Introduction
In Arkansas, grain sorghum is often grown adjacent to rice (Oryza sativa L.), corn (Zea mays L.), soybean [Glycine max (L.) Merr.], or cotton (Gossypium hirsutum L.). Due to its ability to perform well in hot, dry climates it may even be planted in nonirrigated field corners of these other crops (Bennet et al. Reference Bennet, Tucker and Maunder1990). When environmental conditions are favorable, herbicides applied to these crops can move off-target, resulting in injury to nearby grain sorghum (Al-Khatib and Peterson Reference Al-Khatib and Peterson1999). Off-target movement of herbicides released from an unshielded sprayer can range from a rate of 1/100× to 1/10× (Al-Khatib and Peterson Reference Al-Khatib and Peterson1999). The injury to nontolerant crops from off-target movement can differ depending on the herbicide, sensitivity of crop, and growth stage of the plant (Hanks Reference Hanks1995; Miller Reference Miller, Matthews and Hislop1993).
Glufosinate (Weed Science Society of America [WSSA] Group 10) is a herbicide often used in cotton and soybean fields to control glyphosate-resistant Palmer amaranth (Amaranthus palmeri S. Wats.). Applications of glufosinate result in decreased production of glutamine synthetase in susceptible plants. Glutamine synthetase is an enzyme necessary in the conversion of glutamate and ammonia to the amino acid glutamine (Coetzer and Al-Khatib Reference Coetzer and Al-Khatib2001; Devine et al. Reference Devine, Duke and Fedtke1993). Glufosinate-resistant crop varieties were created using the gene bialophos (bar) from Streptomyces hygroscopius, a bacterium. Phosphinothricin acetyltransferase enzyme is expressed by the bar gene, conferring resistance to glufosinate (Culpepper et al. Reference Culpepper, York, Roberts and Whitaker2009). Currently, glufosinate-resistant grain sorghum varieties have not yet been developed, therefore all varieties are sensitive. In 2015, 341,000 ha of cotton and soybean combined were treated with glufosinate (USDA-NASS 2016). Glufosinate being sprayed on fields neighboring grain sorghum in 2015 often resulted in off-target movement and visible injury to grain sorghum (T. Barber, personal communication).
Nicosulfuron was applied in corn to control numerous weedy grass species prior to the introduction of glyphosate-resistant (GR) corn. It is an acetolactate synthase (ALS)-inhibiting (WSSA Group 2) herbicide. This site of action was arguably one of the most widely used in agriculture prior to the introduction of GR crops (Tranel and Wright Reference Tranel and Wright2002). The ALS enzyme is the first in the biosynthetic pathway of branched chain amino acids leucine, isoleucine, and valine (Ray Reference Ray1984). By inhibiting this pathway, susceptible plants can be starved of branched chain amino acids, leading to mortality.
Previous research has been conducted using corn and grain sorghum to show the effect of low rates of glufosinate, glyphosate, imazethapyr, and sethoxydim (Al-Khatib et al. Reference Al-Khatib, Claassen, Stahlman, Geier, Regehr, Duncan and Heer2003). However, this research examined these herbicides only when they were applied to susceptible crops at the 3- to 4-leaf growth stage. During this research, it was observed that symptoms from imazethapyr were similar to those reported for nicosulfuron (Al-Khatib and Peterson Reference Al-Khatib and Peterson1999; Al-Khatib and Tamhane Reference Al-Khatib and Tamhane1999). However, because both herbicides inhibit ALS, these results were not surprising (Beyer et al. Reference Beyer, Duffy, Hay, Schlueter, Kennedy and Kaufman1988; Stidham and Singh Reference Stidham, Singh, Shaner and O’Conner1991). Response of grain sorghum to 1/10× the labeled rate glufosinate applied at the V6 and flagleaf growth stages did not result in yield loss when pooled together (Hale et al. Reference Hale, Bararpour, Kaur, Seale, Singh and Wilkerson2019). However, this research did not report grain sorghum yield following glufosinate application at the individual growth stages. The objective of this field test was to evaluate the tolerance of grain sorghum to low rates of glufosinate and nicosulfuron at varying growth stages to determine the most sensitive period for severe injury and/or yield loss to occur.
Materials and Methods
Research was conducted at the Lon Mann Cotton Research Station (LMCRS) near Marianna, AR, and the Agricultural Research and Extension Center in Fayetteville, AR, in 2016 and 2017; the Northeast Research and Extension Center in Keiser, AR in 2016; and the Pine Tree Research Station near Colt, AR in 2016 to evaluate response of grain sorghum to low rates of nicosulfuron and glufosinate. Soil texture near Colt, AR, was a Herbert silt loam (fine-salty, mixed, active, thermic Aeric Epiaqualf) with 16% sand, 67% silt, 17% clay, pH 7.1, and 2.2% organic matter (OM). The Keiser, AR, site was a Sharkey clay (very fine, montmorillonitic, nonacid, thermic, Vertic Haplaquept) with 22% sand, 25% silt, 53% clay, pH 6.7, and 1.7% OM. Near Marianna, AR, the soil texture was a Calloway silt loam (fine-silty, mixed, active, thermic Aquic Fraglossudalfs) with 2% sand, 82.3% silt, 15.6% clay, pH 5.5, and 2.2% OM. The soil texture at the Fayetteville, AR, site was a Captina silt loam (fine-silty, siliceous, active, mesic Typic Fragiudults) with 22% sand, 64% silt, 14% clay, pH 5.8, and 1.8% OM. A Dekalb® (Monsanto Company, St. Louis, MO) grain sorghum hybrid, DKS 53-67, was planted at 217,000 seeds ha−1 at all locations. DKS 56-67 was chosen because it is a nontraited hybrid that confers no tolerance to either glufosinate or nicosulfuron. Plots were four rows wide at all locations. This field test was arranged as a three-factor factorial including herbicide, rate, and timing of application. The herbicide factor was either glufosinate or nicosulfuron. A proportional rate of 656 g ai ha−1 of glufosinate and 35 g ai ha−1 of nicosulfuron at 1/10×, 1/50×, and 1/250× was applied. Growth stages of V3, V8, flagleaf, heading, and soft dough grain sorghum were chosen to determine at which stage of growth the highest sensitivity exists. All applications were made using an air-pressurized four-nozzle spray boom equipped with TeeJet® Air Induction XR 110015 nozzles, traveling at 4.8 km h−1 and calibrated to deliver 140 L ha−1 (TeeJet® Technologies, Wheaton, IL). At RRS and LMCRS plots were 9 m long, and at NREC and AAREC they were 6 m long. To maintain weed-free plots, an application of atrazine (Aatrex, Syngenta Crop Protection, LLC, Greensboro, NC) at 1,120 g ha−1 and S-metolachlor (Dual II Magnum, Syngenta Crop Protection, LLC) at 1,070 g ha−1 were applied at planting. Any escapes from this application were controlled by a single postemergence application of the same mix, applied 4 wk after initial application. Further escapes were removed by hand for the remainder of the field test. Fertilizer and pest management decisions were based on University of Arkansas extension recommendations (Espinoza Reference Espinoza2015; McLeod and Greene Reference McLeod and Greene2015).
In 2016, visible crop injury was rated at 2 and 4 wk after application (WAA) and grain yield was collected at crop maturity. In 2017, visible crop injury was rated at 2 and 4 WAA, along with crop canopy heights (cm), days to 50% heading, and yield at crop maturity. Visible crop injury relative to nontreated checks was rated on a scale of 0% to 100%, with 0% being no injury and 100% being complete plant mortality. In each plot, five random grain sorghum plants were measured in centimeters, then averaged together and divided by the average of the nontreated plots and recorded as relative crop canopy heights. The center two rows at each location were harvested using a small-plot research combine and recorded as kilograms per hectare after moistures were adjusted to 14%. Reductions or increases in relative yield were calculated by dividing yield of plots by the average yield of nontreated plots.
All data collected were subjected to ANOVA using JMP software (JMP PRO 13, SAS Institute Inc., Cary, NC), with significant means separated using Fisher’s protected LSD (α = 0.05). Herbicide, rate, and timing of application were included as fixed effects, with location and year being random effects. The nontreated in each replication was excluded from the analysis because they were included only for relative comparisons.
Results and Discussion
A three-way interaction for factors herbicide, rate, and timing was observed for visible injury (P < 0.0001) and canopy heights (P = 0.0002) at 2 WAA (Table 1). Response of grain sorghum differed between herbicide, with nicosulfuron generally causing more visible injury than glufosinate. However, increasing herbicide rate resulted in an increase of visible injury for both herbicides (Table 2). At 2 WAA, injury from glufosinate at the 1/10× rate ranged from 14% to 36% across all growth stages. The greatest visible injury (32%–36%) from glufosinate was observed following applications to V3 and flagleaf stages (Table 2). Grain sorghum injury from glufosinate was <12% for the 1/50× and 1/250× rates regardless of growth stage.
Table 1. Analysis of variance for grain sorghum injury, canopy heights, and grain yield from low rates of postemergence-applied glufosinate and nicosulfuron applications from 2016 and 2017. a,b

a Injury experiments for 2016 conducted near Colt, AR; in Keiser, AR; near Marianna, AR; and in Fayetteville, AR.
b Injury experiments for 2017 conducted near Marianna, AR, and in Fayetteville, AR.
c Abbreviations: DF, degrees of freedom; WAA, weeks after application.
d *Denotes significance.
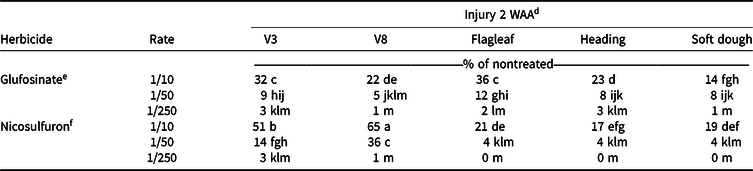
a Injury experiments for 2016 conducted near Colt, AR; in Keiser, AR; near Marianna, AR; and in Fayetteville, AR.
b Injury experiments for 2017 conducted near Marianna, AR, and in Fayetteville, AR.
c Abbreviations: WAA, weeks after application.
d Means followed by the same letter are not different (α = 0.05).
e Glufosinate rates are proportional to 656 g ai ha−1.
f Nicosulfuron rates are proportional to 35 g ai ha−1.
The greatest visible injury (65%) observed at 2 WAA resulted from applications of nicosulfuron at the 1/10× rate applied to V8 sorghum. At this rate and crop stage, along with the flagleaf stage (21%), growth was halted and death of the shoot occurred. Visible injury from glufosinate was high at this growth stage (≤36%) but did not result in death of the growing point. Hale et al. (Reference Hale, Bararpour, Kaur, Seale, Singh and Wilkerson2019) reported less visible injury to grain sorghum following glufosinate application at the V6 growth stage when compared to those of the flagleaf growth stage. This was similar to these results at the V8 growth stage. Increased injury at the flagleaf growth stage could be a result of grain sorghum using sugars and energy toward the metabolism of herbicides and not to the development seed producing blooms (Saeed et al. Reference Saeed, Francis and Clegg1986). Results from nicosulfuron injury were similar to symptoms of imazethapyr reported in other research (Al-Khatib et al. Reference Al-Khatib, Claassen, Stahlman, Geier, Regehr, Duncan and Heer2003). Injury from nicosulfuron was greater than that by glufosinate at the 1/50× rate, ranging from 14% to 36%, with the highest occurring from applications to the V8 growth stage. All other injury was ≤4% at this rate. Visible injury to grain sorghum caused by the 1/250× rate of glufosinate and nicosulfuron was minimal, not exceeding 6% no matter the growth stage of sorghum at the time of application (Table 2).
Glufosinate at the 1/10× rate resulted in height reductions at all growth stages 2 WAA, except for V8 (Table 3). Height reductions were found with the 1/10× rate of nicosulfuron at V3 (21%) and flagleaf (31%) growth stages. Generally, no reduction in height occurred with applications at the 1/50× or 1/250× rate of either herbicide, except for 1/50× rate of glufosinate applied at V3 (9%) and a 1/50× rate of nicosulfuron applied at flagleaf (8%; Table 3).
Table 3. Relative plant heights from grain sorghum in 2017 at various growth stages, 2 WAA of nicosulfuron and glufosinate at low rates in near Marianna, AR and Fayetteville, AR. a
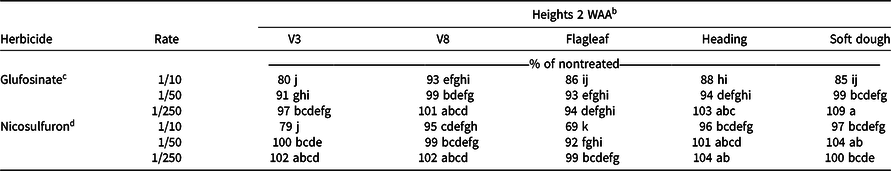
a Abbreviations: WAA, weeks after application.
b Means followed by the same letter are not different (α = 0.05).
c Glufosinate rates are proportional to 656 g ai ha−1.
d Nicosulfuron rates are proportional to 35 g ai ha−1.
As reported by Hale et al. (Reference Hale, Bararpour, Kaur, Seale, Singh and Wilkerson2019), visible injury decreased by 4 WAA, so only plots where the 1/10× rate was applied were included in the statistical analysis, because visible injury from glufosinate or nicosulfuron at the 1/50× or 1/250× rate did not exceed 4%. A two-way interaction of herbicide and timing (P < 0.0001) was observed (Table 1). Glufosinate applied at the 1/10× rate to flagleaf sorghum resulted in 19% injury. At 4 WAA, the least amount of visible injury (3%) was observed when glufosinate at the 1/10× rate was applied to soft dough sorghum (Table 4). The greatest injury (78%) was recorded with the 1/10× rate of nicosulfuron applied to V8 sorghum, which was significantly higher than injury (22%) with the same rate at flagleaf (Table 4). By 4 WAA there was a difference in canopy height found in the main effect of herbicide (P = 0.0053; Table 1). Plots where glufosinate was applied were taller than plots applied with nicosulfuron (data not shown).
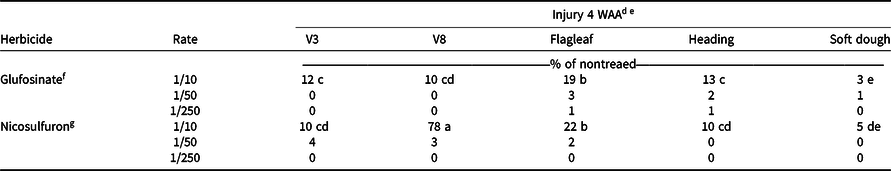
a Injury experiments for 2016 conducted near Colt, AR; in Keiser, AR; near Marianna, A;, and in Fayetteville, AR.
b Injury experiments for 2017 conducted near Marianna, AR, and in Fayetteville, AR.
c Abbreviations: WAA, weeks after application.
d Means followed by the same letter are not different (α = 0.05).
e Rate was not included in statistical analysis due to low levels of visible injury.
f Glufosinate rates are proportional to 656 g ai ha−1.
g Nicosulfuron rates are proportional to 35 g ai ha−1.
No delay in heading was observed in plots applied with glufosinate. However, plots applied with the 1/10× rate of nicosulfuron to V8 and flagleaf sorghum often did not mature into a headed plant (data not shown). Al-Khatib and others (2003) found similar effects from low rates of imazethapyr. The number of seeds per head of grain sorghum can be greatly affected if plants are using sugars and energy toward the metabolism of herbicides (Saeed et al. Reference Saeed, Francis and Clegg1986). A three-way interaction of herbicide, rate, and timing was found for the response variable relative yield (P < 0.0001; Table 1). Injury caused by glufosinate applications only resulted in a yield reduction of greater than 10% when applied at the 1/10× rate to flagleaf sorghum (Table 5). At the 1/10× rate of glufosinate on flagleaf grain sorghum, a 16% yield reduction occurred; however, this reduction did not differ from that of the 1/10× rate of glufosinate on heading and soft dough grain sorghum, which resulted in 6% and 8% reductions, respectively (Table 5). The greatest yield reduction of 96% was collected from plots where nicosulfuron was applied at a 1/10× rate to V8 and flagleaf grain sorghum. When nicosulfuron was applied to heading sorghum at the same 1/10× rate a 41% yield reduction was found. All other applications of nicosulfuron only resulted in a 16% or less reduction in yield (Table 5). Nicosulfuron at the 1/50× rate applied to V8 and flagleaf sorghum did cause a 14% and 16% yield reduction, respectively (Table 5). These results show that the V8 and flagleaf growth stages appear to be the most sensitive stages for yield loss to occur from off-target nicosulfuron or glufosinate herbicide movement and that grain sorghum is not sensitive to yield loss from low rates of glufosinate.
Table 5. Relative yield of grain sorghum after applications of low rates of nicosulfuron and glufosinate. a,b

a Injury experiments for 2016 conducted near Colt, AR; in Keiser, AR; near Marianna, AR; and in Fayetteville, AR.
b Injury experiments for 2017 conducted near Marianna, AR and in Fayetteville, AR.
c Means followed by the same letter are not different (α = 0.05).
d Yield relative to the nontreated check average of 8,174 kg ha−1.
e Glufosinate rates are proportional to 656 g ai ha−1.
f Nicosulfuron rates are proportional to 35 g ai ha−1.







