Introduction
As thermoconforming ectotherm organisms, fish may have their development influenced by the abiotic factors of the water in which they are found (Aride et al., Reference Aride, Gomes, Azevedo, Sangali, Silva, Lavander, Souza, Polese, Mattos, Bassul, Cardoso, Oliveira and Faggio2021; Ribeiro et al., Reference Ribeiro, Oliveira and Carvalho2021). The initial stages of development are the most affected by these factors. For this reason, fish embryos and larvae are more vulnerable to the adverse conditions of the environment in which they live when compared to adult fish (Rijnsdorp et al., Reference Rijnsdorp, Peck, Engelhard, Mollmann and Pinnegar2009; Tyus, Reference Tyus2011).
The high sensitivity presented by embryos and larvae can be explained by several factors, such as incomplete morphological and physiological development, dependence on specific environmental conditions, and low energy reserves (Finn and Kapoor, Reference Finn and Kapoor2008). The environmental variable that most affects the embryonic and larval development of fish is temperature, and it is able to modify the size and duration of these phases when organisms are susceptible to changes in the pattern of ontogenic dynamics (Elliott and Elliott, Reference Elliott and Elliott2010). The length of the embryonic period and consequently the consumption of the yolk material are influenced by water temperature after spawning, and irregular formation of embryonic structures may occur if the reserve material is consumed before the end of development.
Studying the early phases is an important strategy for maximizing the fish’s reproductive system and provides information that allows us to improve different phases of the hatchery. Such information is used for understanding the survival process during incubation and the larval culture process (Pereira et al., Reference Pereira, De Andrade, Radael, Fosse Filho, De Azevedo, Mattos and Vidal Junior2016).
Understanding the influence of temperature provides information related to the organisms’ resistance to natural and captive habitat variations, and this becomes an important tool. Studies associated with initial feeding and the influence of physicochemical variables on early exogenous nutrition in larval fish are still scarce, despite knowledge of this aspect being of great importance in the production during the larval culture phase (Pereira et al., Reference Pereira, De Andrade, Radael, Fosse Filho, De Azevedo, Mattos and Vidal Junior2016; Radael et al., Reference Radael, Cardoso, Andrade, Ferreira, Mattos, Motta and Vidal2015; Mattos et al., Reference Mattos, Cardoso, Fosse, Radael, Fosse Filho, Manhães, Andrade and Vidal2014).
Cichlids are fish that are widely produced in fish farms around the world, both for human consumption and use in aquariums and ornamentation of small ponds (Ladislau et al., Reference Ladislau, Ribeiro, Castro, Pantoja-Lima, Aride and Oliveira2021). The discus fish Symphysodon aequifasciatus, one of the species in this genus, is found in the Amazon basin (Wattley, Reference Wattley1991). It is an ornamental species with high commercial value; however, information regarding its captive requirements is rare (Mattos et al., Reference Mattos, Cardoso, Fosse, Radael, Fosse Filho, Manhães, Andrade and Vidal2014).
Although S. aequifasciatus is a species that is native to Brazil, it is currently produced worldwide. Different color patterns have been developed, and it is now among the species that are most appreciated by the aquarium market. Despite the prominence of the species in the ornamental fish market, there are still few studies addressing issues related to its reproduction and larviculture in captivity and in the natural environment (Mattos et al., 2022; 2014; 2016; Reference Mattos, Screnci-Ribeiro, Cardoso and Vidal Junior2017).
The aim of the present study was to describe the effect of temperature during the development of S. aequifasciatus embryos, as well as determine the time required for the occurrence of morphophysiological events at each tested temperature.
Materials and methods
All experiments were conducted according to local and ARRIVE guidelines (Percie Du Sert et al., Reference Percie du Sert, Hurst, Ahluwalia, Alam, Avey and Baker2020). The eggs and larvae used during the experiment were taken from the natural spawning of S. aequifasciatus. The experiment was divided into two parts: the first one evaluated the influence of temperature on the embryonic and larval development of discus fish. The second one evaluated the influence of temperature on the onset of endogenous feeding (yolk) from exogenous feeding.
During the experimental phase, the eggs were distributed immediately after the spawning and during the second cleavage process. First, they were acclimated and arranged into floating incubators inside the experimental aquarium, which had a volume of 40 L. Each tank had thermostats attached to heaters (Termostato Hopar H-386 50W-Petz) to control the temperature. The tanks were also equipped with a water aeration and oxygenation system.
A total of 225 newly fertilized eggs were used, which were divided into five treatments, totaling 45 eggs for each treatment and 15 eggs for every repetition. All eggs were observed regularly using an optical microscope (Nikon, Eclipse e200) in order to identify the embryonic phase and possible deformations and were later returned to the incubators in the experimental units. The observations took place hourly during the first 24 hours after fertilization and then every three hours after 48 hours had elapsed until early horizontal swimming.
The effect of temperature was noted during the early phases of embryonic development and after the larval development: cleavage, blastula, gastrula, organogenesis, and the mixotrophic period in which the larvae switched from endogenous feeding from the reserve material of the yolk to exogenous feeding.
After the blastopore closure of embryos in each experimental unit, the procedures for evaluating the fertilization rate were conducted according to the following formula: Fertilization rate = [E/(E + i)] × 100, where E represents the number of viable embryos and i represents the number of inviable eggs. The larvae hatching rate was calculated after all the eggs had hatched using the formula HR = AL/FR × 100, where HR represents the hatching rate, AL represents the amount of larvae, and FR represents the fertilization rate.
To carry out the second stage of the experiment, 150 larvae were used, which were distributed among the five treatments and their repetitions. The larvae used in this stage originated from eggs incubated at different temperatures for each treatment, and the time of the beginning of the oriented swimming was determined as time point zero for beginning to offer exogenous food. Two kinds of exogenous food were used: newly hatched brine shrimp (Artemia salina) and wet food (chicken’s eggs cooked for three minutes and decapsulated brine shrimp eggs with a pasty consistency).
The food was offered every hour until all larvae had been fed. After observation every 30 minutes, the incubators were drained with the aid of a siphon to remove leftovers and avoid damaging the water. After 30 minutes of feeding, five larvae were observed with the aid of an optical microscope to assure food intake. The time of first feeding was determined when all larvae showed food in their digestive tract.
The physicochemical variables of the water, such as temperature, oxygen levels, and pH were regularly measured during the entire experimental period with the aid of a thermometer, oximeter, and digital pH meter (Stages 1 and 2).
The experimental design was completely randomized, and statistical analysis was performed (Variance Analysis [ANOVA]) followed by Tukey’s test. The data were considered significant when p > 0.05.
Results
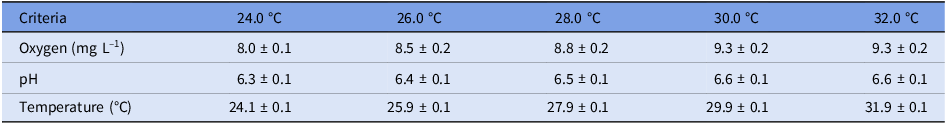
Although increases in both oxygen and pH were observed with increasing temperature (Table 1), the upper values for each were within the comfort range of this species (oxygen: 8.0–9.3 mg L–1; pH: 6.3–6.6). Among all treatments, temperatures were maintained within the target value.
Table 1. Averages of the physicochemical variables of the water in the experimental aquariums (n = 20 at each temperature evaluated)
In the treatments used, no significant variation was noted during the first few hours after fecundation, i.e., the period between zygote and cleavage (zygote: 0; cleavage cells: 3.0–5.5; cleavage 32 cells: 3.5–5.5). However, there was a significant alteration (p<0.05) in the time required to fully develop the embryo, which occurred during gastrulation (gastrula 10%: 13.0–24.0; gastrula 30%: 15.5–28.5; gastrula 80%: 21.5–39.5) and lasted until the initial phase of organogenesis (Table 2).
Table 2. Embryonic events and their respective time (hours) after fecundation of appearance in different temperatures (n = 20 at each temperature evaluated)
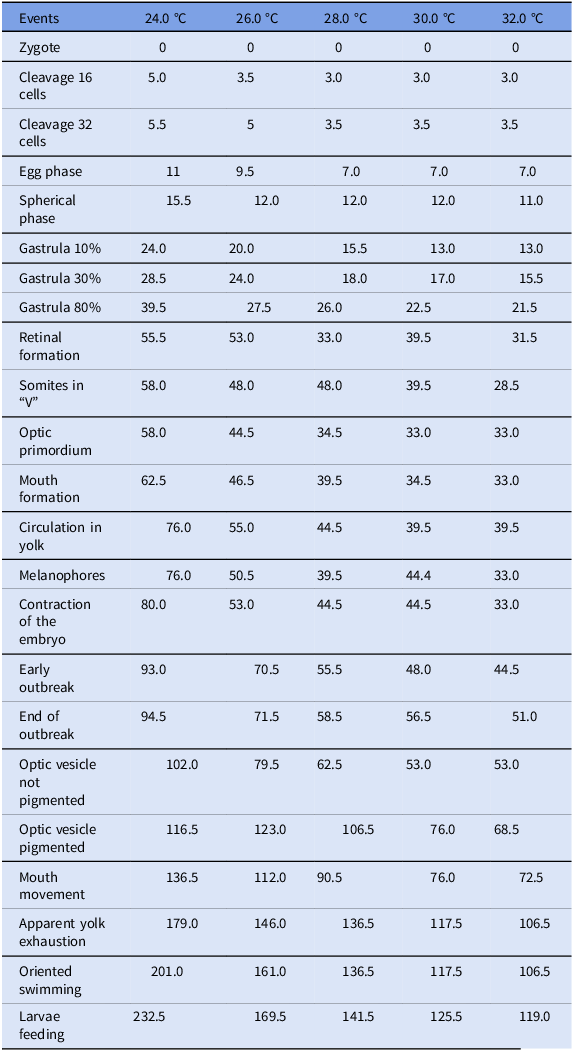
During the organogenesis phase, the effect of temperature was more evident in different treatments when compared to the early phases of development, such as cleavage and gastrula. The decrease in circulation in yolk also followed the same pattern, with a faster reduction in treatments at higher temperatures and with higher concentrations during the organogenesis phase.
During the organogenesis phase, it was observed that the beginning of oriented swimming in the larvae was inversely proportional to the increase in temperature. Among the different treatments, the beginning and the end of the outbreak changed according to the temperature, in which the eggs at a higher temperature had earlier outbreaks when compared to eggs at lower temperatures. The total time between the beginning of each phase and structures after fecundation is shown in Table 2.
During the regular evaluations using an optical microscope, no deformity was perceived in the incubated embryos or larvae at 26.0, 28.0, 30.0, and 32.0 °C. However, 15.5% of the embryos and larvae exposed to 24.0 °C in the incubators presented some kind of visible deformity (Figure 1A and 1B) as well as abnormalities, such as somatic malformation, late development when compared to a healthy individual from the same treatment, and passive hyperemia in the cranial region (Figure 1C and 1D).
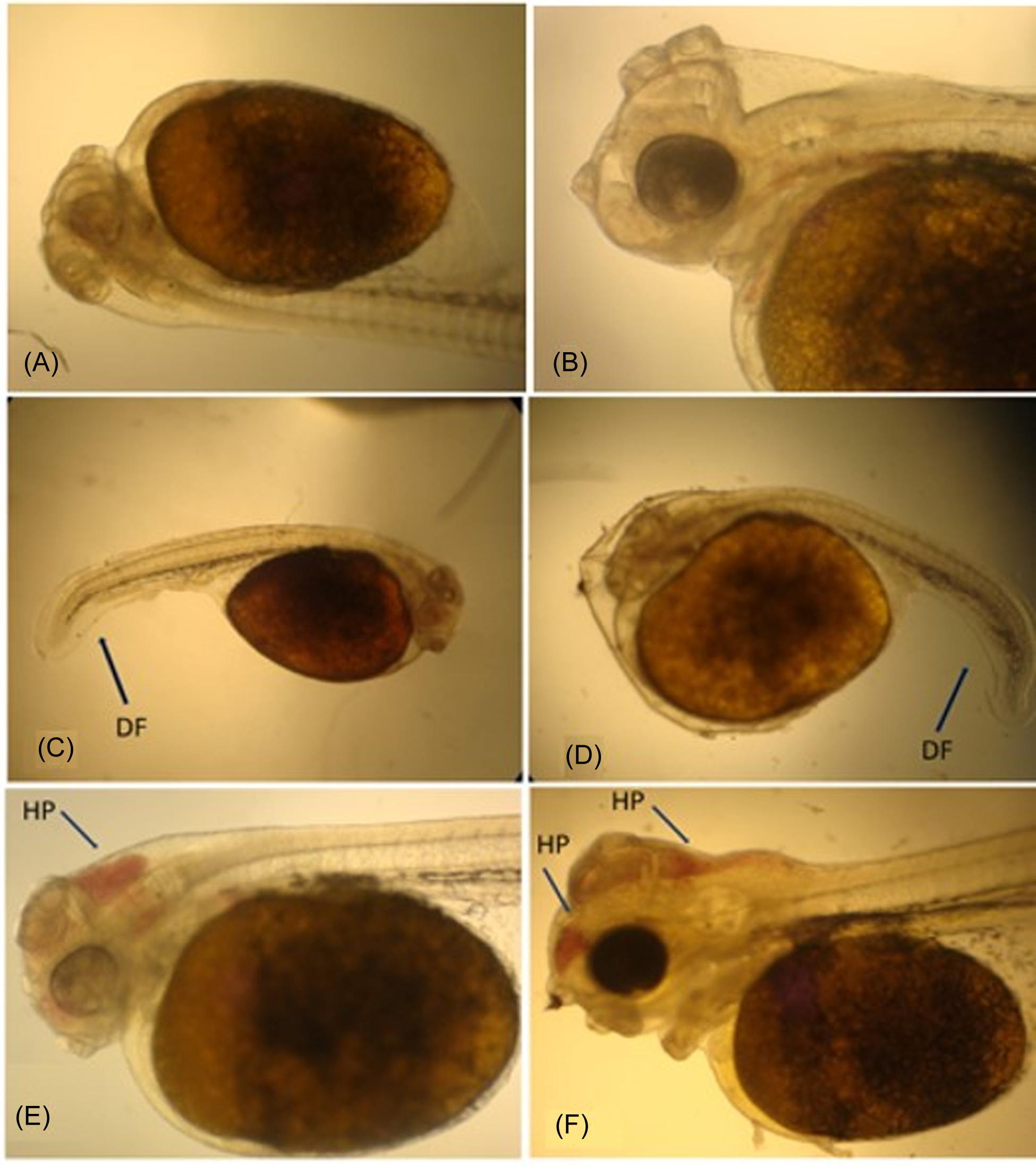
Figure 1. Larvae of S. aequifaciatus presenting deformity in the caudal region of the body and blood congestion in the cranial region (arrows). A and B are experiment control images. C and D (DF) deformity fin, and E and F (HP) hyperemia passive.
The fertilization rate did not show any significant difference among the treatments (p > 0.05); they all presented a 90.0% rate of fertilization data not shown. Regarding the outbreak rate, it was observed that temperature influenced the percentage of hatchability in both the initial and final times of outbreaks. The highest levels of outbreaks were found in embryos incubated at 32.0 °C (82.6%), followed by 26.0 °C (69.5%). The incubators at 28.0 and 30.0 °C showed similar percentages—65.2% and 60.8%, respectively. Concerning the treatments at 24.0 °C, the hatching rate was 40.0%, which was the lowest average.
The feeding transition occurred first with eggs incubated at 32.0 °C, followed by 30.0, 28.0, 26.0, and 24.0 °C, respectively (p < 0.05). After statistical analysis, it was observed that there was on temperature over the time allotted to start the feeding process. The gradual increase in temperature led to a decrease in development time and, later, in the feeding transition.
The exact period of feeding transition was observed with 232.5 hours at 24.0 °C, 169.5 hours at 26.0 °C, 141.5 hours at 28.0 °C, 125.5 hours at 30.0 °C, and 119.0 hours at 32.0 °C. Statistical differences in the averages for the developed time, time to first feeding, and hatching rate are described in Table 3.
Table 3. Time required for complete development, initiation of exogenous feeding, and hatching rate (n = 20 at each temperature evaluated)

* Different letters indicate a statistically significant difference between the means (p < 0.05).
Discussion
In this experiment, there was no significant variation in the physicochemical parameters of water, such as pH and dissolved oxygen according to the different temperatures; therefore, this factor did not influence the growth of embryos and larvae (Fishbase, 2016).
The acceleration of development of young forms of fish is directly related to metabolic acceleration caused by the increase in temperature (Sadati et al., Reference Sadati, Shakuriam, Hasani, Pourali, Pourashadi and Yousefi2011). Different environmental factors can influence various developmental stages in fish throughout their life cycle (Lopes et al., Reference Lopes, Araújo-Dairiki, Kojima, Val and Portella2018). According to Pereira et al. (Reference Pereira, De Andrade, Radael, Fosse Filho, De Azevedo, Mattos and Vidal Junior2016), the time required for embryonic development in fish is closely related to temperature.
According to Johnston et al. (Reference Johnston, Lee, Macqueen, Paranthaman, Kawashima, Anwar, Kinghorn and Damay2009), both organogenesis and somatic development are controlled by gene expression, and they are directly affected by changes in temperature. The metabolism of fish is linked to specific temperature ranges, known as thermal comfort ranges, which is when fish show normal development (Radael et al., Reference Radael, Cardoso, Andrade, Ferreira, Mattos, Motta and Vidal2015). The tolerance in different species varies according to the source environment, and fish of tropical origins have a greater thermal comfort range than fish from temperate and cold weather.
The variation in temperature, even by a small degree, may cause malformation in embryos and larvae (Boltaña et al., Reference Boltaña, Sanhueza, Aguilar, Gallardo-Escarate, Arriagada, Valdes, Soto and Quiñones2017). The presence of malformed embryos at 24.0 °C may be associated with the fact that S. aequifasciatus comes from a tropical region with high temperatures during the four seasons of the year. Sub-optimal conditions can increase the occurrence of fatal deformities and mortality as an effect of the stress caused by impaired development time. Okamoto (Reference Okamoto2004) noticed some deformities on recently hatched larvae of sole (Paralichthys orbignyanus) when incubated in extreme temperatures outside their comfort zone, which is 23.0 °C. Dionísio et al. (Reference Dionísio, Campos, Valente, Conceição, Cancela and Gavaia2012) also noted that incubation temperature is able to cause skeletal deformities in Solea senegalensis.
Blood congestion, or passive hyperemia, evidenced in the cranial region of larvae maintained at 24.0 °C, is constituted by intravascular blood accumulation, which may originate in disorders related to venous return or heart failure (Almeida et al., Reference Almeida, Netto, Montenegro, Franco, Bacchi and Almeida2010; Musso and Pereira, Reference Musso, Pereira, Filho and Vila Mariana2013). Cardiovascular disorders are recurrent in larval development when at extreme temperatures. Low temperatures affect not only physiology but also morphology and cardiovascular development (Burggren and Bagatto, Reference Burggren, Bagatto, Finn and Kapoor2008; Perrichon et al., Reference Perrichon, Pasparakis, Mager, Stieglitz, Benetti, Grosell and Burggren2017). Consequently, stagnation of circulation or reduction of blood flow results in varying degrees of hypoxia in the tissues that have their irrigation affected (Werner, Reference Werner2015).
Concerning the hatching rate, the higher values with high temperatures can be explained by the fact that the species studied in this investigation is found in rivers of the Amazon basin, in tropical environments, and have a higher tolerance to temperature variation. According to Val and Almeida-Val (Reference Val and De Almeida-Val1995), the temperature does not change significantly in the Amazon, and the Solimões River presents 29.0 ± 1.0 °C and the Negro River presents 30.0 ± 1.0 °C, with high temperatures throughout the year.
The timing between the beginning and the end of hatching was also significantly influenced by temperature, when the increase in temperature during the tested intervals reduced the required time to start hatching. The same pattern was observed by Radael et al. (Reference Radael, Cardoso, Andrade, Ferreira, Mattos, Motta and Vidal2015) in their work with eggs and larvae of rainbowfish (Melanotaenia boesemani).
Similar to the results found for S. aequifasciatus, Ferreira et al. (Reference Ferreira, Vidal Junior, Andrade, Yasui, Mendonça and Mattos2009) stated in their work with Glossolepis incisus that the process of absorption of the yolk sac occurs with greater intensity during the organogenesis phase. This characteristic may be explained by the fact that increased energy is demanded during this process, given that different structures are formed throughout this phase, such as eyes, heart, fins, muscle modifications, and, subsequently, changes in embryonic structures. For many different fish species, temperature may cause a great influence on the muscle cells of the embryo, on incubation, and later on in development (Johnston, Reference Johnston2006).
Kamler (Reference Kamler1992) described it as a period of mixed feeding, which may define the period of feeding transition. The same author highlighted it as a decisive stage, in which there is a need for the larvae to find specific food for their survival before depletion of the yolk.
When temperature influenced the development time of discus fish, it also changed the time required to start exogenous feeding, in other words, the transition from feeding on the endogenous yolk to exogenous feeding (artemia and wet food). Inadequate nutrition at this stage can be considered one of the main factors of mortality. However, when given at the right time and in the right quantity, food intake can actually minimize loss and significantly increase survival. According to Portella and Dabrowski (Reference Portella, Dabrowski, Cyrino, Bureau and Kapoor2008), the transition period from endogenous to exogenous feeding is the most critical moment for larvae survival because the digestive system of many species is still in differentiation both in its structure and in operation.
The exogenous feeding of the fish starts when there is complete depletion of the yolk reserves (Gisbert and Williot, Reference Gisbert and Williot1997), while, in other cases, they start before the food supplement is over (Ferreira et al., Reference Ferreira, Vidal Junior, Andrade, Yasui, Mendonça and Mattos2009). According to Shan et al. (Reference Shan, Quan and Dou2008), the initial feeding of fish larvae can be influenced by endogenous factors, such as egg size, and exogenous factors, such as water temperature. The discus fish larvae began exogenous feeding when they were out of food reserves, indicating the need for exogenous feeding as soon as the endogenous food is fully consumed. The larvae maintained at higher temperatures started their food search earlier when compared to larvae maintained at lower temperatures. This happened due to the effect of the temperature on the full development time of the larvae, and, consequently, it reduced the time to first feeding. Being cold-blooded organisms, fish vary their growth and development according to the temperature of the water in which they are grown, which can alter metabolic rates, thus influencing food and digestion (Burton et al., Reference Burton, Killen, Armstrong and Metcalfe2011).
Conclusion
The results observed permit the elucidation of the relevant characteristics of production of discus fish in captivity, thus contributing to a better understanding of larval dynamics and the feeding particularities of the species according to the temperature in which they are reared. It can be concluded that the temperature of 32.0 oC provided the best hatching rate and the shortest time to start the exogenous feeding and is therefore indicated for the incubation of S. aequifasciatus eggs in captivity. Temperatures close to 24.0 oC should be avoided when hatching eggs of this species, as they can lead to a low hatch rate and the appearance of deformities and anomalies in the circulatory system.
Data availability
The datasets in this study are available from the corresponding author on reasonable request.
Acknowledgements
The authors would like to thank North Fluminense State University Darcy Ribeiro for assisting and financing this experiment. AT Oliveira has a research fellowship from CNPq/Brazil (Process 315713/2020-8).
Author contribution
DCM, LDC, RSR, and MCR conceived the study. DCM, ATO, BOM, PHRA, JHSM, and MVVJ designed the study. DCM, LDC, and RSR undertook laboratorial analyses. ATO, BOM, PHRA, and MVVJ drafted the paper with contributions from all other authors. All authors read and approved the final manuscript.
Competing interests
The authors declare no conflict of interest.
Ethical standard
The experiment was developed in accordance with the rules of ethical principles for animal experimentation approved by the National Council for the Control of Animal Experimentation (CONCEA), subject to approval by the Ethics Commission on the Use of Animals (ECUA) of the Federal University of Amazonas under approval No. 005/2016 and ECUA of the Nilton Lins University under approval No. 003/2017. All experiments were conducted according to local and ARRIVE guidelines.







