Published online by Cambridge University Press: 02 June 2009
“Sustained” visual units, i.e. nonerasable (R1) and erasable (R2) units as commonly described, were recorded in the superficial layers of the rostral optic tectum of Rana esculenta and their neuronal activity (expressed as the mean firing frequency 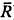 ) was quantitatively analyzed The mean value of the exponent α of the velocity function of the erasable R1 units was found to be 0.46 and the constant k to be about 24.7. The optimal stimulus diameter was equal to 2.6 deg. For the whole population of the erasable units, the exponent α was found to be between 0.36–0.75 (mean value ≈ 0.56, i.e. largely different from the values currently reported for such units) and was independent of the stimulus diameter (D = 0.6–6 deg). However, the constant k of the velocity function (range: 8.2–37.7) varied with D. Most of the erasable units (called “mixed R1-R2” units) therefore showed an R1-type velocity function with an exponent α ≈ 0.5 and a constant k ≈ 25 but reacted to other parameters as did “typical” R2 units: (i) their neuronal response was related to the stimulus diameter by a logarithmic function with a maximum obtained for D = 2.4 deg; and (ii) a variation of about 4–17% on either side of the average value of
) was quantitatively analyzed The mean value of the exponent α of the velocity function of the erasable R1 units was found to be 0.46 and the constant k to be about 24.7. The optimal stimulus diameter was equal to 2.6 deg. For the whole population of the erasable units, the exponent α was found to be between 0.36–0.75 (mean value ≈ 0.56, i.e. largely different from the values currently reported for such units) and was independent of the stimulus diameter (D = 0.6–6 deg). However, the constant k of the velocity function (range: 8.2–37.7) varied with D. Most of the erasable units (called “mixed R1-R2” units) therefore showed an R1-type velocity function with an exponent α ≈ 0.5 and a constant k ≈ 25 but reacted to other parameters as did “typical” R2 units: (i) their neuronal response was related to the stimulus diameter by a logarithmic function with a maximum obtained for D = 2.4 deg; and (ii) a variation of about 4–17% on either side of the average value of 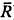 was observed, independent either of the stimulus velocity or of the diameter of the stimulus. Results are compared to known quantitative data, and the use of stimulus-response relationships as a tool for classification is discussed.
was observed, independent either of the stimulus velocity or of the diameter of the stimulus. Results are compared to known quantitative data, and the use of stimulus-response relationships as a tool for classification is discussed.