Introduction
Lowbush blueberry (Vaccinium angustifolium Aiton) is a rhizomatous perennial berry species (Hall et al. Reference Hall, Aalders, Nickerson and Vander Kloet1979) and is an economically important fruit crop in Canada that contributed Can$47.4 million to farm gate value in 2017 (Anonymous 2019). Lowbush blueberry fields are developed from natural stands (Anonymous 2019) that are managed under a 2-yr production cycle in which plants are pruned to ground level by flail mowing in the first year (nonbearing year) and emerged shoots flower and produce berries in the second year (bearing year) (Eaton et al. Reference Eaton, Glen and Wyllie2004; Wood Reference Wood2004). Fields are thus maintained as perennial no-till monocultures, making weed management difficult (McCully et al. Reference McCully, Sampson and Watson1991). The weed flora of lowbush blueberry fields is dominated by herbaceous and woody perennials (Jensen and Yarborough Reference Jensen and Yarborough2004; Lyu et al. Reference Lyu, McLean, McKenzie-Gopsill and White2021; McCully et al. Reference McCully, Sampson and Watson1991), with many species of perennial grasses being of particular concern (Anonymous 2019; Boyd et al. Reference Boyd, White and Rao2014; White and Zhang Reference White and Zhang2019) due to potential to reduce yields and inhibit harvest (Jensen and Specht Reference Jensen and Specht2004; Jensen and Yarborough Reference Jensen and Yarborough2004).
Hair fescue (Festuca filiformis Pourr.) is a caespitose, sod-forming perennial grass introduced from Europe and now well established in eastern and northwestern North America (USDA 2020). This grass occurs as a weed in lowbush blueberry fields, where it forms perennial sods that reduce yield by >50% (White Reference White2019; Zhang Reference Zhang2017; Zhang et al. Reference Zhang, White, Randall Olson, Pruski and Willenborg2018) and inhibit mechanical harvest. Occurrence of this weed in Nova Scotia lowbush blueberry fields decreased between 1984 to 1985 and 2000 to 2001 (McCully et al. Reference McCully, Sampson and Watson1991; KIN Jensen and MG Sampson, personal communication), likely due to control of this species by two photosystem II inhibitors, hexazinone and terbacil (Weed Science Society of America [WSSA] Group 5) (Jensen Reference Jensen1985a, Reference Jensen1985b; Sampson et al. Reference Sampson, McCully and Sampson1990; Smagula and Ismail Reference Smagula and Ismail1981). This grass, however, is now widespread in Nova Scotia lowbush blueberry fields (Lyu et al. Reference Lyu, McLean, McKenzie-Gopsill and White2021; White Reference White2018; White and Zhang Reference White and Zhang2020b; Zhang Reference Zhang2017; Zhang et al. Reference Zhang, White, Randall Olson, Pruski and Willenborg2018), and hexazinone no longer provides effective control (White Reference White2019; Yarborough and Cote Reference Yarborough and Cote2014; Zhang Reference Zhang2017). Terbacil efficacy is also variable (White and Zhang Reference White and Zhang2020a; Zhang Reference Zhang2017; Zhang et al. Reference Zhang, White, Randall Olson, Pruski and Willenborg2018) and generally limited to single-season suppression (White Reference White2019). Hexazinone resistance in F. filiformis populations in lowbush blueberry fields has been suspected since the early 2000s (Jensen and Yarborough Reference Jensen and Yarborough2004), though no work has been conducted to confirm the presence of resistant biotypes of this weed species in Nova Scotia.
Mechanisms of herbicide resistance in weeds can be classified into target-site resistance (TSR) and/or non–target site resistance (NTSR) (Jugulam and Shyam Reference Jugulam and Shyam2019; Powles and Yu Reference Powles and Yu2010). The TSR mechanisms involve mutation(s) in the target site of action of a herbicide, resulting in a target protein that is insensitive or less sensitive to the herbicide (Powles and Yu Reference Powles and Yu2010). The NTSR mechanisms include reduced herbicide uptake/translocation, increased herbicide metabolism, decreased rate of herbicide activation, and/or sequestration (Devine and Eberlein Reference Devine, Eberlein, Roe, Burton and Kuhr1997). Hexazinone has been used since the early 1980s as the primary preemergence herbicide in lowbush blueberry (Jensen Reference Jensen1985a; Yarborough Reference Yarborough2004; Yarborough and Jemison Reference Yarborough and Jemison1997). This herbicide inhibits photosynthesis by binding to D1 proteins encoded by the psbA gene, affecting CO2 fixation and the production of energy needed for plant growth. Selection for naturally occurring target-site mutations in the psbA gene that confer resistance to hexazinone are not widely reported in the literature, though prolonged use of hexazinone has selected for such target-site mutations in populations of shepherds’s-purse [Capsella bursa-pastoris (L.) Medik.] in alfalfa (Medicago sativa L.) fields in Oregon (Perez-Jones et al. Reference Perez-Jones, Intanon and Mallory-Smith2009) and red sorrel (Rumex acetosella L.) in lowbush blueberry fields in Nova Scotia (Li et al. Reference Li, Boyd, McLean and Rutherford2014). Similar mutations may therefore have also been selected for in F. filiformis populations following prolonged hexazinone use in lowbush blueberry fields.
Although most herbicide resistance cases reported concern annual weeds (Beckie Reference Beckie2006; Holt and LeBaron Reference Holt and LeBaron1990; Holt et al. Reference Holt, Powles and Holtum1993), the phenomenon is also common in perennial weeds (Hodgson Reference Hodgson1970; Patton et al. Reference Patton, Weisenberger and Schortgen2018; Vila-Aiub et al. Reference Vila-Aiub, Balbi, Distefano, Fernandez, Hopp, Yu and Powles2012; Yanniccari et al. Reference Yanniccari, Istilart, Giménez and Castro2012), including some in lowbush blueberry fields (Li et al. Reference Li, Boyd, McLean and Rutherford2014). Herbicides continue to be the primary method for weed control in lowbush blueberry fields (Jensen and Yarborough Reference Jensen and Yarborough2004), and limited herbicide availability for perennial grass management is resulting in repeated use of herbicides with similar sites of action (White Reference White2019; White and Zhang Reference White and Zhang2019). This is a major factor contributing to development of herbicide resistance (Norsworthy et al. Reference Norsworthy, Ward, Shaw, Llewellyn, Nichols, Webster, Bradley, Frisvold, Powles, Burgos, Witt and Barrett2012), and it is imperative that herbicide-resistant weed biotypes be identified in lowbush blueberry so that priority weed species can be emphasized in future research and growers can adjust management practices accordingly.
The objectives of this study were to utilize (1) dose–response experiments to confirm suspected hexazinone resistance in a F. filiformis population collected from a lowbush blueberry field in Nova Scotia and (2) advanced molecular tools to identify the mechanism of resistance.
Materials and Methods
Material Source
Festuca filiformis seeds were collected from a commercial lowbush blueberry field in North River, Nova Scotia (45.464933°N, 63.212557°W) (suspected resistant biotype) and from a roadside population located at Glenholme, Nova Scotia (45.441523°N, 63.529923°W) (negative control). Seeds were maintained in the laboratory at room temperature in Nova Scotia for approximately 1 mo before being shipped to the Agriculture and AgriFood Canada Saint-Jean-sur-Richelieu Research & Development Center, Saint-Jean-sur-Richelieu, QC, Canada, for dose–response experiments and molecular analysis.
Seed Germination
Seeds of each biotype were germinated on moistened Whatman filter paper (Grade 2, GE Healthcare Life Sciences, Baie D’Urfé, QC, Canada) in petri dishes before planting. Petri dishes were sealed with parafilm (Parafilm M, Bemis Company, Neenah, WI, USA) and placed in a growth chamber set at 20 C with a photoperiod of 16 h and 70% relative humidity. Seeds germinated after 20 d, and seedlings were transferred into 9 cm by 9 cm pots containing Pro-Mix potting soil (Premier Tech, Rivière-du-Loup, QC, Canada) and placed in the growth chamber under the same conditions as were used for germination. After 25 d of growth (8 to 10 leaves), the plants were treated with herbicides for the dose–response experiment.
Dose–Response Experiment
The Group 5 herbicides hexazinone (Velpar® DF, Tessenderlo Kerley, Phoenix, AZ, USA) and terbacil (Sinbar® WDG, Tessenderlo Kerley) were used for the dose–response experiment. Recommended field rates for hexazinone (Velpar®) and terbacil (Sinbar®) were 1.92 and 2 kg ha−1, respectively. The experiment was arranged as a randomized complete block design, and 18 individuals of each biotype and repetition were treated with hexazinone at eight different doses (0X, 0.03X, 0.08X, 0.25X, 0.5X, 1X, 2X, and 4X times the label rate) and terbacil at nine different doses (0X, 0.01X, 0.03X, 0.08X, 0.25X, 0.5X, 1X, 2X, and 4X times the label rate) using a DeVries Manufacturing (Hollandale, MN, USA) moving-nozzle cabinet sprayer equipped with an 8001E-VS even-banding nozzle (TeeJet®, Springfield, PA, USA) calibrated to deliver 164 L ha−1 of spray solution at 207 kPa. The dose–response experiment was repeated three times for both herbicides. Treated plants were transferred to a greenhouse set at 25/20 C (day/night) with a photoperiod of 16 h. Plant dry biomass was determined by collecting all aboveground plant material in each pot at 21 d after treatment (DAT) and drying it at 70 C for 4 d before weighing. Log-logistic dose–response analysis was performed as described by Seefeldt et al. (Reference Seefeldt, Jensen and Fuerst1995), wherein the response in dry biomass (Y) is related to the herbicide dose (x) and used to determine the value of the slope (b) and GR50 value that would best fit the distribution of the values according to Equation 1:
where C is the lower asymptote, D is the upper asymptote, b is the slope of the line at GR50, and GR50 is the herbicide dose generating a 50% reduction in dry biomass (Seefeldt et al. Reference Seefeldt, Jensen and Fuerst1995). The resistance factor was calculated by dividing the GR50 value of the resistant biotype by the GR50 value of the susceptible biotype. Calculations were performed with the R (R Core Team 2020) drc package (Ritz et al. Reference Ritz, Baty, Streibig and Gerhard2015).
DNA Extraction, psbA Gene Amplification, and Sequencing
Extraction of genomic DNA was performed using Qiagen DNeasy Plant Mini Kit (Qiagen, Mississauga, ON, Canada) according to the manufacturer’s instructions. The primer pair (FESTE-psbA FWD: 5′-CGTGAGCCTGTTTCTGGTTCTT-3′ and FESTE-psbA REV: 5′-CAGCTAGGTCTAGAGGGAAGTTGT-3′) was designed to amplify the gene region known to harbor resistance-causing mutations using the psbA gene sequence of red fescue (Festuca rubra L.) from GenBank (accession number: MN309826.1). Amplification of an 844-bp fragment was performed using Titanium® Taq DNA Polymerase (Takara Bio USA, CA, USA) under the following conditions: 95 C for 5 min; 35 cycles of denaturation for 30 s at 95 C, annealing for 30 s at 58 C, elongation at 68 C for 35 s, and a final elongation for 8 min at 68 C. The PCR products were visualized on agarose gel at 1% and sequenced at Genome Quebec Innovation Centre (Montreal, QC, Canada) using the dideoxynucleotide chain termination method (Sanger et al. Reference Sanger, Nicklen and Coulson1977). The partial sequences of psbA for both susceptible and resistant biotypes are available in GenBank (accession numbers: MZ394740 and MZ394739, respectively).
Protein Structure and Docking Analysis
Docking experiments used the wood fescue (Festuca altissima All.) psbA sequence (GenBank accession AFV62627.1 from the plastid sequence JX871939.1) with a phenylalanine to isoleucine change at position 255. Partial sequencing of the F. filiformis psbA gene revealed that amino acids residues 83 to 339 are identical (results not shown). Protein structure was determined by protein homology using the modeling server CPHmodels (v. 3.2; Nielsen et al. Reference Nielsen, Lundegaard, Lund and Petersen2010), and the structure of the active ingredients was obtained from the Toxin and Toxin-Target (T3) database (Wishart et al. Reference Wishart, Arndt, Pon, Sajed, Guo, Djoumbou, Knox, Wilson, Liang, Grant, Liu, Goldansaz and Rappaport2015). Determination of the binding area and visualization was done with AutoDock tools (Morris et al. Reference Morris, Huey, Lindstrom, Sanner, Belew, Goodsell and Olson2009). Blind docking calculation was done with AutoDock (Morris et al. Reference Morris, Huey, Lindstrom, Sanner, Belew, Goodsell and Olson2009). Results were visualized using PyMOL (PyMOL Molecular Graphics System, v. 2.0, Schrödinger, New York, NY, USA).
Development of a Genotyping Assay
A competitive allele-specific PCR (KASP) assay was used in this study to distinguish between susceptible and resistant biotypes. The partial sequence of the psbA gene (GenBank accession MT362604) was sent to LGC Genomics (Biosearch Technologies, Hoddesdon, Ware, UK) for the design and synthesis of the specific primers. The primer for the resistant allele (5′-AGCATATTGGAAGATTAATCGGCCAAT-3′) was synthetized with the addition of 3′ terminal 6-carboxyfluorescein (FAM) and the primer for the susceptible allele (5′-AGCATATTGGAAGATTAATCGGCCAAA-3′) was labeled with HEX fluorophore at the 3′ terminal. A common primer was used for both biotypes (5′-ACTTATAATATTGTGGCTGCTCATGGTTAT-3′). The genotyping reaction contained 0.14 μl of the assay mix, 5 μl of KASP Master mix, 1 μl of genomic DNA at 1 ng μl−1, and 4 μl of ddH2O. The KASP assay was performed on an AriaMx real-time PCR (Agilent, Santa Clara, CA, USA) with the following conditions: initial denaturation at 94 C for 15 min and 10 touchdown cycles of 20 s at 94 C and 1 min at 61 to 55 C (decreasing 0.6 C per cycle), followed by a second PCR amplification of 26 cycles at 94 C for 20 s and 55 C for 1 min and a final elongation at 30 C for 1 min 30 s.
Results and Discussion
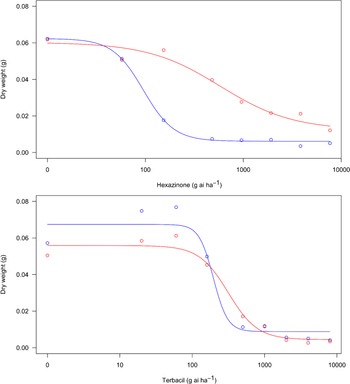
A dose–response experiment was conducted with the s-triazine herbicide hexazinone and the uracil herbicide terbacil to assess potential resistance to these herbicides in a F. filiformis population from a lowbush blueberry field in Nova Scotia. Dry biomass of the F. filiformis plants from a roadside (susceptible) decreased more rapidly with increasing hexazinone dose than that of the F. filiformis plants from the blueberry field (resistant) (Figure 1). The estimated GR50 values were 582.1 and 95.1 g hexazinone ha−1 for the resistant and susceptible biotypes, respectively (Table 1), resulting in a resistance index of 6.1. In contrast, reductions in biomass of each biotype in response to increasing terbacil dose were similar (Figure 1), and the estimated GR50 values were 314.2 and 195.9 g terbacil ha−1 for the resistant and susceptible biotypes, respectively (Table 1), for a resistance index of only 1.6. Dose–response results therefore indicate that the F. filiformis population sampled from a lowbush blueberry field is resistant to hexazinone, which may explain the lack of hexazinone efficacy now observed on this weed species (White Reference White2019; Yarborough and Cote Reference Yarborough and Cote2014; Zhang Reference Zhang2017). Additional research, however, is needed to determine occurrence of resistance throughout the remainder of Nova Scotia, as our results are limited to one field population and factors other than resistance may affect hexazinone efficacy (Anonymous 2017; Minogue et al. Reference Minogue, Zutter and Gjerstad1988). Results also indicate that the F. filiformis population sampled from a lowbush blueberry field is susceptible to field rates of terbacil, despite incomplete control of this weed species with terbacil in lowbush blueberry fields (White and Zhang Reference White and Zhang2019; Zhang Reference Zhang2017; Zhang et al. Reference Zhang, White, Randall Olson, Pruski and Willenborg2018). Reasons for this are unclear, though it could be related to plant size at the time of application under field conditions. Festuca filiformis seedlings are susceptible to terbacil (White Reference White2018), but plants accumulate new leaves and tillers quickly (White and Kumar Reference White and Kumar2017), and plants in lowbush blueberry fields are usually established tufts with hundreds of leaves (Zhang et al. Reference Zhang, White, Randall Olson, Pruski and Willenborg2018; SNW, personal observation). Large (12-leaf) field violet (Viola arvensis Murray) plants absorbed and translocated less terbacil than small (3-leaf) plants (Doohan et al. Reference Doohan, Monaco and Sheets1992). Large plants also metabolized more terbacil than small plants, and Doohan et al. (Reference Doohan, Monaco and Sheets1992) indicated that field rates of terbacil commonly controlled V. arvensis seedlings but not established plants. A similar effect could occur with terbacil in F. filiformis in lowbush blueberry fields and, based on our results, should be explored further.

Figure 1. Effect of hexazinone (top) and terbacil (bottom) dose on dry biomass of suspected resistant (red) and susceptible (blue) Festuca filiformis biotypes. Lines are the predicted dry biomass obtained from a log-logistic dose–response equation of the form Y = C + {(D − C)/[1 + (x/GR50)b]}, where C is the lower asymptote, D is the upper asymptote, b is the slope of the line at GR50, and GR50 is the herbicide dose generating a 50% reduction in dry biomass (Seefeldt et al. Reference Seefeldt, Jensen and Fuerst1995) as calculated with the drc package in R (Ritz et al. Reference Ritz, Baty, Streibig and Gerhard2015).
Table 1. Regression parameters of a four-parameter logistic dose–response equation explaining the relationship between herbicide dose (g ai ha−1) and Festuca filiformis dry weight at 21 d after treatment (DAT) with hexazinone or terbacil. a

a The equation was of the form Y = C + {(D − C)/[1 + (x/GR50)b]}, where C is the lower asymptote, D is the upper asymptote, b is the slope of the line at GR50, and GR50 is the herbicide dose generating a 50% reduction in dry biomass (Seefeldt et al. Reference Seefeldt, Jensen and Fuerst1995).
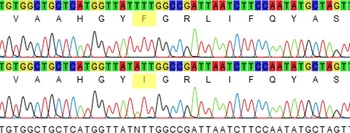
With the advance of molecular techniques, the state of herbicide resistance can be quickly determined even when the mechanism is a priori unknown in certain cases. Several reviews of resistance-conferring mutations have been published (Beckie and Tardif Reference Beckie and Tardif2012; Murphy and Tranel Reference Murphy and Tranel2019), and there is enough information in the literature for the genotype of resistant individuals to be determined, usually via DNA sequencing. This information can be used to identify orthologous mutations in closely related species, especially when the gene targeted by the herbicide mode of action is evolutionarily conserved. The gene encoding the D1 protein of PSII targeted by WSSA Group 5 and 7 herbicides is evolutionarily conserved, and as such, the psbA gene sequence from the related species F. rubra was used to decipher the mechanism of resistance of F. filiformis. Amplification and sequencing of the mutation-containing region of psbA of the suspected hexazinone-resistant F. filiformis biotype from the lowbush blueberry field revealed a phenylalanine change to isoleucine at position 255 (Figure 2) (GenBank accession MT362604). This mutation is reported for the first time in F. filiformis. It was previously reported in C. bursa-pastoris (Perez-Jones et al. Reference Perez-Jones, Intanon and Mallory-Smith2009) and shown to confer resistance to hexazinone, a symmetrical triazine, and metribuzin, an asymmetrical triazine, but did not confer resistance to atrazine, terbacil, or diuron. In contrast, the psbA Phe-255-Val mutation that conferred resistance to hexazinone in R. acetosella (Li et al. Reference Li, Boyd, McLean and Rutherford2014) is different from the one we report, indicating that a range of mutations can confer resistance to hexazinone and that residue 255 plays a key role in the binding of the herbicide to the D1 protein.

Figure 2. Partial Festuca filiformis psbA sequence at the Phe-255-Ile locus. Comparison between the susceptible allele (top sequence) and the resistant allele (bottom sequence). The sequence TTT coding for phenylalanine is found in the wild-type sequence from F. filiformis, while the sequence ATT coding for isoleucine is found in the mutant allele conferring hexazinone resistance in F. filiformis.
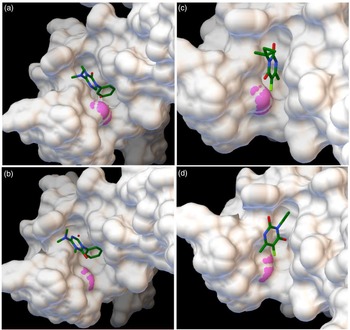
Molecular docking (Figure 3) results indicate a higher affinity of hexazinone to the wild-type D1 protein (−6.45 kcal mol−1) than for the mutated form (−6.22 kcal mol−1) (Table 2), indicating hexazinone is more tightly bound to its ligand in the wild type and can better compete with plastoquinone at the QB binding pocket (Lambreva et al. Reference Lambreva, Russo, Polticelli, Scognamiglio, Antonacci, Zobnina, Campi and Rea2014). A smaller difference in affinity of 0.18 kcal mol−1 can be observed when terbacil is modeled as the ligand, in favor of the susceptible biotype (Table 2). Comparing inhibition constants revealed that 48% (27.38 vs. 18.56 μM) more hexazinone is needed to control the resistant biotype, while a smaller increase, 36% (185.28 vs. 136.85 μM) more terbacil is needed to achieve similar control. This lower difference in concentration values obtained in docking simulations may explain the similar response of both biotypes to terbacil and again indicates susceptibility of the F. filiformis population from lowbush blueberry to this herbicide.
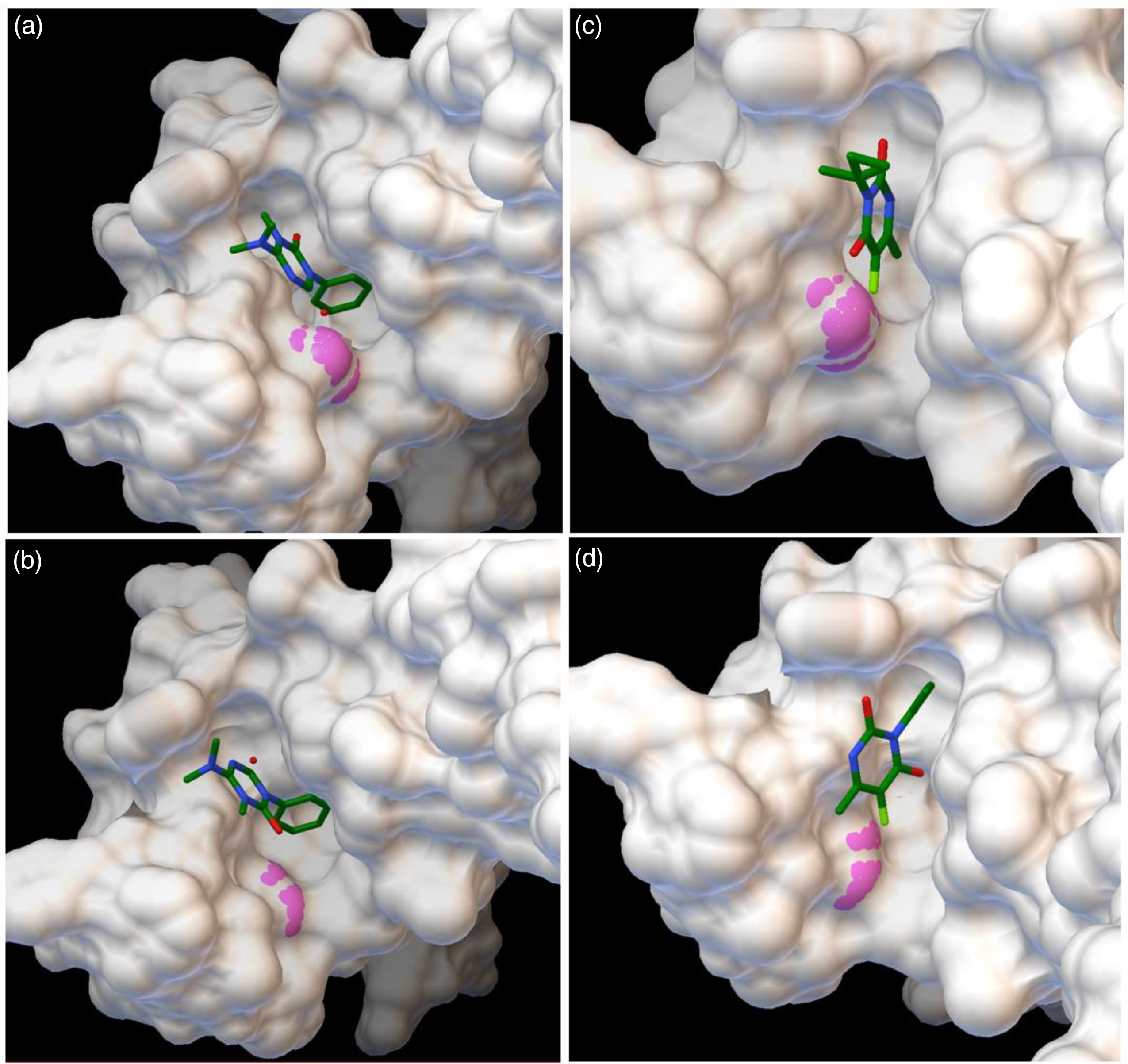
Figure 3. Protein surface visualization of in silico docking of the active ingredients hexazinone (A and B) and terbacil (C and D) to the target protein D1. Protein structure deduced from wild-type allele (phenylalanine, A and C) and resistant allele (isoleucine, B and D). Residue 255 is indicated in pink. Protein deduced from the plastid psbA sequence JX871939.1.
Table 2. Affinity and inhibition constant (Ki) of the active ingredients hexazinone and terbacil to the target D1 protein with and without the Phe-255-Ile amino acid substitution at 25 C as calculated with AutoDock.

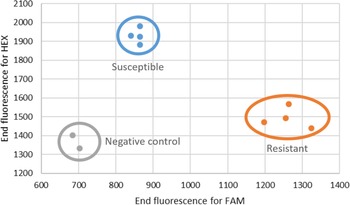
A competitive PCR genotyping assay was developed to aid with the diagnosis of hexazinone resistance in F. filiformis. The fact that the gene coding for the targeted protein D1, psbA, is chloroplast encoded and thus maternally inherited facilitates genotyping, as no heterozygous state is expected. Individuals susceptible to hexazinone, when submitted to this assay, will show increased HEX fluorescence, whereas a resistant biotype will have higher FAM fluorescence (Figure 4). Because each dye is associated with a specific allele, an increase in the detection of FAM is indicative of the presence of the resistance allele, and therefore lack of control with hexazinone should be expected, and producers should take different mitigating actions.

Figure 4. Results of the competitive allele-specific PCR (KASP) assay performed on DNA extracted from two negative controls (gray dots) and four susceptible (blue dots) and four resistant (orange dots) individuals. Fluorescence was measured at the end of cycling on an AriaMx (Agilent, Santa Clara, CA, USA).
From a practical standpoint, identification of hexazinone-resistant F. filiformis provides an important warning for a cropping system that currently has no option for tillage or crop rotation as part of a weed management program. Festuca filiformis seeds lack primary dormancy and readily germinate following dispersal in late summer and fall (White Reference White2018). Festuca filiformis seeds can be killed by short-term exposure to temperatures of 200 and 300 C (White and Boyd Reference White and Boyd2016), thus the replacement of burning by flail mowing for pruning (Eaton et al. Reference Eaton, Glen and Wyllie2004; Yarborough Reference Yarborough2004) may be contributing to increased seed survival and dispersal of resistant biotypes. Furthermore, these seeds are common on lowbush blueberry harvesters (Boyd and White Reference Boyd and White2009), and movement of viable, readily germinable seeds on equipment likely leads to the rapid spread of F. filiformis that is commonly observed within and between fields. While a need for improved equipment sanitation procedures is acknowledged (Anonymous 2019), adoption of machinery cleaning as routine practice remains limited, and the spread of new herbicide-resistant biotypes can be expected unless practices change.
In conclusion, an F. filiformis biotype collected from a lowbush blueberry field was 6.1 times more tolerant to hexazinone than a biotype collected from a roadside F. filiformis population. The mutation conferring this resistance was a phenylalanine change to isoleucine at position 255, similar to a mutation reported in a hexazinone-resistant biotype of C. bursa-pastoris. Terbacil susceptibility, however, was similar in both F. filiformis populations, indicating resistance to hexazinone but not terbacil in the F. filiformis population sampled from a lowbush blueberry field. Confirmation of hexazinone resistance in this F. filiformis population may explain recent failure of hexazinone to control this weed and provides the impetus for developing improved cultural practices around the reduction of human-mediated secondary weed seed dispersal in lowbush blueberry production.
Acknowledgments
The authors want to thank Marie Ciotola, Mélanie Cadieux, Carolane Audette, Maxime Gauthier, Pierre-Yves Veronneau, and Sylvain Fortin for their help in performing the dose–response experiments. This work was funded by Agriculture and AgriFood Canada project J-001751. No conflicts of interest have been declared.