Introduction
Palmer amaranth (C4 dioecious) is one of the most problematic summer annual broadleaf weed species in the United States (Van Wychen Reference Van Wychen2017), including Kansas (Kumar et al. Reference Kumar, Liu and Stahlman2020a). It is native to southwestern United States and northwestern Mexico (Sauer Reference Sauer1957) and belongs to the Amaranthaceae family. Palmer amaranth has widely spread across major crop production regions in the United States, including the southcentral Great Plains (SGP; Ward et al. Reference Ward, Webster and Steckel2013). Palmer amaranth possesses several unique biological traits, including an extended emergence period, rapid growth rate (0.10 to 0.21 cm per growing day), high photosynthetic rate (80 μmol CO2 m2 s−1), high competitiveness, ability to tolerate shade and water stress conditions, and prolific seed production (up to 600,000 seeds per female plant; Burke et al. Reference Burke, Schroeder, Thomas and Wilcut2007; Chahal et al. Reference Chahal, Ganie and Jhala2018; Horak and Loughin Reference Horak and Loughin2000; Keeley et al. Reference Keeley, Carter and Thullen1987; Ward et al. Reference Ward, Webster and Steckel2013). Palmer amaranth can disperse seeds over landscape through waterways, animals, and common agricultural activities such as plowing and harvesting (Costea et al. Reference Costea, Weaver and Tardif2004, Reference Costea, Weaver and Tardif2005). For instance, rainwater runoff dispersed Palmer amaranth seeds as far as 114 m downslope from the original area of introduction in a cotton field within a year (Norsworthy et al. Reference Norsworthy, Griffith, Griffin, Bagavathiannan and Gbur2014). Due to high outcrossing nature, Palmer amaranth also exhibits pollen-mediated gene flow, resulting in high genetic diversity within and among field populations (Adhikary and Pratt Reference Adhikary and Pratt2015; Sosnoskie et al. Reference Sosnoskie, Webster, Kichler, MacRae, Grey and Culpepper2012). Season-long interference of Palmer amaranth at densities ranging from 0.11 to 10.55 plants m−2 reduced grain yields of corn (Zea mays L.), cotton (Gossypium hirsutum L.), sorghum (Sorghum bicolor L.), and soybean [Glycine max (L.) Merr.], by 11% to 91% (Klingaman and Oliver Reference Klingaman and Oliver1994; Massinga and Currie Reference Massinga and Currie2002; Moore et al. Reference Moore, Murray and Westerman2004; Morgan et al. Reference Morgan, Baumann and Chandler2001).
Herbicide-resistant Palmer amaranth has become a serious management concern for producers in the SGP region. Palmer amaranth populations resistant to herbicides that inhibit microtubule assembly (MTA), acetolactate synthase (ALS), 4-hydroxyphenylpyruvate dioxygenase (HPPD), 5-enolpyruvyl shikimate-3-phosphate synthase (EPSPS), photosystem II (PS II), and protoporphyrinogen oxidase (PPO) have been widely reported (Heap Reference Heap2021). More recently, Palmer amaranth with resistance to 2,4-D in Kansas, to dicamba in Tennessee, and to glufosinate in Arkansas have also been reported (Barber et al. Reference Barber, Norsworthy and Butts2021; Heap Reference Heap2021; Kumar et al. Reference Kumar, Liu, Boyer and Stahlman2019). In addition, multiple resistance to five to six different herbicide sites of action has also been reported in Palmer amaranth populations in Kansas and Arkansas (Heap Reference Heap2021; Kumar et al. Reference Kumar, Liu, Boyer and Stahlman2019, Reference Kumar, Liu and Stahlman2020a).
Previous researchers have documented the emergence pattern of Palmer amaranth under diverse management practices (Aulakh et al. Reference Aulakh, Price, Enloe, Wehtje and Patterson2013; Chahal et al. Reference Chahal, Barnes and Jhala2021; DeVore et al. Reference DeVore, Norsworthy and Brye2012, Reference DeVore, Norsworthy and Brye2013; Jha and Norsworthy Reference Jha and Norsworthy2009). For instance, Aulakh et al. (Reference Aulakh, Price, Enloe, Wehtje and Patterson2013) reported a reduction in early-season Palmer amaranth emergence with a double disk tillage operation compared to disking followed by chisel plow, disking followed by field cultivator, and no-tillage (NT) in Alabama. In contrast, Chahal et al. (Reference Chahal, Barnes and Jhala2021) reported that an early-season shallow tillage increased cumulative emergence of Palmer amaranth seedlings compared with mid- to late-season shallow tillage timings in Nebraska. In a separate study, Jha and Norsworthy (Reference Jha and Norsworthy2009) observed two to three consistent emergence periods (early May through mid-July) of Palmer amaranth and concluded that emergence was reduced by 73% to 76% in plots with soybean (due to light interception by soybean canopy) compared to plots without soybean in South Carolina. A combination of deep tillage with a cereal rye cover crop in soybean or soybean double-cropped with wheat reduced Palmer amaranth emergence by 73% to 98% in Arkansas (DeVore et al. Reference DeVore, Norsworthy and Brye2013). All these aforementioned studies were conducted in moisture-enriched environments of various regions in the United States. Currently, there is a lack of published information on the Palmer amaranth emergence pattern in NT semiarid regions of the SGP.
Winter wheat–fallow (WW-F) or winter wheat–summer crop–fallow (WW-S-F) are predominant crop rotations in the SGP region (Peterson and Westfall Reference Peterson and Westfall2004). Due to shallow soil and limited annual rainfall, NT is often practiced for soil and moisture conservation in the region. Weed seeds are generally concentrated in the upper 5 cm of the soil profile in NT system compared to deeper dispersion in conventional tillage systems (Buhler Reference Buhler1992; Cardina et al. Reference Cardina, Regnier and Harrison1991; Clements et al. Reference Clements, Benoit, Murphy and Swanton1996). Small-seeded weeds such as Palmer amaranth can emerge more easily from shallow depths (Buhler et al. Reference Buhler, Mester and Kohler1996; Oryokot et al. Reference Oryokot, Murphy and Swanton1997; Webb et al. Reference Webb, Smith and Schulz-Schaeffer1987). Knowledge on Palmer amaranth emergence biology (emergence pattern and periodicity) in the NT semiarid SGP region can aid in developing effective Palmer amaranth control strategies by optimizing herbicide applications and cultural practices (crop planting dates, reduced row spacing, strategic tillage, cover crops, etc.; Jha and Norsworthy Reference Jha and Norsworthy2009; Nazarko et al. Reference Nazarko, Van Acker and Entz2005). Understanding the emergence pattern of geographically distant Palmer amaranth populations from the SGP region can help direct management practices to target the peak emergence period and emergence duration of Palmer amaranth for depleting soil seed bank, which is critical for mitigating the further spread of herbicide resistance among field populations. The main objectives of this research were to 1) characterize the emergence pattern of Palmer amaranth populations collected from the SGP region in a common garden study and 2) determine the emergence periodicity of these populations under field conditions. It was hypothesized that variation in annual weather conditions might impact the emergence characteristics (especially emergence duration) of selected Palmer amaranth populations from the SGP region.
Materials and Methods
Seed Source
Fully matured seeds of nine Palmer amaranth populations (20 to 30 female heads per population) were collected during fall 2017 from five states in the SGP region, including Colorado, Oklahoma, Kansas, Texas, and Nebraska (Figure 1). All populations were collected from NT dryland fields under 2- or 3-yr crop rotations, except populations from Nebraska. The Nebraska populations were collected from irrigated corn fields. The Palmer amaranth populations were designated as CO1, CO2 (from Colorado); OK (from Oklahoma); KS1, KS2 (from Kansas); TX (from Texas); and NE1, NE2, NE3 (from Nebraska). Seeds of each Palmer amaranth population were manually cleaned and shipped to the Kansas State University Agricultural Research Center (KSU-ARC) near Hays, KS. The sensitivity of all nine populations to commonly used herbicides was unknown. All seeds were stored in coin envelops at 4 C until the initiation of field experiments. Seed viability of all nine Palmer amaranth populations was tested using a crush test (Jha and Norsworthy Reference Jha and Norsworthy2009) and was found to be ≥96% (data not shown).

Figure 1. Field locations (in approximation) in Colorado, Kansas, Nebraska, Oklahoma, and Texas, from where seeds of Palmer amaranth populations used in common garden study were collected in 2017.
Field Study
A common garden study (Berend et al. Reference Berend, Haynes and MacKenzie2019) was conducted in 2018 and repeated in the 2019 growing season at the KSU-ARC near Hays, KS (38.85196°N, 99.34279°W). Soil type at the study site was a Roxbury silt loam, pH 7.6, and 2.1% organic matter. Study site was in a 3-yr crop rotation (wheat-sorghum-fallow) for >10 yr under NT dryland conditions prior to initiation of this study. The study was established in adjacent sorghum stubble fields during the fallow phase of the 3-yr rotation. In both experimental years, seeds of Palmer amaranth populations collected in fall of 2017 were sown. Winter annual weeds were controlled using glyphosate at 870 g ae ha−1 along with ammonium sulfate (AMS) at 2% wt/vol. The study site had no previous history of Palmer amaranth infestation. The study was conducted in a randomized complete block design with four replications. Each experimental unit was made up of a white colored polyvinylchloride (PVC) cylinder (30-cm diameter, 12.5-cm tall) open at both ends. Each cylindrical PVC ring was pushed 10-cm deep into NT soil. An approximately 2.5-cm cylinder lip was left above the soil surface to prevent off-site movement of Palmer amaranth seeds. Two hundred seeds from each population were randomly counted and spread on the soil surface inside each ring (one population per ring) on March 30, 2018, and April 6, 2019.
Data Collection
Newly emerged Palmer amaranth seedlings were counted and manually removed by hand at a weekly interval during the 2018 and 2019 growing seasons. The end date was chosen based on no further emergence over a 15-d period. Weather data, including daily minimum and maximum air temperatures and precipitation for each growing season, were obtained from a permanent weather station located 20 m from the study site (38.8495°N, 99.3446°W). Growing degree days (GDD) and cumulative GDD (cGDD) were calculated from daily minimum and maximum air temperatures using Equations 1 and 2 (McMaster and Wilhelm Reference McMaster and Wilhelm1997):
GDDdaily is the daily GDD (Cd), T min is the daily minimum air temperature (C), T max is the daily maximum air temperature (C), T base is the base temperature, and n is the number of days for which the emergence counts were recorded in each year. A Tbase of 10 C was chosen for calculating the GDDdaily for Palmer amaranth emergence (Norsworthy et al. Reference Norsworthy, Oliveira, Jha, Malik, Buckelew, Jennings and Monks2008).
Statistical Analyses
Cumulative emergence of each Palmer amaranth population was calculated using the sum of emergence on a sampling date and all previous sample dates as a percentage of the total emergence during the season. Data on percent cumulative emergence were subjected to ANOVA using the MIXED procedure in SAS software (v. 9.3; SAS Institute, Cary, NC). Data were checked for ANOVA assumptions before analyses, and all data met assumptions of normality of residuals and homoscedasticity of error variances. The percent cumulative emergence data were fitted by a three-parameter log-logistic model using the drc package in R software (Ritz and Streibig Reference Ritz and Streibig2005; Seefeldt et al. Reference Seefeldt, Jensen and Fuerst1995):
The model selection was based on Akaike’s information criterion (Ritz and Spiess Reference Ritz and Spiess2008). Parameter estimates and standard errors were also determined in R software. In Equation 3, Y is the percent cumulative Palmer amaranth emergence; x is the cGDD; E50 is the cGDD value (C) to reach 50% cumulative emergence; and the parameter b represents the slope at the inflection point, E50. The slope parameter indicates the emergence rate of each Palmer amaranth population over cGDD. For instance, a slope with a high negative value means the Palmer amaranth population had a rapid emergence, whereas a positive value signifies prolonged emergence. Since the percent cumulative emergence was based on the total emergence, parameter d in the model (the maximum percent cumulative emergence) was fixed at “100” for all populations. A lack-of-fit test (P > 0.05) indicated that the nonlinear regression model (Equation 3) adequately described the percent cumulative emergence data for each Palmer amaranth population in this study (Ritz and Streibig Reference Ritz and Streibig2005).
The daily emergence of each Palmer amaranth population was calculated by dividing the seedling count number from each PVC cylinder on a sampling date with the number of days in between the previous sampling dates. The peak emergence period of each Palmer amaranth population was obtained using a quality control method (Jha and Norsworthy Reference Jha and Norsworthy2009; Montgomery et al. Reference Montgomery, Runger and Hubele2001). The peak emergence period was considered when the daily Palmer amaranth emergence was greater than the result of total emergence at the end of the season divided by the duration of the days between the first and last emergence date plus standard deviation of the daily emergence (Jha and Norsworthy Reference Jha and Norsworthy2009 Montgomery et al. Reference Montgomery, Runger and Hubele2001).
Results and Discussion
The daily minimum and maximum air temperatures at the test site were similar over 2018 and 2019 growing seasons; however, precipitation amount and frequency varied between growing seasons (Figure 1). Therefore, cumulative emergence data for all Palmer amaranth populations were analyzed and presented separately by each year to account for any environmental differences between 2018 and 2019 growing seasons (Tables 1 and 2).
Table 1. Regression parameters estimated from the log-logistic model (Equation 3) for cumulative percent emergence of Palmer amaranth populations in the 2018 growing season.

a Abbreviations: b, slope at inflection point of each curve; E10, E50, and E90, cumulative GDD required for 10%, 50%, and 90% Palmer amaranth emergence for each population, respectively; CI, confidence interval.
b Palmer amaranth populations CO1, CO2 from Colorado; OK from Oklahoma; KS1 and KS2 from Kansas; TX from Texas; NE1, NE2, and NE3 from Nebraska.
c Total number of seedling counts out of 200 seeds for each population observed in 2018.
Table 2. Regression parameters estimated from the log-logistic model (Equation 3) for cumulative percent emergence of Palmer amaranth populations in 2019 growing season.

a Abbreviations: b, slope at inflection point of each curve; E10, E50, and E90 are cumulative GDD required for 10%, 50%, and 90% Palmer amaranth emergence for each population, respectively; CI, confidence interval.
b Palmer amaranth populations CO1, CO2 from Colorado; OK from Oklahoma; KS1 and KS2 from Kansas; TX from Texas; NE1, NE2, and NE3 from Nebraska.
c Total number of seedling counts out of 200 seeds for each population observed in 2019.
Palmer Amaranth Emergence Pattern
Percent cumulative emergence of each Palmer amaranth population in relation to cGDD was well fitted by the 3-parameter log-logistic model based on a lack-of-fit test (P > 0.05). In addition to regression parameters, the fitted model also provided biological parameters such as rate of emergence (parameter b), cGDD needed for 10% (E10), 50% (E50), and 90% (E90) cumulative emergence, and duration of emergence (E90 − E10) for each population (Tables 1 and 2). The percent emergence of each Palmer amaranth population was consistent across replications in each year, which resulted in smaller variance. During the 2018 growing season, CO1 and KS1 populations had rapid emergence rates as indicated by greater negative values of parameter b (−5.4, and −5.3, respectively), followed by CO2 (−4.8), KS2 (−4.8), NE2 (−4.5), TX (−4.2), and NE1 (−4.0) populations (Table 1). Among all tested populations, the OK and NE3 populations had the slowest emergence rates, with parameter b of −3.9 and −3.8, respectively. All Palmer amaranth populations from CO, KS, and NE took 125 to 131 cGDD to reach 10% cumulative emergence, whereas the TX and OK populations took 137 and 144 cGDD, respectively. Similarly, a higher cGDD (254 and 232, respectively) was needed to reach 50% cumulative emergence for OK and TX populations as compared with other populations (cGDD of 190 to 227). The OK population took the greatest cGDD (445) to reach 90% cumulative emergence followed by NE3 population (400), whereas the CO1 population took the fewest cGDD (285). The OK population exhibited a prolonged emergence duration (E90 − E10 = 301 cGDD) during 2018 growing season. In contrast, the CO1 had the shortest emergence duration (E90 − E10 = 158 cGDD). The emergence duration for rest of the populations ranged from 166 to 276 cGDD.
In 2019 growing season, the TX, KS1, NE2, and NE3 populations had rapid emergence rates as indicated by the greater negative values of parameter b (−9.4 to −12.2; Table 2). The emergence rate of CO1 and CO2 (b value = −4.6), KS2 (b value = −3.3), NE1 (b value = −2.4) and OK (b value = −2.3) populations was relatively slower. Most Palmer amaranth populations took 61 to 70 cGDD to reach 10% cumulative emergence, except for the NE2, NE3, and TX populations (73 to 74 cGDD). The NE1 population took the fewest cGDD (54) to reach 10% cumulative emergence. To reach 50% cumulative emergence, the OK population took the greatest number of cGDD (160) followed by NE1 (135), KS2 (121), CO1 (112), and CO2 (111) populations. In contrast, the KS1, NE2, TX, and NE3 populations took the fewest cGDD (88 to 89) to reach 50% cumulative emergence. Similarly, the OK population needed the greatest number of cGDD (420) to reach 90% cumulative emergence, whereas the TX and NE3 populations needed the fewest cGDD (105 and 106). The other six populations took 109 to 338 cGDD to reach 90% cumulative emergence. Consistent with the 2018 growing season, the emergence duration of the OK population was the longest (E90 − E10 = 359) among all populations followed by NE1 (284) population. However, the KS1, TX, NE2, and NE3 populations had significantly shorter emergence duration (E90 − E10 = 32 to 42) in 2019 compared to 2018 growing season.
Palmer Amaranth Emergence Peaks
During the 2018 growing season, all Palmer amaranth populations emerged from May 11 to August 2, with peak emergence periods from early/mid-May through early June (Figure 2). The CO1, CO2, KS1, and KS2 populations had two emergence peaks, which occurred between May 12 and May 27. However, the OK, TX, NE2, and NE3 populations had three emergence peaks, which occurred between May 11 and June 8. Among all populations, only the NE1 population had four emergence peaks, which occurred between May 13 and June 8 (Figure 3). Total rainfall received during the 2018 growing season was 379 mm. Out of total eight rainfall events (each event receiving more than 10 mm rainfall), three events occurred during the peak emergence period of Palmer amaranth (Figure 2). The mean temperature was above 25 C during the week of emergence initiation, indicating optimum temperature conditions for Palmer amaranth germination and emergence (Guo and Al-Khatib Reference Guo and Al-Khatib2003; Steckel et al. Reference Steckel, Sprague, Stoller and Wax2004; Wright et al. Reference Wright, Coble and Raper1999).

Figure 2. Daily minimum and maximum air temperature (C) and precipitation (mm) during Palmer amaranth emergence period in 2018 (A) and 2019 (B) growing seasons.
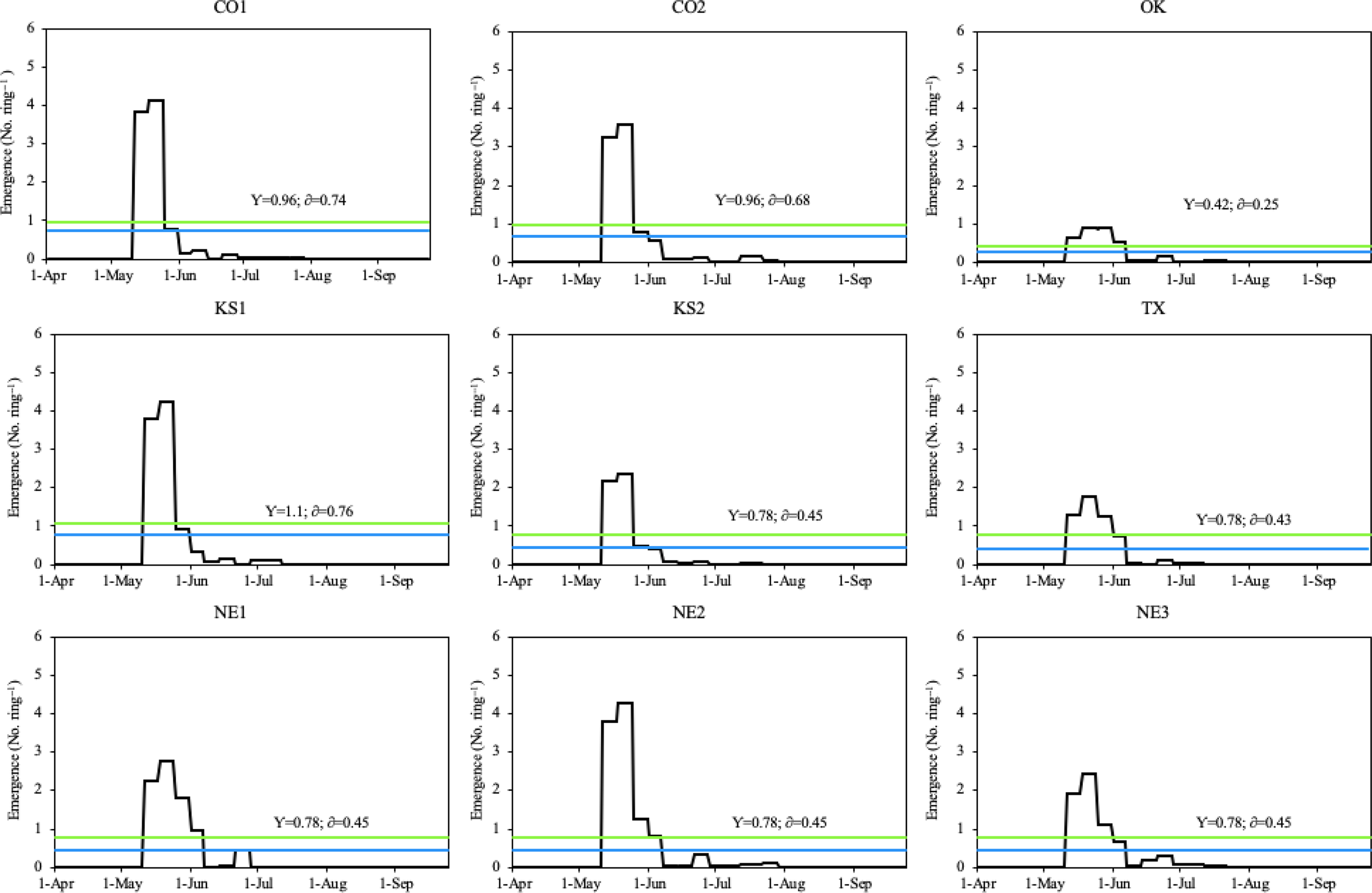
Figure 3. Daily emergence of Palmer amaranth populations in Hays, KS in 2018. The blue line within each graph represents the daily mean emergence of each population, respectively (y); the green line represents the mean plus the standard deviation of a population (δ). Palmer amaranth populations were designated as CO1, CO2 from Colorado; OK from Oklahoma; KS1, KS2 from Kansas; TX from Texas; and NE1, NE2, NE3 from Nebraska.
During the 2019 growing season, all Palmer amaranth populations emerged between April 30 and September 8, with peak emergence periods throughout May (Figure 4). The peak emergence period of all Palmer amaranth populations was slightly longer in 2019 (33 d) than 2018 (29 d; Figures 3 and 4). Six out of nine Palmer amaranth populations (OK, KS1, TX, NE1, NE2, and NE3) had two emergence peaks, which occurred between April 30 and May 15. The other three Palmer amaranth populations (CO1, CO2, and KS2) had three emergence peaks, which occurred between April 30 and June 1. There were more than twice as many rainfall events in 2019 than in 2018. Out of total 19 rainfall events (each event with more than 10 mm rainfall) received in the 2019 growing season, seven events occurred during peak emergence periods (Figures 2 and 4). The mean air temperature during the week of emergence initiation was 21 C.
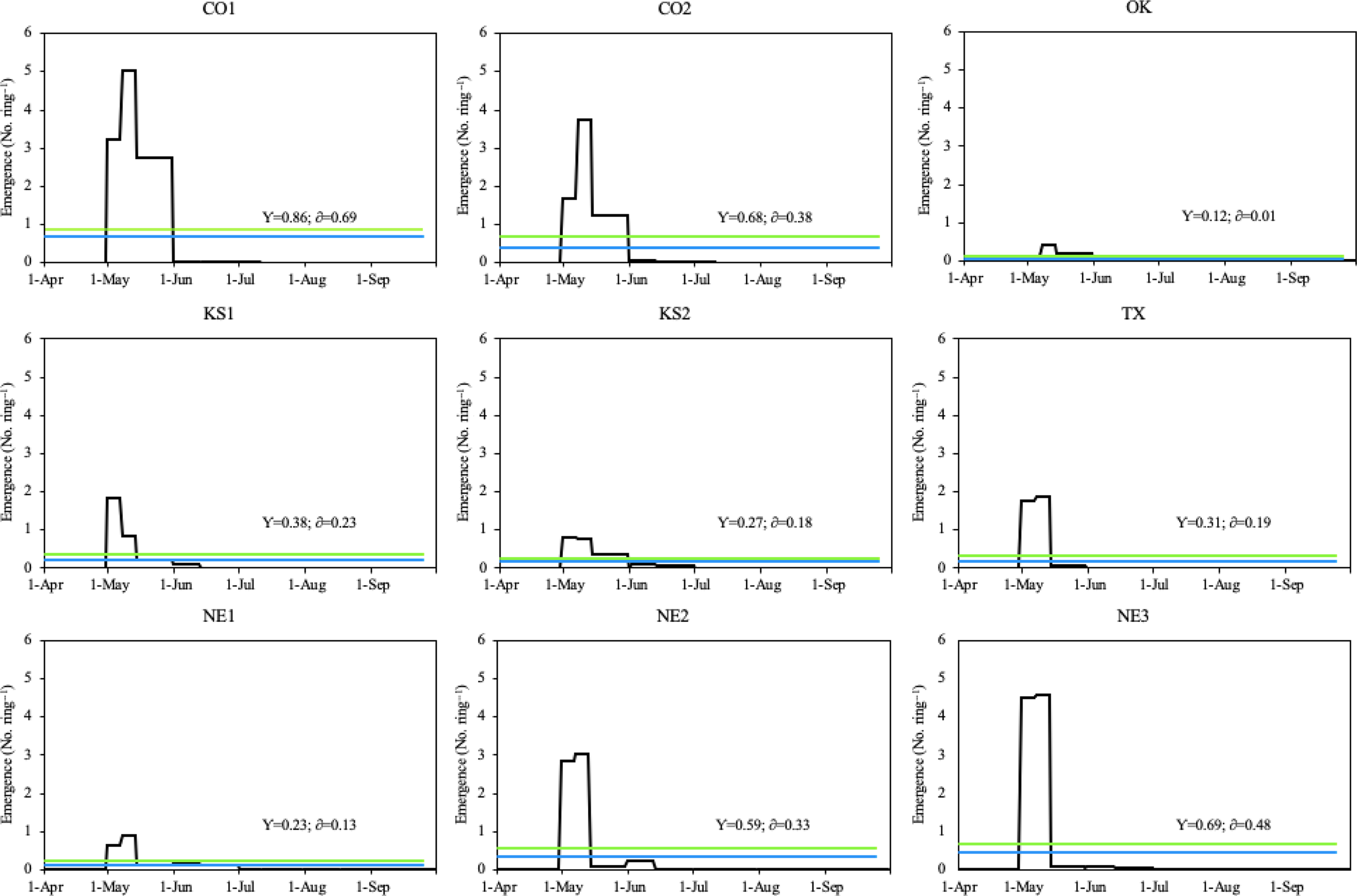
Figure 4. Daily emergence of Palmer amaranth populations in Hays, KS in 2019. The blue line within each graph represents the daily mean emergence of each population, respectively (y); the green line represents the mean plus the standard deviation of a population (δ). Palmer amaranth populations are designated as CO1, CO2 from Colorado; OK from Oklahoma; KS1, KS2 from Kansas; TX from Texas; and NE1, NE2, NE3 from Nebraska.
Environmental conditions such as soil moisture and high temperature favor seed germination and emergence of pigweeds (Guo and Al-Khatib Reference Guo and Al-Khatib2003; Hartzler et al. Reference Hartzler, Buhler and Stoltenberg1999; Jha and Norsworthy Reference Jha and Norsworthy2009), whereas low soil moisture can delay pigweed emergence (Hartzler et al. Reference Hartzler, Buhler and Stoltenberg1999; Jha and Norsworthy Reference Jha and Norsworthy2009). In current study, emergence initiation of all nine Palmer amaranth populations was slightly delayed in 2018 (first emergence observed on May 11) vs. 2019 (first emergence observed on April 30) growing season. However, peak emergence in both years coincided with periods of summer crop planting. This was probably because the daily mean air temperature prior to the month of May was lower in 2018 compared with 2019. The optimum temperature and soil moisture conditions occurred earlier in 2019 than 2018 might have resulted in the shift of peak emergence periods by almost a week earlier in 2019.
Practical Implications
Results from this study indicate that emergence pattern differ among Palmer amaranth populations from five states (Colorado, Oklahoma, Kansas, Texas, and Nebraska) and peak emergence of most populations occurred from early May through early June across 2018 and 2019. The rate of emergence, the cGDD needed to reach 10%, 50%, and 90% emergence, and the emergence duration, also varied across the populations. Spaunhorst et al. (Reference Spaunhorst, Devkota, Johnson, Smeda, Meyer and Norsworthy2018) previously reported that Palmer amaranth populations from geographically distant locations varied in growth and development and exhibited environmental plasticity. This implies the need for site-specific tactics to manage Palmer amaranth seedbanks in the SGP region. The timing of corn planting in the NT dryland SGP region extends from late April to mid-May. For soybean, sorghum, or sunflower, it extends from mid-May through mid-June. Based on the findings from current research and prevailing environmental (conducive temperature and soil moisture) conditions, the peak emergence period of Palmer amaranth populations may coincide with the emergence of summer crops in the SGP region. Therefore, management practices should target these peak emergence period of Palmer amaranth in the early season (early May to early June) of crop growth and development. The use of effective preemergence or postemergence herbicides (multiple sites of action) in conjunction with improved cultural practices (competitive crop cultivars, optimum seeding rates, narrow row spacing, proper nutrients management, etc.) can help in managing these in-crop early-season cohorts of Palmer amaranth. In contrast to established corn or soybean, two to three peak emerging cohorts of Palmer amaranth may occur before sorghum or sunflower planting in mid-June. These peak emergence cohorts provide an opportunity to control Palmer amaranth with nonselective burndown herbicides before sorghum or sunflower planting. The extended emergence period of Palmer amaranth (late April to early September) further warrants the need for a season-long integrated weed management strategy. Considering the rapid evolution of multiple resistance to herbicides applied postemergence, other weed control tactics such as cover crops, strategic tillage, and harvest weed seed control (chaff lining and weed seed destructor) should be integrated for managing Palmer amaranth seedbanks in this NT dryland region. For instance, double cropping or planting summer cover crops (sorghum sudangrass or forage millet) after winter wheat harvest may help in suppressing Palmer amaranth cohorts in postharvest wheat stubble (Kumar et al. Reference Kumar, Obour, Jha, Liu, Manuchehri, Dille, Holman and Stahlman2020b). Similarly, fall-planted cover crops (cereal rye or winter triticale) in postharvest wheat stubble or spring-planted cover crops (spring oats or barley) in summer crop (sorghum/corn/soybean/sunflower) stubble in a typical 3-yr rotation (wheat-summer crop-fallow rotation) would also augment other weed control tactics in depleting Palmer amaranth seedbanks in this region.
Future studies should assess the long-term impact of nonchemical weed control strategies (such as cover crops, competitive crop rotations, harvest weed seed control methods—chaff line or weed seed destructor) alone or in combination with effective preemergence or postemergence herbicides on the emergence dynamics of Palmer amaranth populations in the NT dryland SGP region. Information obtained from this research will also help in developing and validating the prediction models for Palmer amaranth emergence in the region.
Acknowledgments
We thank Taylor Lambert and Natalie Aquilina for their assistance in maintaining the study site and data collection. We also thank Todd Gaines from Colorado State University, Misha Manuchehri from Oklahoma State University, Nevin Lawrence from University of Nebraska, and Muthukumar Bagavathiannan from Texas A&M University for providing seeds of Palmer amaranth populations from each respective state. This work was supported by the USDA–National Institute of Food and Agriculture through Hatch project number 1019671. No conflicts of interest have been declared. This publication is contribution no. 22-055-J from the Kansas Agricultural Experiment Station, Manhattan, KS.