Triquetrous murdannia is an annual weed and a member of the Commelinaceae family. This weed is widely distributed over east, central, and south China and is commonly found in ditch borders, floodplain wetlands, and farmlands, such as rice fields (Supplementary Figure 1). Generally, seedlings appear in late February, flowering occurs in mid-September, and seed dispersal begins in mid-October. Besides propagating by seeds, triquetrous murdannia can also develop new shoots by stem fragments buried in paddy or wet soil (Li Reference Li1998; Xu et al. Reference Xu, Qi, Lu, Yang and Xie2014). Triquetrous murdannia can produce up to 1,000 seeds and 20 to 30 branches per plant. It is highly competitive with rice and hard to eradicate by manual control (Lu Reference Lu1991).
Rice is one of the most important staple food crops in China, where it is grown under flooded conditions. As a result of looming water scarcity, shortage of labor, and increasing farming costs, farmers were disposed to shift from conventional flooded transplanting systems to systems involving labor- saving technologies, such as direct-seeded rice (DSR) and wheat–rice interplanting (Liu et al. Reference Liu, Zhang, Lin and Zhang2014; Rao et al. Reference Rao, Johnson, Sivaprasad, Ladha and Mortimer2007). Weed infestation is a serious problem in labor-saving systems because of simplified tillage and the absence of standing water, which would have a suppressive effect on weed growth during rice emergence and provide a seedling size advantage for rice seedlings (Chauhan Reference Chauhan2012; Opeña et al. Reference Opeña, Chauhan and Baltazar2014). Changes in the establishment method of transplanted rice to DSR have led to shifts in weed flora (Ahmed et al. Reference Ahmed, Salim and Chauhan2014; Chauhan and Johnson Reference Chauhan and Johnson2010; Singh et al. Reference Singh, Bharadwaj, Thakur, Pachauri, Singh and Mishra2009). Triquetrous murdannia has become a dominant species in the weed community in DSR and/or wheat–rice rotated fields in China (Bao Reference Bao2003; He et al. Reference He, Sun and Zhou1999; Tian et al. Reference Tian, Shen, Lu, Gu and Wen2015; Wang et al. Reference Wang, Yu, Li, Liao, Wang, Li and Qiu2013).
Cultural practices such as crop rotation or adequate tillage can help to manage weeds; however, it has been the practice of many farmers to use herbicides as the most economic and effective weed management option in rice fields (Mahajan and Chauhan Reference Mahajan and Chauhan2013; Suria et al. Reference Suria, Juraimi, Rahman, Man and Selamat2011). Usually, triquetrous murdannia emerges by April, which is before the planting of single-cropping rice or late double-cropping rice in central and south China, thus escaping the efficacy of PRE herbicides (Supplementary Figure 2). Therefore, it is critical to control this weed species by using POST herbicides. Previous studies have reported that early POST applications of pyribenzoxim, fluroxypyr, bispyribac-sodium, and MCPA were particularly effective in controlling triquetrous murdannia in rice fields (He et al. Reference He, Sun and Zhou1999; Tan Reference Tan2014; Tian et al. Reference Tian, Shen, Lu, Gu and Wen2015). However, the efficacy of POST herbicides is dependent on the growth stage of the targeted weed. Herbicide efficacy is reduced when applied on older plants (Opeña et al. Reference Opeña, Chauhan and Baltazar2014; Singh and Singh Reference Singh and Singh2004). Response of triquetrous murdannia at different growth stages to POST herbicides is not available in the literature.
Successful establishment of a weed species depends heavily on its ability to germinate and emerge under a wide range of environmental conditions. Seed germination and seedling emergence are usually influenced, directly or indirectly, by many environmental factors, such as temperature, light, soil pH, moisture and salinity stress, seed burial depth, and amount of crop residue in the field (Chachalis and Reddy Reference Chachalis and Reddy2000; Chauhan Reference Chauhan2012; Koger et al. Reference Koger, Reddy and Poston2004). The development of effective integrated weed management strategies depends on a detailed knowledge of weed seed biology. Wang et al. (Reference Wang, Yu, Li, Liao, Wang, Li and Qiu2013) and Tian et al. (Reference Tian, Shen, Lu, Gu and Wen2015) studied the occurrence characteristics of triquetrous murdannia in rice fields. The seed germination and emergence behavior were affected by various environmental factors that may determine the presence of triquetrous murdannia in the field. To date, detailed study of the germination and emergence biology of triquetrous murdannia has not been reported.
Therefore, the objectives of this study were: (1) to determine the effects of temperature, light, osmotic and salt stress, burial depth, flooding depth, and amount of rice residue on triquetrous murdannia germination and emergence; and (2) to evaluate the efficacy of commonly available POST herbicides on different growth stages of triquetrous murdannia.
Material and Methods
Experiments were conducted at the laboratory and screenhouse (an 8 by 20 m chamber framed with 1 cm iron mesh and covered overhead with a transparent plastic cover to prevent rain damage) of the China National Rice Research Institute (CNRRI), Hangzhou, China (30.04°N, 119.55°E), from February to June 2015. Seeds of mature triquetrous murdannia were collected in October 2014 from the upland rice fields at CNRRI. After collection, seeds were cleaned, dried in the shade, and stored in paper bags at room temperature until being used in the experiments. All experiments were conducted in a randomized complete block design with four replications. Each replication was considered a block and was arranged on a different shelf in the incubator or bench in the screenhouse. Each experiment was conducted twice.
General Germination Test
Seed germination of triquetrous murdannia was determined by placing 25 seeds in 9-cm-diameter petri dishes containing two layers of Whatman No.1 filter paper and 5 ml of distilled water or a treatment solution. Petri dishes were sealed with Parafilm (American National Can, Greenwich, CT) to reduce loss of water and were placed in an incubator (RTOP-310D; Zhejiang Top Instrument, Hangzhou, China) at fluctuating day/night temperatures of 25/15 C. Photoperiod was set at 12 h to coincide with the high-temperature period. These conditions were found optimum in the temperature and light experiments. The visible protrusion of the radicle was the criterion for germination (Chauhan and Johnson Reference Chauhan and Johnson2008c). Germination values were calculated as the total number of seeds germinated divided by the total number of seeds in the petri dish.
Effect of Temperature and Light on Germination
The objective of this study was to find the optimum temperature and light regime for seed germination of triquetrous murdannia. Germination was determined by incubating seeds under fluctuating day/night temperatures (35/25, 30/20, 25/15, and 20/10 C) in both light/dark and dark regimes. For the dark treatment, the petri dishes were wrapped in three layers of aluminum foil to prevent any light penetration. Four incubators (RTOP-310D) were used in the test, and each temperature regime was conducted in a separate incubator. The number of germinated seeds in the light/dark regime was counted daily for up to 15 d, whereas in the dark regime, the germination was recorded only after 15 d. Seeds that failed to germinate in the dark after 15 d were moved to the light/dark regime with 5 ml of distilled water added for another 15 d period, at the end of which the germinated seeds were counted.
Effect of Osmotic and Salt Stress on Germination
The effect of water stress on triquetrous murdannia seed germination was studied by placing 25 seeds in 5 ml solutions with osmotic potentials of 0, −0.1, −0.2, −0.4, −0.6, or −0.8 MPa. The solution concentrations were prepared by dissolving 0, 99.4, 140.6, 198.8, 243.4, and 281 g of polyethylene glycol 8000 (Sigma-Aldrich, St. Louis, MO) in 1 L of distilled water (Michel Reference Michel1983; Opeña et al. Reference Opeña, Chauhan and Baltazar2014). The effect of salt stress on germination of triquetrous murdannia was determined by placing 25 seeds in petri dishes containing 5 ml solutions of 0, 25, 50, 100, 150, 200, and 250 mM NaCl. The solutions were prepared by dissolving 0, 1.5, 2.4, 5.8, 8.8, 11.7, and 14.6 g of NaCl per 1 L of distilled water. The petri dishes were incubated under fluctuating day/night temperatures of 25/15 C with a 12 h photoperiod condition to test germination. The number of germinated seeds was counted 15 d after sowing.
Effect of Burial Depth on Seedling Emergence
The effect of burial depth on seedling emergence was determined in a pot experiment conducted in a screenhouse.The soil used in all pot experiments was collected from upland rice fields at CNRRI with a pH of 6.0 and consisted of 40% sand, 32% silt, and 28% clay. The soil was autoclaved and passed through a 3 mm sieve. Twenty-five seeds were sown at depths of 0, 0.5, 1, 2, 4, and 6 cm below the soil surface in plastic pots (12 cm diameter, 10 cm height) that had drainage holes at the bottom. In order to prevent soil from leaking out, a piece of filter paper was placed inside at the bottom of each pot. Pots were initially irrigated with an overhead sprinkler and were later subirrigated. In all pot experiments, visible coleoptiles on the soil surface indicated emergence. The number of seeds that emerged was counted 20 d after planting.
Effect of Rice Residue Amount on Seedling Emergence
This study was conducted in the same screenhouse as described in the previous experiment. Twenty-five seeds were sown on the soil surface in plastic pots containing finely chopped rice straw (stems and leaves) at rates equivalent to 0, 1, 2, 4, and 6 103 kg ha−1. The amounts of rice straw used in this study reflect the amount of straw produced in single-cropping rice fields (Wei et al. Reference Wei, Wang and Xie2012). The condition of the soil, the pots used, and emergence counting in this experiment were done as described above for the seed burial depth experiment.
Effect of Flooding Depth on Seedling Emergence
Twenty-five seeds were sown on the soil surface in small plastic pots (9 cm diameter, 8 cm height) containing the same soil described in the previous screenhouse experiments. The pots containing the seeds were placed inside larger plastic pots (12 cm diameter, 10 cm height) to retain water and maintain flooding depths of 0, 1, 2, 4, and 6 cm. After seeding, the pots were kept at saturated conditions for 5 h to let the seeds absorb water and to prevent them from floating. Flooding to the desired depth was introduced 5 h after seeding, and pots were kept flooded at the aforementioned depths for 20 d. The number of emerged seedlings was counted after 20 d of flooding.
Effect of POST Herbicides on Seedling Survival and Growth
Twelve seeds were sown on the soil surface in pots (9 cm diameter, 8 cm height) and covered with a thin layer of sterilized soil. Seedlings were thinned to six plants per pot at 7 d after sowing. Ten POST herbicides labeled for use in rice fields for annual broadleaf and non-grass monocot weed control were selected (Table 1). Herbicides were sprayed at the 2-, 4-, and 6-leaf stages using a laboratory spray tower (3WPSH-700E; Nanjing Institute of Agricultural Mechanization Ministry of Agriculture, Nanjing, China) equipped with a flat-fan nozzle (Teejet 80015) to deliver 300 L ha−1 at 230 kPa. The POST herbicides used included bensulfuron-methyl (10% WP; Jiangsu Jiangnan Agrochemical, Changzhou, China) at 375 g ai ha−1; bentazone (48% SL; BASF, Shanghai, China) at 1200 g ai ha−1; bispyribac-sodium (10% SC; Kumiai Chemical Industry, Nanjing, China) at 27 g ai ha−1; ethoxysulfuron (15% WDG; Jiangsu Jiangnan Agrochemical, Changzhou, China) at 21 g ai ha−1; fluroxypyr (20% EC; Dow Agro Sciences, Nanjing, China) at 338 g ai ha−1; MCPA (56% SP; Shandong Qiaochang Chemical, Binzhou City, China) at 506 g ai ha−1; pyrazosulfuron (10% WP; Nissan Chemical Industries, Tianjin, China) at 23 g ai ha−1; MCPA+bentazone (20% AS; Mefront Agricultural Chemicals, Wenzhou, China) at 540 g ai ha−1; MCPA+fluroxypyr (42.5% EC; Lier Chemical, Mianyang, China) at 248 g ha−1; pyrazosulfuron+mefenacet (75% WP; Mefront Agricultural Chemicals) at 563 g ai ha−1. There was a nontreated control for each leaf stage. The number of seedlings that survived (with green stem or with green leaves on the plant) was counted 21 d after herbicide application. The shoots (leaf and stem) of the plants were cut from the base of the soil, cleaned, bagged, and dried in an oven at 70 C for 72 h to obtain dry biomass.
Table 1 Effect of POST herbicides on survival (%) and shoot biomass (g pot-1) of triquetrous murdannia when sprayed at 2-, 4-, and 6-leaf stages of the weed.

Statistical Analyses
The data of the repeat experiments were pooled, because no experiment by treatment interaction was revealed. All data met normality conditions. Data obtained from rice residue and herbicide treatment experiments were subjected to analysis of variance with the use of SPSS software, version 13.0 (SPSS, Chicago, IL). Mean comparison was performed using Fisher’s protected least significant difference test, where the overall differences were significant (P≤0.05). Data were analyzed using regression analysis to determine relationships among different temperature regimes, salt and osmotic concentrations, burial depths, residue amounts, and flooding depths. These were best fit to a functional three-parameter sigmoid model using Origin, version 8.0 (OriginLab, Northampton, MA). The model (Ahmed et al. Reference Ahmed, Opeña and Chauhan2015) fit to the fluctuating temperatures was:
where G is the total germination (%) at time T, G max is the maximum germination (%), T 50 is the time required for 50% of maximum germination, and G rate indicates the slope. The model fit to varying osmotic and salt concentrations was:
where G is the total germination (%) at osmotic potential or salt concentration x, G max is the maximum germination (%), x 50 is the osmotic potential or salt concentration required for 50% inhibition of maximum germination, and G rate indicates the slope. An OriginPro exponential decay model in the form of:
was fit to seedling emergence (%) obtained at different burial and flooding depths, where E represents cumulative emergence (%) at depth x, E 0 is the offset, A is the amplitude, and t is the decay constant.
Results and Discussion
Effect of Temperature and Light
Germination of triquetrous murdannia seeds was influenced by the combined effect of light and temperature (P<0.05); germination in continuous darkness was significantly lower than seeds in light/dark in all the tested temperature regimes (Figure 1). In the continuous-dark conditions, seed germination was lower at 35/25 C (46%) than at 30/20, 25/10, and 20/10 C (55 to 61%). Also, when triquetrous murdannia seeds were exposed to the light/dark conditions, higher cumulative germination was observed at 30/20, 25/15, and 20/10 C than at the 35/25 C temperature regimes with 94, 96, and 96% versus 78%, respectively.

Figure 1 Cumulative germination of triquetrous murdannia incubated at alternating day/night temperatures (20/10, 25/15, 30/20, and 35/25 C) and light (light/dark and dark). Vertical bars represent standard errors of the mean. Bars designated by different lowercase letters are significantly different according to Fisher’s protected LSD at P≤0.05.
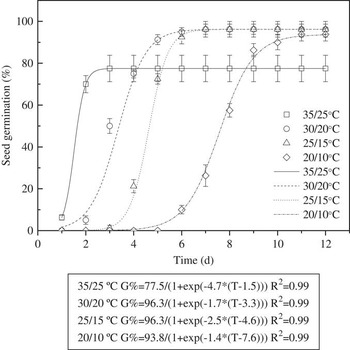
Seeds of triquetrous murdannia exposed to light/dark conditions at the lowest temperature regime took longer time to reach 50% germination (Figure 2). The time it took for 50% germination (of the maximum germination) at 20/10, 25/15, 30/20, and 35/25 C was 8, 5, 3, and 2 d, respectively. However, as mentioned earlier, cumulative germination (%) at 15 d was similar for 30/20, 25/15, and 20/10 C temperature regimes.

Figure 2 Effect of alternating day/night temperatures (20/10, 25/15, 30/20, and 35/25 C) and light (light/dark) on germination of triquetrous murdannia seeds over a 12 h photoperiod for 15 d. Vertical bars represent standard errors. The line represents a three-parameter sigmoid model, G=G max/{1+exp[−(T−T 50)/G rate]}, fit to the data, where G is the total germination (%) at time T, G max is the maximum germination (%), T 50 is the time required for 50% of maximum germination, and G rate indicates the slope at T=T 50.
Temperature is considered an important factor affecting germination of several weed species. In a similar study, germination in doveweed [Murdannia nudiflora (L.) Brenan] was significantly greater at 35/25 C than it was at 30/20 C (Ahmed et al. Reference Ahmed, Opeña and Chauhan2015). The ability of triquetrous murdannia to germinate at all temperatures suggests that this species could emerge throughout the year at low altitudes in warm areas of China.
Varied germination responses to light have been reported among different weed species. Previous studies found that some weed species, such as texasweed [Caperonia palustris (L.) St. Hil.] (Koger et al. Reference Koger, Reddy and Poston2004), American sloughgrass [Beckmannia syzigachne (Steud.) Fernald] (Rao et al. Reference Rao, Dong, Li and Zhang2008), and rose natalgrass [Melinis repens (Willd.) Zizka] (Stokes et al. Reference Stokes, MacDonald, Adams, Langeland and Miller2011) have no light requirement for germination. In contrast, weed species such as common ragweed (Ambrosia artemisiifolia L.) (Baskin and Baskin Reference Baskin and Baskin1980), common lambsquarters (Chenopodium album L.) (Bouwmeester and Karssen Reference Bouwmeester and Karssen1993), and eclipta [Eclipta prostrata (L.) L.] (Chauhan and Johnson Reference Chauhan and Johnson2008b) require light for germination.
The current study supports the findings of Chauhan and Johnson (Reference Chauhan and Johnson2008c, Reference Chauhan and Johnson2008b) and Opeña et al. (Reference Opeña, Chauhan and Baltazar2014) that temperature and light are important factors influencing weed seed germination. In a previous study, Chauhan and Johnson (Reference Chauhan and Johnson2008b) found that eclipta germination at 30/20 C under light/dark conditions was significantly higher than at 25/15 and 35/25 C temperature regimes. Germination of goosegrass [Eleusine indica (L.) Gaertn.] (Chauhan and Johnson Reference Chauhan and Johnson2008c) and Echinochloa glabrescens (Munro ex Hook.) (Opeña et al. Reference Opeña, Chauhan and Baltazar2014) was significantly higher in light/dark conditions than in dark conditions. In our study, seeds could germinate in the dark, which indicates that light is not a prerequisite for germination of triquetrous murdannia. Such results also imply that seeds of this weed species are not photoblastic and may germinate even when buried at shallow depths in the soil and after canopy closure in rice and other crops. Germination of triquetrous murdannia stimulated by light suggests that this species could be a problematic weed in no-till systems, wherein much of the weed seedbank remains on the soil surface (Chauhan and Johnson Reference Chauhan and Johnson2009).
In our study, when nongerminated seeds from the dark treatment were transferred to light/dark conditions at 30/20 C, their germination reached over 90% (unpublished data). These results indicate that the exposure of previously buried triquetrous murdannia seeds to light may trigger germination in field conditions.
Effect of Osmotic Stress on Germination
Seed germination of triquetrous murdannia was strongly influenced (P<0.05) by water potential (Figure 3). Germination decreased from 96 to 21% as osmotic potential decreased from 0 to −0.6 MPa and was completely inhibited at ≥−0.8 MPa. The osmotic potential required to inhibit 50% germination was −0.5 MPa. Similar results were reported for doveweed and redroot pigweed (Amaranthus retroflexus L.), in which −0.40 MPa reduced seed germination by 50% for both weed species (Ahmed et al. Reference Ahmed, Opeña and Chauhan2015; Ghorbani et al. Reference Ghorbani, Seel and Leifert1999). These results indicate that the seeds of triquetrous murdannia were not adversely affected by exposure to low osmotic potentials for up to 15 d. They also suggest that most of the seeds will germinate in moist conditions, while seeds maintained under dry conditions may wait until adequate moisture conditions occur before germinating.

Figure 3 Effect of osmotic potential on germination of triquetrous murdannia seeds incubated at 25/15 C with a 12 h photoperiod for 15 d. Vertical bars represent standard errors. The line represents a three-parameter sigmoid model, G=G max/{1+exp[−(x−x 50)/G rate]}, fit to the data, where G is the total germination (%) at osmotic potential x, G max is the maximum germination (%), x 50 is the osmotic potential required for 50% inhibition of maximum germination, and G rate indicates the slope at x=x 50.
Effect of Salt Stress on Germination
Maximum germination of 96 and 95% was observed in seeds incubated at 0 and 25 mM NaCl concentration, respectively, and no germination was observed at ≥200 mM (Figure 4). Germination was greater than 75% at 100 mM and decreased to 18% at 150 mM. The salt concentration required for 50% reduction in germination was 122 mM.

Figure 4 Effect of NaCl concentration on germination of triquetrous murdannia seeds incubated at 25/15 C with a 12 h photoperiod for 15 d. Vertical bars represent standard errors. The line represents a three-parameter sigmoid model, G=G max/{1+exp[−(x−x 50)/G rate]}, fit to the data, where G is the total germination (%) at NaCl concentration x, G max is the maximum germination (%), x 50 is the NaCl concentration required for 50% inhibition of maximum germination, and G rate indicates the slope at x=x 50.
Seed germination response to salt stress differs according to weed species. The NaCl concentration for 50% reduction in germination for giant false sensitive plant (Mimosa diplotricha C. Wright) and Japanese brome (Bromus japonicas Thunb.) was 255 and 202 mM NaCl, respectively (Chauhan and Johnson Reference Chauhan and Johnson2008a; Li et al. Reference Li, Tan, Li, Yuan, Du, Ma and Wang2015). The concentration for 50% germination reduction for Ceratocarpus arenarius (L.) was 400 mM NaCl (Ebrahimi and Eslami Reference Ebrahimi and Eslami2012). On the other hand, some weed seeds, such as texasweed and goosegrass were found to be sensitive to high NaCl concentrations (Chauhan and Johnson Reference Chauhan and Johnson2008b; Koger et al. Reference Koger, Reddy and Poston2004). Our results suggest that triquetrous murdannia has the ability to germinate under moderate soil salinity, and this species might not be a problematic weed in high-saline soils.
Effect of Seed Burial Depth on Seedling Emergence
Seedling emergence of triquetrous murdannia was significantly affected by seed burial depth (Figure 5). Cumulative seedling emergence declined with increasing burial depth. Highest seedling emergence (68%) was observed when seeds were sown on the soil surface, and only 2% emergence occurred when the seeds were sown at 4 cm depth. No emergence occurred when seeds were sown at ≥6 cm depth. It has been reported for many weed species that seedling emergence decreased with increasing burial depth (Bello et al. Reference Bello, Hatterman-Valenti and Owen2000; Fandrich and Mallory-Smith Reference Fandrich and Mallory-Smith2006; Rao et al. Reference Rao, Dong, Li and Zhang2008). Seedling emergence from deep in the soil is usually inhibited by the absence of light, limitation on soil gas diffusion, and inadequate nutritional content of weed seeds to support emergence (Benvenuti and Macchia Reference Benvenuti and Macchia1995; Bewley and Black Reference Bewley and Black1994).

Figure 5 Effect of seed burial depth on emergence of triquetrous murdannia seedlings in the screenhouse (20 d after sowing). Vertical bars represent standard errors. The line represents an exponential decay model, E=Aexp(−x/t)+E 0, where E represents cumulative emergence (%) at seed burial depth x, E 0 is the offset, A is the amplitude, and t is the decay constant.
Similar to triquetrous murdannia, weed species such as horseweed [Conyza canadensis (L.) Cronq.] (Nandula et al. Reference Nandula, Eubank, Poston, Koger and Reddy2006), Echinochloa glabrescens (Opeña et al. Reference Opeña, Chauhan and Baltazar2014), and romerillo [Bidens alba (L.) DC.] (Ramirez et al. Reference Ramirez, Jhala and Singh2012) had their highest emergence rates when seeds were sown on the soil surface. Greater emergence on the soil surface in triquetrous murdannia is consistent with stimulation of germination by light. These results also suggest that no-till or strip-till DSR systems may favor its emergence in the field. When seeds are buried deeper than 2 cm, establishment of this weed might be more difficult, as the emergence was lower. Thus, plowing seeds to a depth below 4 cm through adequate tillage operations might be useful for managing triquetrous murdannia.
Effect of Rice Residue Amount on Seedling Emergence
Triquetrous murdannia seedling emergence was significantly affected by the amount of rice residue. The addition of residue at 1 to 2 103 kg ha−1 increased the emergence of seedlings (Figure 6). Maximum seedling emergence (93%) was observed when 1 103 kg ha−1 residue was applied, which was significantly (P<0.05) different from all other treatments. Cumulative seedling emergence across different amounts of residue (1 to 6 103 kg ha−1) decreased from 93 to 35%.

Figure 6 Effect of rice residue amount on emergence of triquetrous murdannia seedlings in the screenhouse (20 d after sowing). Vertical bars represent standard errors of the mean. Bars designated by different lowercase letters are significantly different according to Fisher’s protected LSD at P≤0.05.
Reduced seedling emergence and biomass with the addition of crop residue has been reported for many weed species. For example, doveweed and barnyard grass [Echinochloa crus-galli (L.) P. Beauv] were reported to have a marked reduction in seedling emergence and biomass with increased amount of rice residue (Ahmed et al. Reference Ahmed, Opeña and Chauhan2015; Chauhan and Johnson Reference Chauhan and Johnson2011). This was probably due to the reduction in light transmittance, lower soil temperatures, and physical obstruction by use of rice residue as mulch to inhibit weed seed germination (Crutchfield et al. Reference Crutchfield, Wicks and Burnside1985; Teasdale and Mohler Reference Teasdale and Mohler1993). In this study, increased seed germination with the addition of 1 to 2 103 kg ha−1 rice residues compared with the absence of residue could be a result of higher availability of soil moisture due to better soil seed contact. Our results also suggest that the addition of rice residue up to 6 103 kg ha−1 may help in suppressing triquetrous murdannia seedling emergence in fields. However, these high amounts of rice residue may not be feasible in China, as the common practice of most famers is to burn or carry away the straw after harvest.
Effect of Flooding Depth on Seedling Emergence
When the seeds were sown on the soil surface, there was a marked reduction in seedling emergence of triquetrous murdannia with increasing flooding depth (Figure 7). Seedling emergence was reduced by 40, 48, 64, and 70% at flooding depths of 1, 2, 4, and 6 cm, respectively. Reduction in emergence of weeds under flooded conditions may be a result of several factors, including reduced O2 levels, accumulation of CO2, and the presence of reduced forms of chemical radicals and toxic gaseous products of anaerobic decomposition, such as methane, nitrogen, nitrous oxides, and sulfides (Smith and Fox Reference Smith and Fox1973).

Figure 7 Effect of flooding depth on emergence of triquetrous murdannia seedlings in the screenhouse (20 d after sowing). Vertical bars represent standard errors. The line represents an exponential decay model, E=Aexp(−x/t)+E 0, where E represents cumulative emergence (%) at seed flooding depth x, E 0 is the offset, A is the amplitude, and t is the decay constant.
Similar to triquetrous murdannia, emergence of many weed species, such as rice flatsedge (Cyperus iria L.), junglerice [Echinochloa colona (L.) Link], and fimbry [Fimbristylis miliacea (L.) Vahl], were reduced greatly with increasing flooding depth (Civico and Moody Reference Civico and Moody1979; Smith and Fox Reference Smith and Fox1973). Our results indicate that shallow flooding can be used to suppress the emergence of triquetrous murdannia. In some areas of eastern and central China, farmers practice a double-cropping system in which rice is planted in June following the wheat harvest. At that time, triquetrous murdannia had already germinated and grown in nonirrigated soil for over 1 mo. To reduce the dispersal of triquetrous murdannia stem fragments in flooded rice fields (Supplementary Figure 3), fields used in double-cropping systems must undergo pretreatment with a broad-spectrum herbicide before rice is planted to manage this weed.
Effect of POST Herbicides on Seedling Survival and Growth
The response of triquetrous murdannia to the tested POST herbicides varied depending on weed growth stage (Table 1). Fluroxypyr and MCPA were able to completely control triquetrous murdannia at any growth stage. None of the triquetrous murdannia plants survived an application of bispyribac-sodium, MCPA+bentazone, or MCPA+fluroxypyr at the 2- and 4-leaf stage; however, when those herbicides were applied at the 6-leaf stage, the number of surviving plants increased to 10, 23, and 15%, respectively. The application of ethoxysulfuron was unable to control triquetrous murdannia at any leaf stage, but it reduced biomass significantly compared with the nontreated control. At the 2-leaf stage, bensulfuron methyl, bentazone, pyrazosulfuron, and pyrazosulfuron+mefenacet had 75, 58, 75, and 88% seedling survival, respectively. These herbicides were unable to control triquetrous murdannia when applied at the 4- and 6-leaf stage, although they significantly reduced shoot biomass.
Manual control of a branching weed such as triquetrous murdannia is very difficult, time-consuming, and expensive. Therefore, farmers have to rely on herbicides to control triquetrous murdannia. The results of our study suggest that farmers can control this weed through the application of fluroxypyr and MCPA up to the 6-leaf stage in rice fields. Bispyribac-sodium, MCPA+bentazone, or MCPA+fluroxypyr can also be used. However, these herbicides should be applied before the 4-leaf stage to achieve more than 90% control. In a similar study, bispyribac-sodium, fluroxypyr, and MCPA applied at 45, 240, and 683 g ai ha−1, respectively, were effective to control triquetrous murdannia at the 2- to 3-branch stages in rice fields (He et al. Reference He, Sun and Zhou1999; Tian et al. Reference Tian, Shen, Lu, Gu and Wen2015).
In summary, triquetrous murdannia germination was stimulated by light, and more than 80% of the seeds germinated when incubated at 30/20, 25/15, and 20/10 C, suggesting that triquetrous murdannia seeds can germinate at low altitudes in subtropical and tropical areas in China. Germination decreased as water stress and salt concentration increased but occurred over a relatively broad range of osmotic potentials and salt concentrations, indicating some degree of tolerance to dry and saline conditions. The highest germination was observed from seeds sown on the soil surface, and emergence was greatly reduced with increases in burial and flooding depths. The use of rice residue as mulch suppressed seedling emergence. The results of our study suggest that adequate tillage and shallow flooding could be effective methods of managing triquetrous murdannia. In the absence of PRE herbicides, the use of POST herbicides such as fluroxypyr and MCPA up to the 5-leaf stage of triquetrous murdannia can provide complete control.
Acknowledgments
This work was supported by the Rice Pest Management Research Group of the Agricultural Science and Technology Innovation Program of China Academy of Agricultural Science, China Agriculture Research System (CARS-01-02A). The authors thank all the workers for assistance in conducting this research.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1614/WS-D-16-00048.1