Introduction
Modern agricultural production has relied on the use of synthetic herbicides as a powerful tool to control infestations of unwanted plants (Oerke Reference Oerke2006). However, repeated herbicide application to the same area is likely to lead to the evolution of herbicide resistance in resident weed populations (Powles and Yu Reference Powles and Yu2010). More than 200 weed species have been reported to have evolved resistance in response to selection with one or more herbicides (Heap Reference Heap2014, Reference Heap2022). The reduced-tillage cropping system in southern Australia tends to increase grower reliance on herbicides for weed control across large fields and farmland areas and has resulted in the selection of numerous annual ryegrass (Lolium rigidum Gaudin) (Busi et al. Reference Busi, Dayan, Francis, Goggin, Lerchl, Porri, Powles, Sun and Beckie2020, Reference Busi, Bates, Beckie, Davey, Porri, Lerchl and Onofri2021) and wild radish (Raphanus raphanistrum L.) populations (Busi and Powles Reference Busi and Powles2017; Owen and Powles Reference Owen and Powles2018) resistant to multiple herbicide modes of action.
In southern Australian grain-growing regions, R. raphanistrum is the most damaging and widespread dicotyledonous weed species (Hashem et al. Reference Hashem, Dhammu, Powles, Bowran, Piper and Cheam2001). It can be a strong competitor with the crop for water, light, and nutrients, resulting in significant crop yield reductions and economic losses for the grower. For example, R. raphanistrum can cause 10% wheat (Triticum aestivum L.) yield loss, even at densities as low as 7 plants m−2 (Hashem and Wilkins Reference Hashem and Wilkins2002; Walsh and Powles Reference Walsh and Powles2007) and is the second most economically damaging weed in Australia, causing losses up to A$53 million yr−1 (Llewellyn et al. Reference Llewellyn, Ronning, Ouzman, Walker, Mayfield and Clarke2016). The weed is known to have negative effects on grain quality, and it is an alternative host for a variety of pests and diseases of grain crops and pastures (Cheam and Code Reference Cheam and Code1995).
Raphanus raphanistrum, as an obligate outcrossing and highly fecund species, is also resistance prone; four random field surveys conducted in Western Australia over the past 20 yr have revealed resistance to herbicides inhibiting acetolactate synthase (ALS), photosystem II (PSII), phytoene desaturase (PDS), and 5-enolpyruvylshikimate-3-phosphate synthase and to the synthetic auxin herbicides (Owen et al. Reference Owen, Martinez and Powles2015). In particular, the frequency of resistance to ALS inhibitors (chlorsulfuron, triasulfuron, imazamox + imazapyr), auxinic herbicides (2,4-D, MCPA), and PDS inhibitors (diflufenican, picolinafen) has dramatically increased to 50% of cropped fields (Owen and Powles Reference Owen and Powles2018). Originally largely confined to the northern regions of the Western Australian cropping region, R. raphanistrum has spread to the south of Western Australia in recent years and has become a troublesome crop weed across the entire state. The species is also reported to persist as a summer (fallow) weed (Borger et al. Reference Borger, Hashem and D’Antuono2019).
Given the widespread resistance of R. raphanistrum to herbicides commonly used for its control, Australian growers in the last decade have relied extensively on preformulated mixtures of Group 4 (MCPA), 6 (bromoxynil), 14 (diflufenican, picolinafen), and alternative sites of action such as 4-hydroxyphenylpyruvate dioxygenase (HPPD)-inhibiting herbicides (Group 27). The HPPD-inhibiting herbicides are a relatively new group of herbicides that effectively control a broad spectrum of broadleaf and some grass weeds. The HPPD inhibitors block the metabolic pathway leading to plastoquinone, carotenoid, and tocopherol synthesis in plants (van Almsick Reference van Almsick2009). Since 2007 in Australia, HPPD-inhibiting herbicides have been used to selectively control multiple-resistant R. raphanistrum populations in wheat and barley (Hordeum vulgare L.). Bicyclopyrone (a member of the triketone class of HPPD inhibitors) was introduced first, followed by pyrasulfotole (pyrazole class) as a preformulated mixture with MCPA in 2008 or with bromoxynil in 2009. Since 2020, two new HPPD-inhibiting herbicides have been introduced for use in Australian wheat and barley crops: topramezone (pyrazole class), with the option of tank-mixing with MCPA or bromoxynil for selective postemergence weed control; and mesotrione (triketone class) registered for preemergence application.
Resistance to this mode of action has been reported in populations of waterhemp [Amaranthus tuberculatus (Moq.) Sauer] (Hausman et al. Reference Hausman, Singh, Tranel, Riechers, Kaundun, Polge, Thomas and Hager2011; Kaundun et al. Reference Kaundun, Hutchings, Dale, Howell, Morris, Kramer, Shivrain and McIndoe2017; Oliveira et al. Reference Oliveira, Jhala, Gaines, Irmak, Amundsen, Scott and Knezevic2017) and Palmer amaranth (Amaranthus palmeri S. Watson) (Küpper et al. Reference Küpper, Peter, Zöllner, Lorentz, Tranel, Beffa and Gaines2018) in the United States that were repeatedly exposed to mesotrione, tembotrione, and/or tompramezone. The expression and magnitude of phenotypic HPPD-inhibitor resistance in A. tuberculatus (Kaundun et al. Reference Kaundun, Hutchings, Dale, Howell, Morris, Kramer, Shivrain and McIndoe2017) and A. palmeri varied at different growth stages, with a general pattern of low-level phenotypic resistance (Küpper et al. Reference Küpper, Peter, Zöllner, Lorentz, Tranel, Beffa and Gaines2018). In Australia, despite anecdotal reports of lower than expected R. raphanistrum control in the field by HPPD-inhibiting herbicides and a study showing reduced sensitivity of R. raphanistrum to extremely low rates of HPPD herbicides (Lu et al. Reference Lu, Yu, Han, Owen and Powles2020a), there are no confirmed cases of field resistance to HPPD herbicides, as documented in the most recent geographic field surveys. Conversely, several studies have reported complex patterns of resistance selection in response to historical use of herbicide Groups 2, 4, 5, and 12 (Owen and Powles Reference Owen and Powles2018).
We report on a series of pot experiments to confirm the putative HPPD-inhibitor resistance detected in two populations subjected to large-scale herbicide-resistance screening and to quantify cross-resistance at the same plant growth stage of R. raphanistrum. Direct measurements of Group 27 herbicide survival in the field from which one of these resistant populations was originally collected were also performed to corroborate the results obtained under controlled conditions. Herbicide treatments and application times in the field study were designed to reflect control strategies for R. raphanistrum in Australia, which involves a range of different use patterns for HPPD-inhibiting herbicides and their integration with other sites of herbicide action.
Materials and Methods
Plant Material
Siliques of R. raphanistrum plants from fields perceived to contain herbicide-resistant populations were submitted by growers from across the Western Australian grain belt at the time of harvest in late 2019. In each case, siliques were collected from multiple plants (a minimum of 30) in the population and bulked to form a representative sample of that population. Seeds were removed from siliques in a mill, sieved, cleaned by forced-air separation, and stored dry at 37 C to permit dormancy release by afterripening.
Initial Herbicide-Resistance Screening at the Recommended Label Rate
Well-characterized populations susceptible to all herbicides and resistant to the specific herbicide tested (when available) were included in all experiments alongside the field-collected populations. The 121 putative-resistant R. raphanistrum populations submitted to the University of Western Australia’s herbicide-resistance testing center were screened with three HPPD-inhibitor herbicides and other herbicide sites of action per their registered use patterns and their respective label rates, as indicated in Table 1.
Table 1. Survival (%) of two putative resistant Raphanus raphanistrum populations (86-2020 and 91-2020) and a susceptible control (WARR36) in response to the recommended label rates of several herbicide sites of action (Group). a

a All herbicides were applied postemergence, with the exception of mesotrione, which was applied preemergence per the recommendation on the label. A χ2 heterogeneity test was used to compare survival rates of populations 86-2020 and 91-2020 vs. WARR36 and P-values are reported.
The three HPPD-inhibiting herbicides were applied as follows: 96 g mesotrione ha−1 (Callisto ®: 480 g mesotrione L−1, Syngenta Australia, Sydney, Australia); a mixture of 12 g topramezone and 285 g MCPA ha−1 (a tank mix equivalent to 200 ml ha−1 of Frequency®, 60 g L−1 topramezone, BASF Australia, Melbourne, Australia; and 500 ml ha−1 of Polo® LVE: 570 g L−1 MCPA, Nufarm Australia, Melbourne, Australia), and a coformulated mixture of 25 g pyrasulfotole and 140 g bromoxynil ha−1 (a premix equivalent to 670 ml ha−1 of Velocity®, 37.5 g L−1 pyrasulfotole and 210 g L−1 bromoxynil, Bayer CropScience Australia, Melbourne, Australia). Seeds (20 per treatment) were sown into 5 by 5 by 15 cm plastic pots containing moist potting mix (50% composted pine bark, 25% peat, 25% river sand) and maintained in a naturally lit glasshouse (22 C mean temperature, 14-h photoperiod of natural light) at the University of Western Australia over February and March 2020. Pots were watered regularly to maintain soil moisture levels up to >80% of soil capacity. Mesotrione was applied preemergence to seeds immediately after sowing, and the seeds were then covered with fresh potting mix and watered. Topramezone + MCPA and pyrasulfotole + bromoxynil were applied postemergence to 3-leaf seedlings. All herbicides were applied using a custom-built dual-nozzle (Tee-Jet® XR11001 flat fan, TeeJet Australasia, Newtown, Australia) cabinet sprayer delivering a water volume of 110 L ha−1. Plant survival was assessed at 21 d after herbicide application. Survivors were those plants that, despite herbicide damage, could recover, grow, and produce flowers.
HPPD-Inhibitor Dose–Response Assays
As two of the 121 populations (populations 86-2020 and 91-2020) used in the initial screening exhibited a cross-resistance profile in response to the three HPPD-inhibiting herbicides tested, with survival ranging from 5% to 32% (Table 1), they were subjected to further herbicide dose–response screening. Median survival to pyrasulfotole + bromoxynil was 0%. A standard population (WARR36), known to be susceptible to HPPD-inhibiting herbicides (Lu et al. Reference Lu, Yu, Han, Owen and Powles2020a), was used as a control. Plants grown as described earlier were sprayed at the 3- to 4-leaf stage with the preformulated commercial mixture pyrasulfotole + bromoxynil (Velocity® herbicide) at rates of 0 + 0, 4.7 + 26.3, 9.4 + 52.5, 18.8 + 105, 25.1 + 140.7, 31.9 + 178.5, or 37.5 + 210 g pyrasulfotole + bromoxynil ha−1. These doses are equivalent to 0, 125, 250, 500, 670 (recommended rate), 850, and 1,000 ml ha−1 of formulated Velocity®. For this and all subsequent experiments, there were three replicates of 20 seeds for each population and herbicide dose, and plant survival was assessed at 21 d after herbicide application; aboveground biomass was also harvested (from survivors and dead plants) at this time and weighed after drying at 60 C for 7 d. In the same study, plants were treated with each active ingredient applied as a stand-alone at the 3- to 4-leaf stage. Pyrasulfotole (supplied by Bayer Crop Science Australia) was applied at rates of 0, 35, 70, 140, or 280 g ha−1, and bromoxynil (supplied by Nufarm Australia) at rates of 0, 100, 200, 300, or 400 g ha−1.
Characterization of HPPD-Inhibitor Cross-Resistance
An additional study was conducted to assess cross-resistance of populations 86-2020 and 91-2020 to different HPPD inhibitors, with 3- to 4-leaf plants treated with the commercial formulations of mesotrione applied at 0, 24, 48, 96, or 192 g ha−1; topramezone applied at 0, 3, 6, 12, or 24 g ha−1; or pyrasulfotole applied at 0, 35, 70, 140, or 280 g ha−1. Mesotrione is not registered for postemergence use in Australia. For this reason, the initial screening for mesotrione resistance was conducted by preemergence application of the herbicide. Conversely, mesotrione was used postemergence in this cross-resistance study to maintain consistency between the three different HPPD-inhibiting herbicide treatments and the subsequent assessment of plant survival.
Field Trial
In May 2021 a trial was established in the same field where population 91-2020 was collected before harvest in 2019 (Canna, WA 6627; 28.900°S, 115.867°E). A wheat crop was sown on May 19, 2021, with a planting rate of 76 kg seed ha−1 at 3.5-cm depth. Row spacing was set to 24 cm, with each plot 2.5-m wide and 12-m long. Each plot represented an experimental unit. There were three replicates for each herbicide treatment in a randomized complete block. Soil analysis (CSBP Soil and Plant Analysis Lab, Perth, Western Australia) indicated a sandy loam soil with texture of sand 83%, silt 5.1%, and clay 11%. Organic matter was 0.8%, pH 4.0, and CEC 1.1 mEq 100g−1. Treatments were applied on May 19 for soil-applied preemergence herbicides; June 17 for early postemergence treatments, with approximately 80% of R. raphanistrum plants at the 2- to 3-leaf growth stage; and on July 2 for later postemergence treatments, with the majority of R. raphanistrum plants at the 3- to 4-leaf stage. Herbicide treatments were designed to reflect industry standards, consisting of two sequential herbicide treatments (preemergence followed by postemergence or two postemergence treatments at an interval of 2 wk) to achieve effective R. raphanistrum control. Final herbicide efficacy assessments were conducted on September 6 (66 d after last herbicide application) by plant counts using a 0.25-m2 quadrat (nine replicated points across the plot) and visually estimating weed control across the whole replicated plot (three replicates each surveyed by three assessors).
Statistical Analysis
In each dose–response experiment, there were three replications (pots) for each herbicide dose, and each pot was the experimental unit. The dose–response studies were repeated once, and data were pooled before nonlinear regression analysis. Plant survival was expressed as a percentage of total plants treated with herbicide, and biomass as a percentage of the biomass of the untreated control.
Survival rates (number of survivors/number of treated plants) as the proportion of survival found in populations 86-2020 and 91-2020 versus the susceptible control (WARR36) were compared using the command prop.test in the statistical software R (R Core Team 2021) and separated by multiple comparisons by a χ2 heterogeneity test (see Table 1).
The drc package of the statistical software R v. 4.1.0 (R Core Team 2021) was used to calculate the herbicide dose causing 50% plant mortality (LD50) or growth reduction (GR50) and to estimate the regression coefficients of a three-parameter log-logistic model:
where d is the upper limit (100%), b is the slope of the curve, x is the herbicide dose, and e is the dose producing a 50% reduction in response. Nonlinear regression was applied to data under the assumption of a continuous Gaussian distribution of errors. Data were normally distributed as visually checked with Q-Q plots (data not shown). Statistically significant differences in estimated LD50 or GR50 values between susceptible and putative-resistant R. raphanistrum populations were assessed using the EDcomp function in the drc package. The data were plotted using GraphPad Prism 8.02 (GraphPad, San Diego, CA, USA).
In the field experiment, values of visual weed control percentages and weed infestation densities (as number of surviving plants m−2) following application of the recommended rates of commercial herbicides were analyzed by ANOVA, and means were separated by multiple comparisons with the post hoc Tukey test. ANOVA assumptions were held under square-root transformation, and back-transformed data are presented.
Results and Discussion
Initial Screening of Resistance to HPPD Inhibitors
In the initial herbicide screening, populations 86-2020 and 91-2020 displayed substantial and the greatest survival to one or more of the HPPD inhibitors tested (Table 1). Population 86-2020 was more resistant to mesotrione or the mixture topramezone + MCPA than population 91-2020, but population 91-2020 was more resistant to the preformulated herbicide mixture pyrasulfotole + bromoxynil (Table 1). Both populations displayed a high level of resistance to the two synthetic auxin herbicides tested and were fully susceptible to the PDS inhibitors diflufenican and picolinafen (Table 1).
Raphanus raphanistrum is known for its enormous potential to rapidly evolve herbicide resistance and accumulate traits for resistance against multiple sites of action when exposed to intense and repeated selection with herbicides. To date, high frequencies of multiple resistance to at least four different herbicide sites of action have been reported in R. raphanistrum populations (Owen et al. Reference Owen, Martinez and Powles2015; Walsh Reference Walsh2019). Resistance to HPPD inhibitors, which may be characterized as a low risk due to the paucity of weed species reported to be resistant, had not been previously reported in R. raphanistrum, but the appearance of resistance after 12 yr of use parallels the time interval between herbicide introduction and the first report of field resistance for most other sites of action (e.g., Walsh et al. [Reference Walsh, Powles, Beard and Porter2004]; Walsh and Powles [Reference Walsh and Powles2007] for the specific case of R. raphanistrum).
Confirmation of Resistance to Pyrasulfotole and Pyrasulfotole + Bromoxynil
The dose–response experiment with pyrasulfotole + bromoxynil on 4-leaf-stage R. raphanistrum plants confirmed that both populations displayed a 4-fold phenotypic resistance as measured by an LD50 resistance index (RI; Table 2; Figure 1; Supplementary Figures S1 and S2). Similarly, the level of phenotypic resistance measured as survival in response to pyrasulfotole applied stand-alone was substantially greater than the susceptible control in both populations (Table 2; Figure 1). As indicated by RI values, population 91-2020 was more pyrasulfotole-resistant than population 86-2020 based on LD50; RI based on GR50 of 91-2020 was also significantly greater than that of the susceptible control (Table 2), indicating limited plant aboveground biomass suppression (Table 2). RIs calculated by the quantification of plant biomass were generally lower than those measured for plant survival rates. This was due to the fact that surviving plants were visibly suppressed by the herbicide action, but also because the aboveground biomass was measured by pooling live and dead plants.
Table 2. Estimated LD50 (dose causing 50% mortality) and GR50 (dose causing 50% growth reduction) values (±SE) for Raphanus raphanistrum populations expressed as grams of active ingredient of pyrasulfotole + bromoxynil per hectare, pyrasulfotole stand-alone, or bromoxynil stand-alone. a
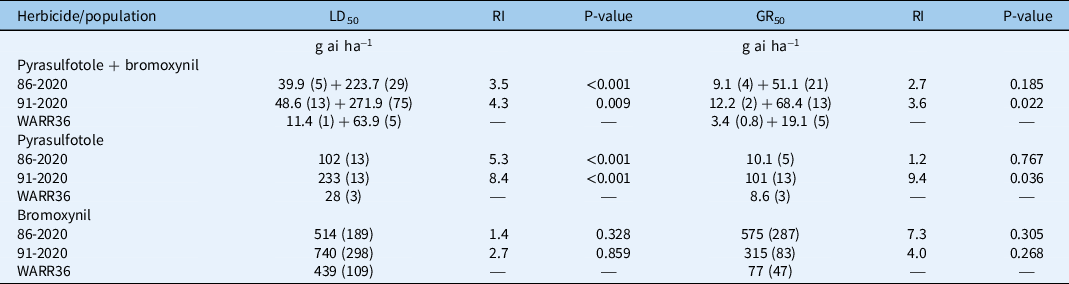
a Resistance indices (RI) are expressed as the ratio of LD50 or GR50 values for the putative resistant (86-2020 and 91-2020) and susceptible (WARR36) populations. Probability values (P) of difference between LD50 values were calculated as a t-test comparison between the putative resistant and the susceptible control using the EDComp function in the drc package of the software program R v. 3.6.1.

Figure 1. Dose–response curves for the two resistant Raphanus raphanistrum populations 86-2020 (solid line, solid circle) and 91-2020 (solid line, solid square) and the standard herbicide-susceptible population WARR36 (solid line, solid triangle) treated postemergence at the 3- to 4-leaf stage with varying doses of (A) Velocity® (formulated mixture of 37.5 g L−1 pyrasulfotole + 210 g L−1 bromoxynil), (B) pyrasulfotole, or (C) bromoxynil in spring 2021. Symbols represent mean percentage survival ±SE (n = 6).
Conversely, there was no significant difference between LD50 and GR50 values of putative HPPD inhibitor–resistant and susceptible control populations in response to bromoxynil stand-alone (Table 2; Figure 1).
HPPD-inhibitor resistance in field populations of A. tuberculatus and A. palmeri was reported in the United States a decade ago (Hausman et al. Reference Hausman, Singh, Tranel, Riechers, Kaundun, Polge, Thomas and Hager2011; Kaundun et al. Reference Kaundun, Hutchings, Dale, Howell, Morris, Kramer, Shivrain and McIndoe2017; Nakka et al. Reference Nakka, Godar, Wani, Thompson, Peterson, Roelofs and Jugulam2017), and it is now documented in R. raphanistrum populations from a cropping area in the northern Western Australian grain belt where multiple-resistant R. raphanistrum infestations have been endemic for more than 20 yr. This study is the first report of field resistance in R. raphanistrum in response to a decade-long selection by HPPD-inhibiting herbicides used every year in the same crop (wheat) for postemergence selective control of R. raphanistrum. In Australia, pyrasulfotole has been the main HPPD inhibitor used for the selective control of multiple-resistant R. raphanistrum since 2008 (Hausman et al. Reference Hausman, Singh, Tranel, Riechers, Kaundun, Polge, Thomas and Hager2011), when it was registered as a preformulated mixture with MCPA or bromoxynil in wheat and barley. Topramezone and mesotrione have only been commercialized since 2020 (in the growing season following weed seed sample collection for this study) and therefore did not contribute to the selection of HPPD-inhibitor resistance reported herein. Thus, the two HPPD inhibitor–resistant populations reported here were selected by the repeated field application of pyrasulfotole + bromoxynil for a minimum of 12 consecutive years. They both displayed phenotypic resistance to the synergistic mixture of pyrasulfotole + bromoxynil when treated at recommended field rates. Bromoxynil (stand-alone) resistance in R. raphanistrum is low, with resistance in <2% of fields detected across about 300 R. raphanistrum populations tested in 2020 to 2022 (RB, unpublished data), but it remains an emerging issue that needs to be monitored every year. It is clear that the two populations of R. raphanistrum resistant to the coformulated mixture of bromoxynil and pyrasulfotole survived mainly because of their evolved pyrasulfotole resistance trait(s). Conversely, the initial characterization of survival responses to topramezone + MCPA and to mesotrione applied preemergence under controlled environmental conditions, which were not significantly different from that of the susceptible control, indicated that cross-resistance to HPPD-inhibiting herbicides can be incomplete, depending on herbicide use patterns and dosages (Table 1).
Pyrasulfotole, Mesotrione, and Topramezone Cross-Resistance
Dose–response survival analysis of R. raphanistrum to the three herbicides mesotrione, pyrasulfotole, and topramezone applied as stand-alone foliar postemergence treatments to 3- to 4-leaf R. raphanistrum plants confirmed cross-resistance to each of the three HPPD inhibitors tested (Table 3; Figure 2). At herbicide dosages equivalent to the recommended label rates of each stand-alone herbicide, we observed greater than 50% survival to mesotrione, close to 100% survival to pyrasulfotole, and greater than 60% survival to topramezone for both populations 86-2020 and 91-2020 (Figure 2). At those doses, the survival of the susceptible control (WARR36) ranged from 0% to 4% (Figure 2). The calculated RIs from LD50 values for 86-2020 were all around 10-fold for the three HPPD-inhibiting herbicides tested (Table 3). However, 91-2020 exhibited a higher and significant (P < 0.03) level of resistance to pyrasulfotole (14-fold) and around 4- to 5-fold resistance to the other two herbicides (Table 3).
Table 3. Estimated LD50 values (±SE) expressed as grams pyrasulfotole, mesotrione, or topramezone per hectare and resistance index (RI) expressed as the ratio of LD50 values for the Raphanus raphanistrum resistant (86-2020 and 91-2020) and susceptible (WARR36) populations. a

a Probability values (P) of the difference between LD50 values were calculated as a t-test comparison between the putative resistant and the susceptible control using the EDComp function in the drc package of the software program R v. 3.6.1.

Figure 2. Dose–response curves for the two resistant Raphanus raphanistrum populations 86-2020 (solid line, solid circle) and 91-2020 (solid line, solid square) and the standard herbicide-susceptible population WARR36 (solid line, solid triangle) treated postemergence at the 3- to 4-leaf stage with varying doses of (A) pyrasulfotole, (B) mesotrione, and (C) topramezone in summer 2021. Symbols represent mean percentage survival ±SE (n = 6).
It appears that HPPD herbicide responses and resistance profiles in Amaranthus from the United States and Raphanus from Australia are comparable. In both genera, mechanistic studies have revealed that mesotrione, tembotrione, and topramezone resistance are predominantly non–target site based and mediated by enhanced herbicide metabolism (Kaundun et al. Reference Kaundun, Hutchings, Dale, Howell, Morris, Kramer, Shivrain and McIndoe2017; Küpper et al. Reference Küpper, Peter, Zöllner, Lorentz, Tranel, Beffa and Gaines2018; Lu et al. Reference Lu, Yu, Han, Owen and Powles2020a; Lygin et al. Reference Lygin, Kaundun, Morris, McIndoe, Hamilton and Riechers2018). Research is warranted to establish the mechanism(s) of resistance to pyrasulfotole, which has been the primary driver for HPPD-inhibitor resistance selection and evolution in the two R. raphanistrum populations investigated here.
Efficacy Trial to Validate HPPD-Inhibitor Resistance under Field Conditions
In the field from which population 91-2020 had been collected after the previous growing season, the lowest level of R. raphanistrum control was observed for postemergence application of a single treatment of pyrasulfotole + bromoxynil (79% control) and also with two repeated treatments with topramezone + bromoxynil (87%; Table 4; Supplementary Figure S3). Conversely, the R. raphanistrum infestation was fully controlled by another HPPD-inhibitor, mesotrione, applied preemergence stand-alone or in a mixture with other herbicide sites of action. The level of early control delivered by preemergence treatments with mesotrione was >97.3% (data not shown) and consistently remained high (>99%) throughout the duration of the study (Table 4). The trial also showed that effective R. raphanistrum control was achieved by a combination of multiple herbicide sites of action applied postemergence, including diflufenican, MCPA, bromoxynil, aclonifen, and pyroxasulfone. It was particularly evident that a single treatment with a postemergence herbicide mixture of four sites of action (pyroxasulfone, diflufenican, aclonifen, and bromoxynil) resulted in 100% weed control efficacy (Table 4).
Table 4. Herbicide efficacy trial conducted in 2021 in a field in Western Australia with a putative HPPD-inhibitor resistant Raphanus raphanistrum population. a, b

a Abbreviations: HPPD, 4-hydroxyphenylpyruvate dioxygenase; PRE, preemergence; POST, postemergence; T, treatment.
b Herbicide treatments listed across rows denote sequential herbicide applications. Final herbicide efficacy assessments were conducted 66 d after last herbicide application. Herbicides were applied at full recommended rates. Different letters for means denote significant difference according to the Tukey test at P < 0.05.
Although the two populations exhibited resistance to mesotrione applied postemergence in the dose–response experiment, this herbicide was effective when applied preemergence. In one population (91-2020), mesotrione cross-resistance was greatly reduced when the herbicide was applied preemergence in pots (Table 1) and under field conditions (Table 4), compared with when it was applied postemergence (Table 3). The use pattern of mesotrione in Australia is only as a preemergence stand-alone herbicide. The same phenomenon of reduced plant survival in response to preemergence as opposed to postemergence mesotrione treatments was observed in HPPD inhibitor–resistant A. tuberculatus: mesotrione resistance was 2-fold in response to preemergence application compared with 44-fold for postemergence treatments (Kaundun et al. Reference Kaundun, Hutchings, Dale, Howell, Morris, Kramer, Shivrain and McIndoe2017). This discrepancy could be partially explained by the complex inheritance of metabolic resistance as documented in Amaranthus spp. (Küpper et al. Reference Küpper, Peter, Zöllner, Lorentz, Tranel, Beffa and Gaines2018; Murphy et al. Reference Murphy, Beffa and Tranel2021); population variability in the detoxification of different HPPD- and PDS-inhibiting herbicides in R. raphanistrum (Lu et al. Reference Lu, Yu, Han, Owen and Powles2020a, 2020b); or variability in herbicide responses likely mediated by a different metabolic capacity of distinct tissues, as shown in A. tuberculatus treated with mesotrione applied pre- versus postemergence (Kaundun et al. Reference Kaundun, Hutchings, Dale, Howell, Morris, Kramer, Shivrain and McIndoe2017).
In conclusion, the first two cases of field resistance to HPPD-inhibiting herbicides (Group 27) in R. raphanistrum are confirmed in a background of previously reported multiple resistance across three sites of action, including Groups 2, 4, and 12 herbicides. Resistance to HPPD inhibitors in R. raphanistrum was verified under field conditions. Moreover, alternative herbicide options that could be utilized for control of multiple-resistant R. raphanistrum were identified. It is remarkable that R. raphanistrum was able to evolve resistance to a synergistic herbicide mixture of two sites of action, pyrasulfotole and the PSII inhibitor bromoxynil. This ability reinforces the need for a proactive approach to detect herbicide resistance early through regular monitoring and resistance testing and to incorporate diverse agronomic tactics to reduce herbicide selection pressure for resistance evolution. Combining multiple herbicide sites of action to delay resistance evolution can only be sustainable if integrated with a robust set of nonchemical strategies (e.g., weed seed destruction at harvest), crop diversity, and manipulation of crop–weed competition.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/wsc.2022.42
Acknowledgments
The Australian Herbicide Resistance Initiative (AHRI) is primarily funded by the Grains Research and Development Corporation (GRDC). Participating farmers and agronomists are kindly acknowledged. No conflicts of interest have been declared.









