland1996). Although it is possible to assess sperm morphology to identify obvious abnormalities of the head, midpiece, tail, and presence or absence of vacuoles within the head, it is extremely difficult to accurately determine sperm head size visually. Some studies have reported that strict sperm morphology selection for ICSI is not related to clinical outcomes such as the rates of fertilization, pregnancy, and abortion (Mansour et al., Reference Mansour, Aboulghar, Serour, Amin and Ramzi1995; Nagy et al., Reference Nagy, Liu, Joris, Verheyen, Tournaye, Camus, Derde, Devroey and Van Steirteghem1995; Svalander et al., Reference Svalander, Jakobsson, Forsberg, Bengtsson and Wikland1996). In contrast, De Vos et al., who classified sperm into normal and abnormal groups based on Kruger’s strict criteria (Figure 1), found that the normal sperm group had significantly higher fertilization and pregnancy rates than the abnormal sperm group (De Vos et al., Reference De Vos, Van De Velde, Joris, Verheyen, Devroey and Van Steirteghem2003). In addition, Cassuto et al. (Reference Cassuto, Bouret, Plouchart, Jellad, Vanderzwalmen, Balet, Larue and Barak2009) reported that the rate of fertilization and embryo development varies among groups of sperm classified by head size and shape. Sperm heads of 3–5 μm length and width of 2–3 μm gave the best results and were set as the standard. More recently, we injected human sperm into enucleated mouse oocytes, and found that a lower aspect ratio results in a decreased male pronuclear formation rate (Figure 2; Nishikawa et al., Reference Nishikawa, Itoi, Nagahara, Jose, Matsunaga, Ueda and Iwamoto2018). The width of the sperm head was found to be more important than its length. These findings indicate that sperm head size could affect the fertilization rate (Nishikawa et al., Reference Nishikawa, Itoi, Nagahara, Jose, Matsunaga, Ueda and Iwamoto2018). In contrast, Zahiri and Ghasemian reported that acrosome size and morphology of the sperm head affected sperm chromatin status as well as fertilization rates and clinical outcomes (Zahiri and Ghasemian, Reference Zahiri and Ghasemian2019). Sperm with small or large acrosomes significantly decreased the fertilization rates compared with those with normal acrosomes. Moreover, sperm heads with large acrosomes decreased the rates of implantation, clinical pregnancy, and live birth. It was further reported that patients with globozoospermia and patients with a predominance of abnormal acrosomes had significantly higher DNA fragmentation, and sex chromosome aneuploidy and disomy, compared with controls (Brahem et al., Reference Brahem, Mehdi, Elghezal and Saad2011). So far, we have described the relationship between sperm head size, shape, acrosome status, fertilization rate, and clinical outcomes. It is also important to ensure that selected sperm have no chromosomal abnormalities. However, most chromosomal tests on sperm are not performed on single spermatozoa without using bright-field images. In general, chromosomal abnormalities are detected using many sperm in combination with various staining techniques, making it impossible to use these sperm for ICSI. Overall, there is very little evidence describing the relationship between sperm head shape and the presence of chromosome abnormalities (Lee et al., Reference Lee, Kamiguchi and Yanagimachi1996). In this respect, it is desirable to perform chromosomal analyses at low magnification without using staining or similar approaches that disable ICSI post analysis.
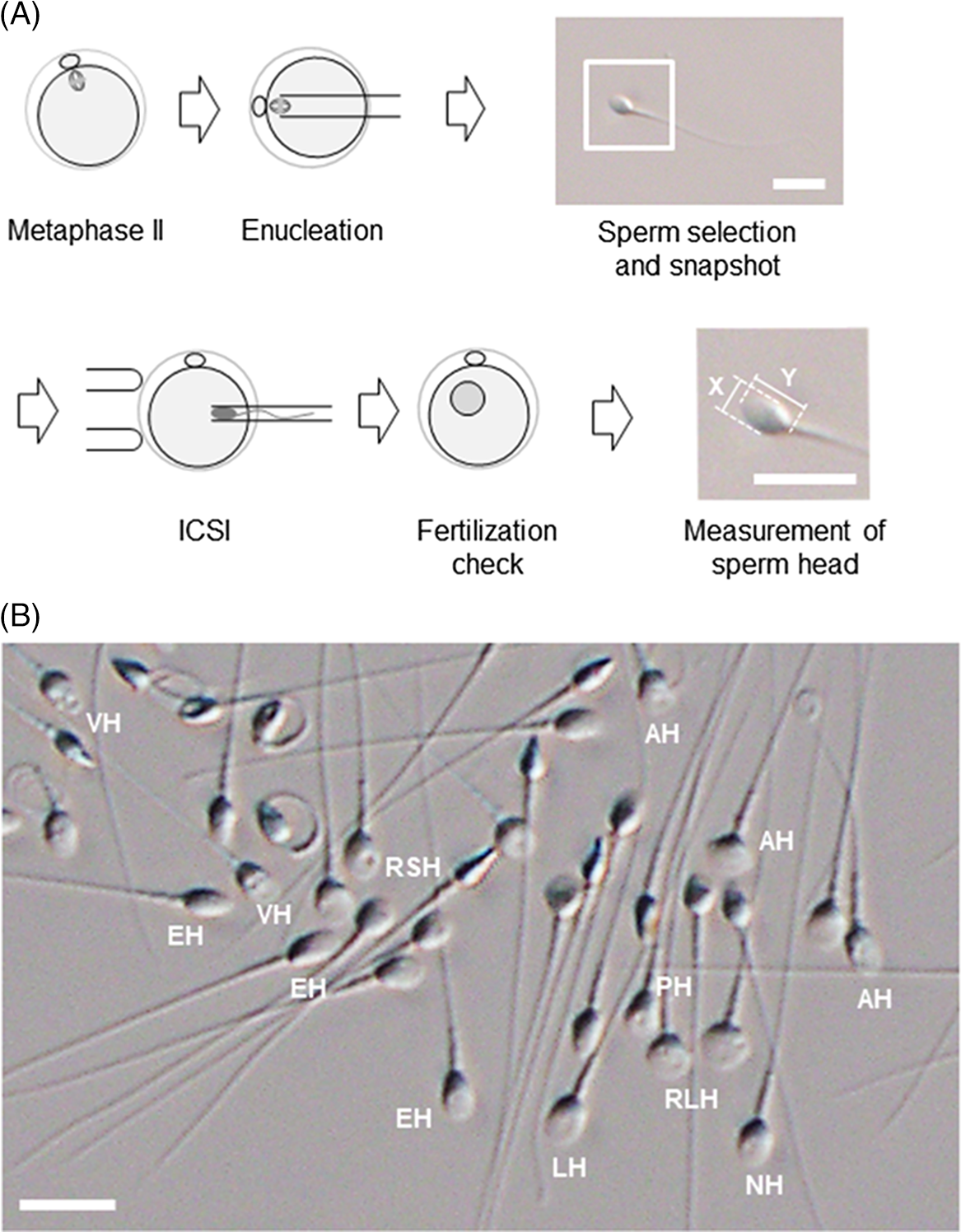
Figure 2. An experimental procedure for assessing the utility of sperm with different head shapes. (A) Enucleated mouse oocytes are injected with human sperm; sperm head size is measured after ICSI and analysed retrospectively. Images of each sperm head are used to calculate the aspect ratio. The magnified image of the inset square in ‘Sperm selection and snapshot’ illustrates how the aspect ratio is calculated from the length (Y) and width (X) of the sperm head. Scale bar represents 10 μm. (B) Spermatozoa viewed under an inverted microscope at ×400 magnification. Spermatozoa with a range of sperm head shapes are present. NH, morphologically normal head; LH, large head; RSH, round small head; RLH, round large head; AH, amorphous head; EH, elongated head; PH, pyriform head; VH, vacuolated head. Scale bar represents 10 μm.
Sperm morphology can also be analysed by motile sperm organelle morphology examination (MSOME) (Table 1). This high-magnification analysis (more than ×6000) detects the presence or absence of vacuoles within the sperm head. Sperm with abnormal morphological characteristics, as determined by MSOME criteria, have an increased risk of DNA fragmentation (Bartoov et al., Reference Bartoov, Berkovitz, Eltes, Kogosowski, Menezo and Barak2002). Subsequently, MSOME criteria have been used to select sperm for use in intracytoplasmic morphologically selected sperm injection (IMSI) (Table 1). Luna et al. (Reference Luna, Hilario, Duenas-Chacon, Romero, Zavala, Villegas and Garcia-Ferreyra2015) reported that IMSI gave a significantly improved implantation rate and an improvement in embryo quality and development. Utsuno et al. (Reference Utsuno, Oka, Yamamoto and Shiozawa2013) described sperm head shape in detail using MSOME and reported that sperm with abnormal ellipticity and angularity had a higher rate of DNA fragmentation than sperm with a normal-shaped head (Utsuno et al., Reference Utsuno, Oka, Yamamoto and Shiozawa2013). Various studies have compared the outcomes of ICSI based on conventional sperm parameters and IMSI (Bartoov et al., Reference Bartoov, Berkovitz and Eltes2001, Reference Bartoov, Berkovitz, Eltes, Kogosovsky, Yagoda, Lederman, Artzi, Gross and Barak2003; Berkovitz et al., Reference Berkovitz, Eltes, Yaari, Katz, Barr, Fishman and Bartoov2005). Although these studies showed an association between sperm heads with or without vacuoles and the rates of implantation and pregnancy (Bartoov et al., Reference Bartoov, Berkovitz, Eltes, Kogosovsky, Yagoda, Lederman, Artzi, Gross and Barak2003; Berkovitz et al., Reference Berkovitz, Eltes, Lederman, Peer, Ellenbogen, Feldberg and Bartoov2006; Hazout et al., Reference Hazout, Dumont-Hassan, Junca, Cohen Bacrie and Tesarik2006; Antinori et al., Reference Antinori, Licata, Dani, Cerusico, Versaci, d’Angelo and Antinori2008), according to later studies, others have concluded that IMSI is only effective when there are indications of couples with multiple abnormal sperm parameters (Schachter-Safrai et al., Reference Schachter-Safrai, Karavani, Reuveni-Salzman, Gil and Ben-Meir2019), including teratozoospermia (Perdrix and Rives, Reference Perdrix and Rives2013), oligoasthenoteratozoospermia (Mangoli et al., Reference Mangoli, Khalili, Talebi, Agha-Rahimi, Soleimani, Faramarzi and Pourentezari2019), or for recurrent implantation failure following ICSI (Boitrelle et al., Reference Boitrelle, Guthauser, Alter, Bailly, Bergere, Wainer, Vialard, Albert and Selva2014). Therefore, IMSI is currently only used in a limited number of clinical situations because of the limited effects.
From these studies, it is reasonable to conclude that the shape and size of the sperm head may affect fertilization rates and subsequent clinical outcomes with respect to the sperm used for ICSI treatments. To apply this knowledge in clinical practice, it is desirable to develop technologies and/or devices that can easily and objectively judge the shape and size, and acrosome status of the sperm head accurately, particularly using the ×400 or ×600 magnification images that are standard in many fertility clinics.
Future perspectives for ARTs
As described above, sperm selection for ICSI in human ART has generally been performed using subjective criteria. Recently, however, attempts have been made to apply an automated classification to sperm selection (Table 1). For example, a dictionary-learning technique (Shaker et al., Reference Shaker, Monadjemi, Alirezaie and Naghsh-Nilchi2017), a deep-learning-based method (McCallum et al., Reference McCallum, Riordon, Wang, Kong, You, Sanner, Lagunov, Hannam, Jarvi and Sinton2019; Riordon et al., Reference Riordon, McCallum and Sinton2019), and other automatic systems have been developed (Mirsky et al., Reference Mirsky, Barnea, Levi, Greenspan and Shaked2017; Chang et al., Reference Chang, Heutte, Petitjean, Hartel and Hitschfeld2017b). Moreover, using the publicly available Human Sperm Head Morphology dataset (HuSHeM), Shaker et al. (Reference Shaker, Monadjemi, Alirezaie and Naghsh-Nilchi2017) compared adaptive patch-based dictionary learning (APDL) and cascade ensemble of support vector machines (CE-SVMs) for sperm selection. They reported that APDL has better accuracy and recall than CE-SVMs. Conversely, Riordon et al. (Reference Riordon, McCallum and Sinton2019) showed that using the convolutional neural network model VGG16, they were able to obtain true positive rates comparable with those of the CE-SVM and APDL methods. The same group used a similar deep-learning technique to develop a method for recognizing sperm DNA fragmentation based on bright-field images, allowing selection for sperm with DNA integrity (McCallum et al., Reference McCallum, Riordon, Wang, Kong, You, Sanner, Lagunov, Hannam, Jarvi and Sinton2019). In addition, automatic classification using a gold standard for morphological sperm dataset (SCIAN-MorphoSpermGS; Chang et al., Reference Chang, Garcia, Hitschfeld and Hartel2017a) and support vector machines (SVMs; Chang et al., Reference Chang, Heutte, Petitjean, Hartel and Hitschfeld2017b), and a combination of interferometric phase microscopy and SVMs have been reported (Mirsky et al., Reference Mirsky, Barnea, Levi, Greenspan and Shaked2017). Recently, Iqbal et al. (Reference Iqbal, Mustafa and Ma2020) established a morphological classification of human sperm heads (MC-HSH) that has higher accuracy than previously reported methods that use the SCIAN or HuSHeM datasets. Unfortunately, most of these analytical methods are based on images captured at ultrahigh magnification (×6300–10,000) using fluorescence microscopy. In addition, these methods are technically difficult to perform in standard fertility clinics. Sperm selection for ICSI is performed using inverted microscopes at approximately ×400 magnification in most ART clinics. While it is desirable to be able to select sperm at this magnification, it is extremely difficult to select sperm of ideal size and shape visually. Furthermore, it is not economically feasible to introduce IMSI systems into small clinics where staff may not be sufficiently experienced with ICSI, as only a small number of cases are processed routinely. By contrast, in large clinics dealing with a high number of ICSI cases, it may be difficult for embryologists and other staff to use IMSI in all cases as it is a time-consuming and laborious method. Considering these difficulties, a definition of strict criteria for sperm selection is essential. However, even if the injected sperm is of poor quality, oocytes can repair some of the damage in the sperm (Sakkas et al., Reference Sakkas, Ramalingam, Garrido and Barratt2015), suggesting that the ICSI operator may require less onerous methods for sperm selection along with other routine work. Recently, Javadi and Mirroshandel developed an automated assessment approach using a deep-learning-based method to detect morphological deformities in different parts of the human sperm head, acrosome, and vacuoles using nonstained and low-resolution images (Javadi and Mirroshandel, Reference Javadi and Mirroshandel2019). The same group also reported an automatic algorithm for detection of human sperm head malformations in real time, with low computation cost (Ghasemian et al., Reference Ghasemian, Mirroshandel, Monji-Azad, Azarnia and Zahiri2015) and for selection of the best sperm for ICSI (Mirroshandel et al., Reference Mirroshandel, Ghasemian and Monji-Azad2016). However, few studies have investigated the relationship between sperm images captured at low magnification for sperm selection for ICSI, and the relationship with embryo development, embryo quality, and clinical outcomes.
Conclusion
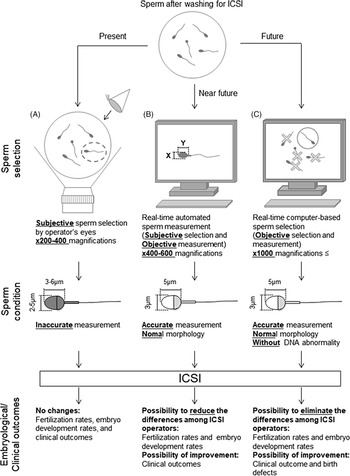
Retrospective analyses are required to investigate the relationship between individual sperm head size, shape, and acrosome status (assessed from bright-field images of a spermatozoon prior to injection into the oocyte) and the resulting embryonic, clinical, neonatal developmental potential, and birth defects. A collaborative analysis involving multiple ART clinics worldwide should allow us to create and define more stringent criteria for sperm selection with noninvasive, objective, real-time methods for ICSI (Figure 3). The information obtained from such studies will ultimately lead to the development of training data for artificial intelligence-based methods. It is important to note that we need to define the formats (file type, magnification, types of data collected) before initiating such international collaborative studies. Furthermore, as infertility is an increasing problem in many countries (Inhorn and Patrizio, Reference Inhorn and Patrizio2015; Calhaz-Jorge et al., Reference Calhaz-Jorge, De Geyter, Kupka, de Mouzon, Erb, Mocanu, Motrenko, Scaravelli, Wyns and Goossens2017; Irahara et al., Reference Irahara, Kuwahara, Iwasa, Ishikawa, Ishihara, Kugu, Sawa, Banno and Saito2017; De Geyter et al., Reference De Geyter, Calhaz-Jorge, Kupka, Wyns, Mocanu, Motrenko, Scaravelli, Smeenk, Vidakovic and Goossens2018; Sunderam et al., Reference Sunderam, Kissin, Zhang, Jewett, Boulet, Warner, Kroelinger and Barfield2020), it will become necessary to construct noninvasive, objective, real-time sperm selection systems for use when embryologists are performing ICSI. These systems should involve the use of an inverted microscope at ×400–600 magnification for accurate sperm head measurements and evaluation. These images can then be applied for deep-learning selection methods. For the evaluation of the normality of genomic DNA integrity by dictionary-learning-based and/or deep-learning-based methods, observation at higher magnification (×1000 or higher) would be necessary. We contend that if these procedures are introduced and established as standard in clinics, not only would there be fewer differences in sperm selection between ICSI operators, but fertilization rates, clinical outcomes, and even neonatal outcomes may also be improved. These approaches may take some time but will eventually improve and advance ARTs in the future.
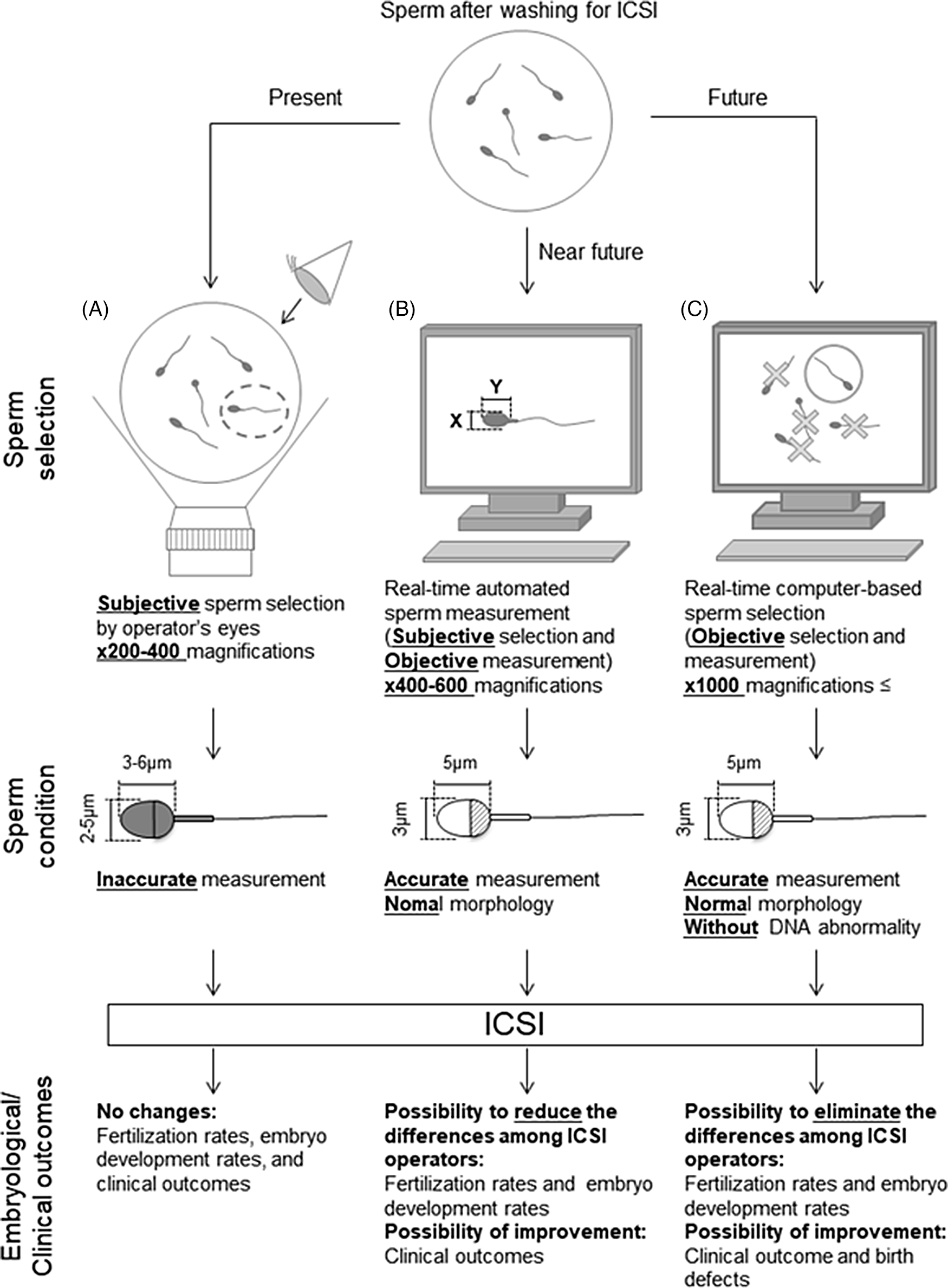
Figure 3. Schematic diagram of proposed sperm selection procedures at present and in the future. (A) Sperm selection methods for ICSI are currently subjective, performed by the operator’s visual inspection. (B) Real-time automated sperm measurement after subjective selection by the operator in the near future. (C) As standardized knowledge increases in the future, real-time computer-based sperm selection may be possible.
Acknowledgement
We thank Daisuke Mashiko for insightful discussion.
Financial support
This work was supported in part by research grants from the Japan Society for the Promotion of Science KAKENHI grants (JP16H01319, JP16K07099 and JP19K06452 to J.U.); the Takeda Science Foundation (J.U.); the Kato Memorial Bioscience Foundation (J.U.); the Akiyama Life Science Foundation (J.U.); and the Joint Research Program of the Institute for Genetic Medicine, Hokkaido University (J.U.).
Conflict of interest
Fumiaki Itoi, Toshinobu Miyamoto, Takehiro Himaki, Hiroyuki Honnma, Miho Sano and Jun Ueda declare that they have no conflict of interest.
Ethical standards
Not applicable.