Introduction
In the United States, almonds [Prunus dulcis (Mill.) D.A. Webb] are a $6 billion commodity grown solely in California, making almonds the second highest grossing commodity in the state, behind only dairy products (CDFA 2020b). As of 2020, there were more than 500,000 bearing hectares of almond trees planted in California that produced 1.3 billion kg of almonds (USDA-NASS 2020).
Almonds are harvested by mechanically shaking the trees, sweeping the almonds into windrows, and picking the nuts up from the orchard floor. Preharvest herbicide programs and mowing are used to control vegetation that would otherwise reduce harvest efficiency (Connell et al. Reference Connell, Colbert, Kreuger, Cudney, Gast, Bettner and Dallman2001; UCANR 2002). Glyphosate has been registered in almonds since the early 1990s, and glufosinate has been registered since the early 2000s (CDPR 2021); these are commonly used herbicides for preharvest orchard preparations because of their broad-spectrum weed control and relatively short preharvest interval (PHI), 3 and 14 d, respectively. In 2018, more than 1 million kg of glyphosate and nearly 300,000 kg of glufosinate-ammonium were applied in almond orchards (CDPR 2018). Because of the harvesting process, there is ample opportunity for the almond hulls, shells, and kernels to be in close contact with herbicide-treated soil.
The majority of California’s almond crop, about two-thirds, is exported and generated more than $4.9 billion in 2019 (CDFA 2020a). Of the exports, 22% were shipped inshell and 78% were shipped shelled (ABC 2019). Asia is the largest aggregate market for inshell almonds, while the majority of shelled almond shipments go to European markets (ABC 2019; CDFA 2020a). Exported shipments of almonds are subject to pesticide residue testing and must be at or below a maximum concentration set by the region’s food safety authority.
The maximum residue limit (MRL) for glyphosate and glufosinate in almonds differs by definition as well as concentration between the European Union (EU) and the United States. In the United States, both glyphosate and glufosinate MRLs, which are commonly called tolerances, are defined to include the parent compound as well as its primary metabolites (Bryant Christie Inc. 2021). For clarity, these MRLs will be referred to as “total glyphosate” or “total glufosinate” if the concentrations of the metabolites are to be summed with the concentration of the parent compound. Total glyphosate is the summation of glyphosate, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), N-acetyl-glyphosate, and N-acetyl-AMPA. Total glufosinate is the summation of glufosinate, N-acetyl-glufosinate, and 3-(methylphosphinico)propionic acid (MPP).
The U.S. MRL for glyphosate is 25 mg kg−1 for almond hulls and 1 mg kg−1 for kernels. There is not a separate U.S. MRL for inshell almonds, because the residue in inshell almonds is determined by shelling the almonds and measuring the residue in only the kernels. The U.S. MRL for total glufosinate in almond hulls and kernels is 0.5 mg kg−1.
In the EU, the MRL for glyphosate is 0.1 mg kg−1 in almond kernels (European Commission 2013). The EU MRL for glufosinate includes its metabolites; the MRL for total glufosinate is 0.1 mg kg−1 (European Commission 2016).
Glyphosate is registered in the EU until December 15, 2022 (European Commission 2017). A recent review completed by the European Food Safety Authority (EFSA 2019) recommended that the MRL for glyphosate be reduced to 0.05 mg kg−1 and that an optional total glyphosate MRL for the summation of glyphosate and its primary metabolites, AMPA and N-acetyl-glyphosate, be set to 0.2 mg kg−1. Hence, it is anticipated that glyphosate MRLs will be reduced in upcoming years, and it is a possibility that the chemical may not be reregistered. According to statute, if at any time the safety of a current MRL is reconsidered, the MRL can be reduced to the lowest limit of analytical detection, which is 0.01 mg kg−1 (European Parliament 2005).
Because of the importance of the European markets to the California almond industry and the importance of glyphosate and glufosinate to preharvest preparations, lab and field studies were conducted to evaluate the herbicide transfer from soil to almonds during harvest. The objectives were to determine whether glyphosate and glufosinate residues can transfer to almonds from soil particles or directly sprayed almonds, to determine whether increasing the PHI could substantially reduce the risk of herbicide in or on almond fractions, and to quantify the concentration of soil-bound herbicide in almond samples.
Materials and Methods
Lab Experiments
Soil Transfer to Whole Almonds
A laboratory experiment was conducted to determine the transfer of glyphosate or glufosinate to different parts of the almond via intimate contact with treated soil particles. The study was carried out using Yolo silt loam (fine-silty, mixed, superactive, nonacid, thermic Mollic Xerolfuvents; California Soil Resource Lab 2019) soil from the Department of Plant Sciences Field Research Facility, University of California, Davis, in Davis, CA (38.54°N, 121.79°W). The loam soil had a bulk density of 1.08 g cm−3, pH of 7.90, and 1.62% organic matter.
A solution of 1.665 MBq [14C]glyphosate (50 mCi mmol−1 glyphosate [phosphono-methyl-14C], American Radiolabeled Chemicals, 101 Arc Drive, St Louis, MO, USA) or 1.665 MBq [14C]glufosinate (6.35 MBq mg−1 [3,4-14C]glufosinate hydrochloride, Bayer Crop Sciences, Alfred-Nobel-Strasse 50, Monheim am Rhein, North Rhein-Westphalia, Germany) in 10-ml HPLC Plus methanol (Sigma Aldrich, 2909 Laclede Avenue, St Louis, Mo, USA) was applied to 16.2 g of soil. The soil was air-dried until all methanol had evaporated. The mass of soil used for the experiment was calculated based on the assumption that nine almonds occupy an area of 150 cm2 and 1-mm depth of soil would be disturbed by the almond sweeper.
The amount of [14C]herbicide that was used was based the limit of quantification of the liquid scintillation counter, which was 16.67 Bq, with the ideal minimum detection being approximately 0.001% herbicide transfer from soil to almond fraction. The total dose added to the soil was 166,500 Bq for both herbicides. The actual amount of glyphosate and glufosinate added to the soil in these experiments is roughly 6% and 10% of the field rate, respectively. Therefore, the intended use of the data generated is monitoring transfer processes and comparison of residue levels in hulls versus shells versus kernels.
Glyphosate and glufosinate were evaluated in separate experiments. In each experiment, four replicates of nine whole (kernel, shell, hull) almonds were exposed to the herbicide-treated soil. The treatments were carried out in 250-ml Nalgene bottles (Thermo Fisher Scientific, 168 3rd Avenue, Waltham, MA, USA) containing nuts and soil treated with [14C]herbicide; the bottles were rotated using a rock tumbler (Dual Drum Rotary Rock Tumbler, 26541 Agoura Road, Chicago Electric Power Tools, Calabasas, CA, USA) (Supplementary Figure S1). The inside of each bottle had four plastic inserts (9 cm by 1 cm by 1 cm) attached to the wall to help pick up the soil and almonds and create dust during the mixing process. The almonds were tumbled for 15 min and let rest for 15 min; excess soil was dusted off the almonds before analysis.
Soil Transfer to Almond Kernels
Another experiment to analyze the surface-associated herbicide involved tumbling four almond kernels directly in the 14C-treated soil. Shelled kernels were tumbled for 15 min in the 14C-treated soil, dusted off, rinsed with water using gentle inverted shaking, and both kernels and rinsate were analyzed for [14C]herbicide.
Almond-to-Almond Transfer with No Soil Contact
This experiment was conducted to determine glyphosate transfer from directly treated almonds to nontreated almonds. It was intended to mimic a situation in which a small number of almonds fall to the ground very early (“windfall” nuts) and could conceivably be directly sprayed with preharvest treatments and then contaminate the later-harvested crop during harvest and handling steps. Two almonds were directly treated with 0.8325 MBq [14C]glyphosate by using a microsyringe to dot the stock solution over the entire almond, including the inside of the split hull and exposed shell. The two treated almonds were tumbled with nine nontreated almonds using the apparatus and methods described earlier. The treated almonds were clearly marked so they could be removed after the tumbling process. The almonds were tumbled using a rock tumbler for 15 min and let rest for 15 min. The treated almonds were removed and discarded before analysis, and the untreated almonds were dissected and analyzed for [14C]glyphosate. This experiment was replicated four times.
[14C]Herbicide Analysis
The whole almonds from each replicate from both soil-transfer experiments and the almond-to-almond transfer experiment were separated for three different analyses: whole-almond rinse, herbicide adsorption to almond fractions, and a surface swipe after a postharvest mimicking process. All samples were analyzed using a liquid scintillation counter (LS6500, Beckman Coulter, 250 South Kraemer Boulevard, Brea, CA, USA). The data were corrected for the background levels of radiation in the scintillation counter.
The rinsate of whole almonds was used to determine how much [14C]herbicide was loosely associated with the surface of the almonds. Three whole almonds were rinsed with water using gentle inverted shaking. The rinsate was collected into glass scintillation vials and evaporated using a vacuum evaporation system at 30 C (RapidVap, Labconco, 8811 Prospect Avenue, Kansas City, MO, USA). Once the samples were evaporated to near dryness, 10 ml of Ultima Gold™ (PerkinElmer, 940 Winter Street, Waltham, MA, USA) was added to each vial. The samples were analyzed using the liquid scintillation counter.
To determine how much herbicide was adsorbed to the almond fractions, three almonds were dissected into their hull, shell, and kernel components. Each component was homogenized using a mortar and pestle and liquid nitrogen. Approximately 500 mg of each homogenized almond fraction was collected into a combustion cone (CombustoPad, Perkin Elmer) and combusted using a sample oxidizer (Model 307, PerkinElmer). The combustion product, [14CO2], was collected in 20 ml of scintillation cocktail composed of 10 ml of Carbo-Sorb E® (PerkinElmer) and 10 ml of Permafluor® (PerkinElmer). Glass scintillation vials containing the 14C samples were analyzed using the liquid scintillation counter.
The remaining three almonds were used in a postharvest mimicking process. The almond hulls were discarded, and the shells were opened by hand cracking through a plastic barrier, then discarded. The plastic was swiped using a filter paper, and the swipe was added to a glass scintillation vial with 10 ml of Ultima Gold™. The swipes were analyzed using the scintillation counter. The kernels were collected, homogenized, and combusted, and the combustion product was mixed with scintillant and analyzed using the scintillation counter as described earlier.
The four almond kernels (no hull or shell) that were tumbled directly in the [14C]herbicide-treated soil were rinsed with 20 ml of water. The rinsate was collected into glass scintillation vials and evaporated to near dryness using vacuum evaporation. Then, 10 ml of Ultima Gold™ was added to the scintillation vial and analyzed using the liquid scintillation counter. The rinsed kernels were homogenized and combusted; the combustion product was mixed with scintillant and analyzed using the liquid scintillation counter.
Field Experiment
To examine the glyphosate and glufosinate residues in almonds at different PHIs, a field study was conducted in a mature almond orchard at the Nickels Soil Laboratory (38.96°N, 122.07°W) located near Arbuckle, CA, USA. The orchard included full rows of nonpareil almonds alternating with rows of several pollenizer varieties; trees were planted 4.9 m apart within the rows, and rows were 6.7 m apart.
The experiment was conducted in the nonpareil rows, and treatments were organized into a randomized complete block design with four replicates. Herbicide treatments included a single herbicide mix applied at timings that correspond to PHIs of 35, 21, 14, 7, and 3 d before shaking. Each plot was 19.6-m long by 4-m wide and contained four almond trees; the width of each herbicide plot started from one side of the tree trunk and extended 4 m, nearly to the next tree row (Supplementary Figure S2). The herbicide treatment for all plots was a tank mix of commercial glyphosate (Anonymous 2020; Roundup WeatherMax®, Bayer Crop Science, 8400 Hawthorne Road, Kansas City, MO, USA) at 1,681 g ae ha−1, commercial glufosinate (Anonymous 2018; Rely® 280, BASF Corporation, 100 Park Avenue, Florham Park, NJ, USA) at 1,681 g ai ha−1, nonionic surfactant at 0.25% v/v (RAINIER-EA®, Wilbur-Ellis, 16300 Christensen Road no. 135, Tukwila, WA, USA), and AMS at 1% v/v (Bronc® Max, Wilbur-Ellis). Applications were made using a CO2-pressurized backpack sprayer with a 2-m boom equipped with four air-induction extended-range nozzles (AIXR 11002, TeeJet® Technologies, 1801 Business Park Drive, Springfield, IL, USA) calibrated to deliver 187 L ha−1 at a pressure of 207 kPa. At each application date, previously fallen almonds were counted in two 1-m2 areas in each plot.
On the day of harvest, the middle two almond trees of each plot were hand shaken using mallets and poles, then the nuts were left on the orchard floor to dry. Approximately 100 g of surface soil was collected from each plot at this time for herbicide analysis before sweeping. Three days after shaking, the nuts were swept into a windrow between tree rows in approximately the center of the herbicide-treated plots using a commercial self-propelled mechanical sweeper. Four days later, approximately 500 g of nuts were collected from each plot windrow, separated by hand from the soil and other debris, and stored frozen until further analysis. This timeline corresponds to typical commercial harvest practices. At almond sampling, approximately 100 g of surface soil from each plot was also collected for herbicide analysis post sweeping.
Almond samples from each plot were dissected into hull, shell, and kernel fractions and sent to a commercial laboratory (Safe Food Alliance, 2037 Morgan Drive, Kingsburg, CA, USA) for analysis. The laboratory used modified methods from QuPPe v. 10 (EURL-SRM 2019) and liquid chromatography–tandem mass spectroscopy (QTRAP® 5500 LC-MS/MS System, 1201 Radio Road, Sciex, Redwood City, CA, USA) equipped with a MicroSolv Congent Diol™ column (4.6 mm by 250 mm by 4 µm, MicroSolv Technology, 9158 Industrial Boulevard, Leland, NC, USA) to quantify glyphosate, N-acetyl-glyphosate, AMPA, N-acetyl-AMPA, glufosinate, N-acetyl-glufosinate, and MPP.
The same compounds were quantified from an unreplicated composite soil sample from each PHI treatment by the same commercial laboratory. The laboratory used modified methods from Druart et al. (Reference Druart, Delhomme, de Vaufleury, Ntcho and Millet2011) and the same LC-MS/MS instrumentation.
Statistical Analysis
The laboratory and field data were subject to ANOVA using R statistical analysis software (R Core Team 2020), and multiple comparisons were performed with Tukey’s HSD with α = 0.05.
Results and Discussion
Lab Experiment
Soil Transfer to Whole Almonds
The rinsate analysis of the washed whole almonds showed a removal of herbicide from the surface of the whole almond averaging 6,667 ± 1,782 Bq of [14C]glyphosate and 6,130 ± 2,319 Bq of [14C]glufosinate (Supplementary Table S1). The swipe of the plastic barrier used to crack the almond shells had a residue of 154 ± 36 Bq of [14C]glyphosate and 109 ± 23 Bq of [14C]glufosinate (Supplementary Table S2).
The kernels of the almonds used for the postharvest mimic process contained 0.138 ± 0.035 Bq mg−1 of [14C]glyphosate and 0.093 ± 0.016 Bq mg−1 of [14C]glufosinate (Supplementary Table S3). The amount of herbicide in the kernel samples from the postharvest mimic process was not significantly different from the amount of herbicide in the kernel samples from the dissection process.
A summary of the results of the whole-almond dissection is presented in Figure 1. Unsurprisingly, the hull fraction contained the most herbicide; [14C]glufosinate averaged 2.350 ± 0.369 Bq mg−1 and [14C]glyphosate averaged 2.308 ± 0.871 Bq mg−1. Shell samples averaged 1.299 ± 0.230 Bq mg−1 [14C]glyphosate and 1.226 ± 0.145 Bq mg−1 [14C]glufosinate. The average [14C]herbicide in the kernels was 0.138 ± 0.035 Bq mg−1 [14C]glyphosate and 0.113 ± 0.040 Bq mg−1 [14C]glufosinate. Of the total [14C]glyphosate found in the almond fractions, roughly 62% was in the hull, 35% was in the shell, and 3% was in the kernel; of the [14C]glufosinate found in the almond fractions, 64% was in the hull, 33% was in the shell, and 3% was in the kernel. The data did not show statistically significant differences between the two herbicides. There were significant differences between residues in the hull, shell, and kernel fractions in the samples treated with [14C]glufosinate. The hull and shell fractions of the [14C]glyphosate samples had significantly more residue than the kernel fraction.
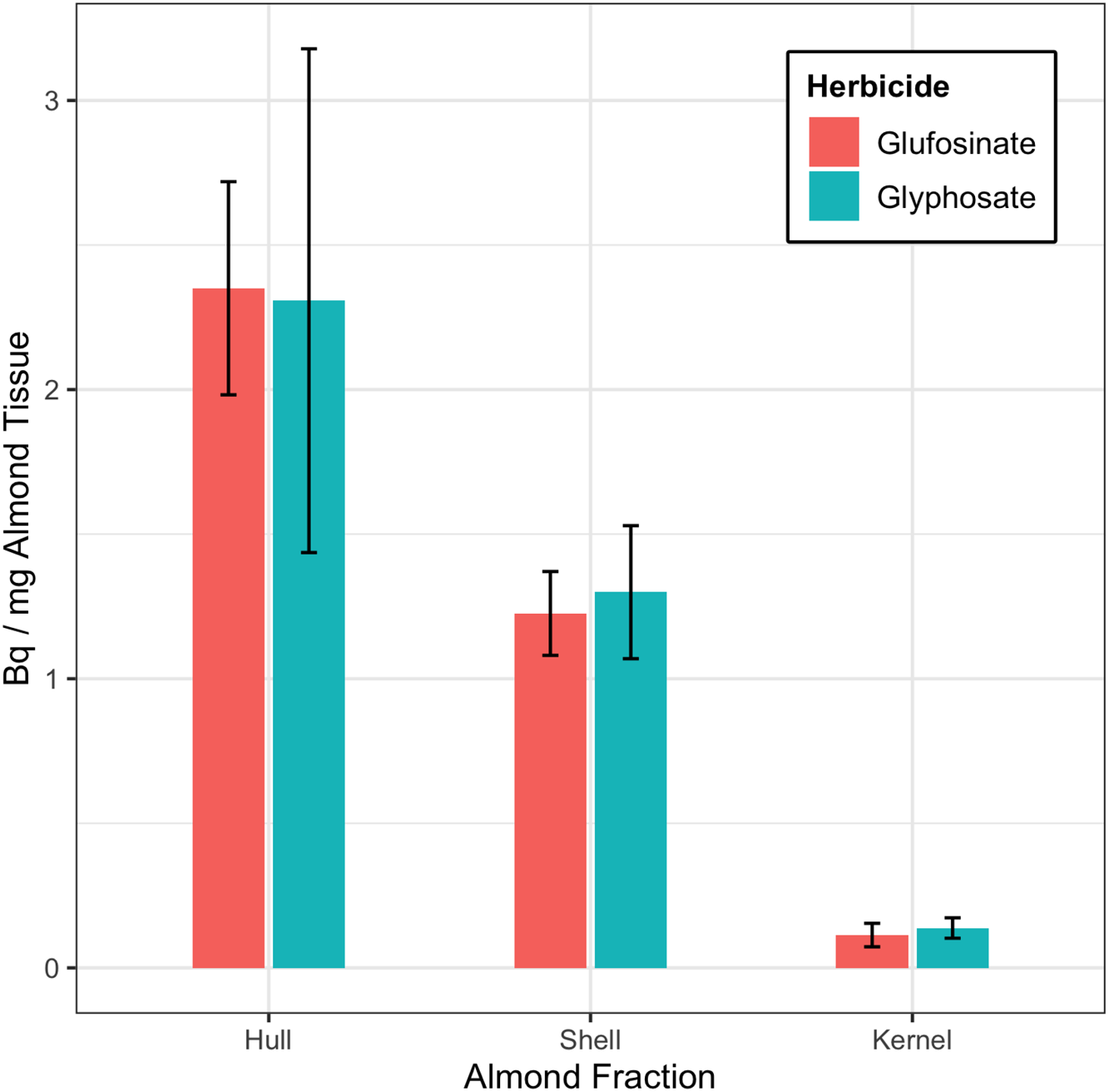
Figure 1. [14C]glyphosate and [14C]glufosinate Bq mg−1 of almond hull, shell, and kernel detected in samples from the soil-transfer experiment. Total dose applied to the soil was 166,500 Bq. Error bars represent the 95% confidence intervals.
Soil Transfer to Almond Kernel
The amount of [14C]glyphosate that remained on the rinsed kernels was 0.040 ± 0.002 Bq mg−1 and the amount of [14C]glufosinate that remained on the rinsed kernels was 0.062 ± 0.004 Bq mg−1 (Figure 2). After this brief water rinse there was significantly less herbicide on the kernels. [14C]glyphosate was reduced by 71% and [14C]glufosinate was reduced by 46% in almond kernel samples. There were no statistical differences between herbicides for 14C in the unrinsed kernels; however, there was less [14C]glyphosate on rinsed kernels than [14C]glufosinate. This is unsurprising, as the log K ow of glyphosate is lower than that of glufosinate, meaning the glufosinate is more attracted to the nonpolar almond surface than glyphosate. From these results, we can conclude that a large proportion of glyphosate and glufosinate residue in almond samples likely is associated with soil particles on the surface of the kernels.

Figure 2. [14C]glyphosate and [14C]glufosinate Bq mg−1 of unrinsed and rinsed almond kernels from the kernel rinsate experiment. Total dose added to the soil was 166,500 Bq. Error bars represent the 95% confidence intervals.
Almond-to-Almond Transfer
The rinsate analysis of the whole washed almonds showed a removal of glyphosate from the surface of the whole nut averaging 1,534 ± 265 Bq (Supplementary Table S4). The swipe of the plastic barrier used to crack the shells was below the detection limit. The kernels of the almonds used for the swipe test were also below the detection limit of [14C]glyphosate.
Contact between directly treated whole almonds and untreated nuts resulted in the untreated hulls having very low levels of herbicide residue. The average untreated hull [14C]glyphosate residue was 0.136 ± 0.033 Bq mg−1, while [14C]glyphosate was below the limit of quantification in the shells and kernels from the untreated almonds. Therefore, transfer from early fallen nuts directly sprayed during preharvest preparations is unlikely to be a major contributor to herbicide residue in whole sample lots of almonds.
Field Trial
The range of fallen nuts in two 1-m2 quadrats within each plot are shown in Table 1. There was no apparent correlation between the number of early fallen nuts and glyphosate or glufosinate residue levels in the subsequently harvested samples (data not shown).
Table 1. The range of nuts on the orchard floor counted in four replicates of two 1-m−2 quadrats (n = 8) at each preharvest interval (PHI). a

a Almonds were hand shaken on August 10, swept on August 13, and collected from the windrow on August 17.
A summary of the glyphosate residues is presented in Table 2. Total glyphosate concentration is presented as the sum of glyphosate, AMPA, N-acetyl-glyphosate, and N-acetyl-AMPA. There were no statistically significant differences in concentration of glyphosate or total glyphosate found in the hull and shell samples. N-acetyl-AMPA was found only in almond hull samples. There was no detection of glyphosate or its metabolites in any of the almond kernel samples. The almond hulls had the highest detection of glyphosate and its metabolites, averaging 0.174 mg kg−1, while still being well below the U.S. MRL. The almond shell samples were above the EU almond kernel residue limit of 0.1 mg kg−1; however, in practice, inshell almonds are shelled before residue analysis. PHI within the tested range did not have a statistically significant effect on glyphosate residues in hull and shell samples.
Table 2. Summary of the concentration of glyphosate and metabolites found in almond hulls, shells, and kernels at each preharvest interval (PHI). a

a Values are represented as mean concentration ± SE. There were no significant differences in glyphosate or total glyphosate concentrations in the hull or shell fractions. The PHI did not significantly influence the residue levels in hulls, shells, or kernels. Glyphosate is the concentration of the parent compound and total glyphosate is the sum of the concentrations of glyphosate, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), N-acetyl-glyphosate, and N-acetyl-AMPA. LOD, limit of detection.
b PHI indicates the preharvest interval before hand shaking on August 10. Almond samples were collected from the windrows of each plot on August 17.
c Three replicates were below the limit of detection.
d Two replicates were below the limit of detection.
A summary of the glufosinate residue data is presented in Table 3. Total glufosinate concentration is presented as the sum of glufosinate, N-acetyl-glufosinate, and MPP. There were no significant differences in residues found in hulls, shells, or kernels, and these samples were all below the U.S. MRL for total glufosinate. The EU total glufosinate MRL was exceeded in almond shells in at least some replicate plots at PHIs of 3, 14, 21, and 35 d. MPP was the only compound detected in almond kernels at PHIs of 3, 14, 21, and 35 d. Although the 3- and 7-d PHIs were off-label applications of glufosinate, there were no significant differences in glufosinate residues among the PHI treatments.
Table 3. Summary of the concentration of glufosinate and metabolites found in almond hulls, shells, and kernels at each preharvest interval (PHI). a

a Values are represented as mean concentration ± SE. There were no significant differences in residue levels in the almond fractions. The PHI did not significantly influence residue levels in hulls, shells, or kernels. PHI of 3 and 7 d is an off-label application of the herbicide. Glufosinate is the concentration of the parent compound and total glufosinate is the sum of the concentrations of glufosinate, N-acetyl-glufosinate, and 3-(methylphosphinico)propionic acid (MPP). LOD, limit of detection.
b PHI indicates the preharvest interval before hand shaking on August 10. Almond samples were collected from the windrows of each plot on August 17.
c One replicate was below the limit of detection.
d Two replicates were below the limit of detection.
e Three replicates were below the limit of detection.
Glyphosate and glufosinate are generally considered to have moderate and short soil half-lives, respectively (Shaner Reference Shaner2014), and the almond orchard soil samples collected from the orchard floor support that degradation pattern. Total glyphosate concentrations remained consistent, apart from an anomalous 7-d pre-sweep value, across all PHIs and pre- and post-sweep samples; the range of total glyphosate in samples taken before sweeping was 2.331 to 2.575 mg kg−1, and the range in samples taken after sweeping was 1.536 to 3.554 mg kg−1 (Table 4). The half-life of glyphosate in soil ranges between 7 and 60 d depending on soil properties (Giesy Reference Giesy2000), and given that samples were taken from soil surface that was dry due to preharvest management practices, it is expected the half-life would be closer to the high end of the given range. Total glufosinate concentration in the soil followed a decreasing trend from the PHI of 3 to 35 d, with the majority of the total glufosinate concentration being attributed to MPP (Table 4). Total glufosinate decreased from 5.339 to 0.210 mg kg−1 in the pre-sweep samples and from 7.687 mg kg−1 to less than the detection limit in the post-sweep samples (Table 4). Glufosinate is rapidly degraded by soil bacteria and has a half-life between 3 and 7 d; the main degradation product is MPP (Gallina and Stephenson Reference Gallina and Stephenson1992). The 7-d pre-sweep sample appears anomalous and likely from a sample processing error in the unreplicated sample, as there were no correspondingly high values in the almond samples from those plots (Graham et al. Reference Graham, Clay, Jackson, Jones, Arthur, Barefoot and Clay2002).
Table 4. Concentrations of total glyphosate, total glufosinate, and 3-(methylphosphinico)propionic acid (MPP) found in soil from the Nickels Soil Laboratory field site pre and post orchard sweeping at each preharvest interval (PHI). a

a Total glyphosate represents the sum of glyphosate, α-amino-3-hydroxy5-methyl-4-isoxazolepropionic acid (AMPA), N-acetyl-glyphosate, and N-acetyl AMPA.
Total glufosinate represents the sum of glufosinate, N-acetyl-glufosinate, and MPP. Pre sweep is the soil sample taken on August 13, before the sweeper went through the orchard, and post sweep is the soil sample taken on August 17, after the sweeper went through the orchard and almonds were in windrows.
b The 7-d preharvest interval sample appears to be a data anomaly assumed to be from a sample collection or processing error, as there were no corresponding high values in the almond samples; however, this cannot be confirmed, because the replicated field plot samples were homogenized and analyzed as a single unreplicated lab sample. Field dissipation studies have shown that zero-time soil measurements of various pesticides have resulted in artificially low residue levels (Graham et al. Reference Graham, Clay, Jackson, Jones, Arthur, Barefoot and Clay2002).
The current labels state the minimum PHI for glyphosate and glufosinate is 3 and 14 d, respectively. The field results showed that increasing the PHI up to 35 d before shaking did not appear to substantially reduce the amount of glyphosate or glufosinate in the samples. Total glyphosate residues in kernels from almonds sampled in the windrow were below the limit of detection at every PHI tested (Table 2). At the minimum 14-d PHI, total glufosinate residues in kernels from almonds sampled in the windrow were 0.037 mg kg−1, while the 35-d PHI residues were 0.089 mg kg−1; these data were not statistically different (Table 3). Based on these data, we conclude increasing the PHI of the herbicides within a range of utility for preharvest operations is unlikely to significantly contribute to lower residue levels.
Before these experiments were conducted, one almond industry concern was that windfall nuts directly sprayed with herbicide might contaminate an entire batch. Windfall nuts typically account for 0% to 1% of the total harvest, and nuts that fall more than 4 wk before harvest are usually of poor quality (Brown et al. Reference Brown, Camargo and Cirhigiri2019) because of immaturity or degradation processes. The number of potential directly treated almonds was relatively low (0 to 46 nuts m−2) in this study, and the earliest-falling and mostly likely to be directly treated would likely be removed from the batch during processing based on the U.S. Department of Agriculture grading standards for size, damage, and color (USDA 1997). The almond-to-almond transfer experiment in the lab suggested low transfer of glyphosate or glufosinate from treated to untreated nuts; therefore, the small portion of directly sprayed windfall nuts that make it through the processing facility are unlikely to have high enough residues to elevate the batch residues above the MRL.
Almond hulls, shells, and kernels were below the U.S. MRLs for both glyphosate and glufosinate as well as their metabolites. If the EU reduces the MRL further based on new hazard and risk assessments, this will pose a challenge to California growers when choosing preharvest herbicides. It is worth noting the almonds in both the field and lab experiments presented here were not commercially processed and thus were not subjected to mechanical and pneumatic cleaning and sorting operations to remove soil and debris; these steps likely would have more effectively removed the soil particles and soil-associated herbicides compared to these research samples. It is also recognized that the limits of detection of the analytical instrumentation methods used are higher than the recommended new MRLs for glyphosate and its metabolites. Future research will focus on pesticide residues at later points in almond processing and will include sampling almonds and soil particles at various points within a commercial hulling and shelling facility.
Acknowledgments
This work was supported by the Almond Board of California, grant no. HORT54.Hanson. The authors would like to thank the Nickels Soil Laboratory for orchard management, Seth Watkins for chemical application, and Steven Haring and Matthew Fatino for harvest support. No conflicts of interest have been declared.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/wsc.2022.20









