Published online by Cambridge University Press: 24 August 2021
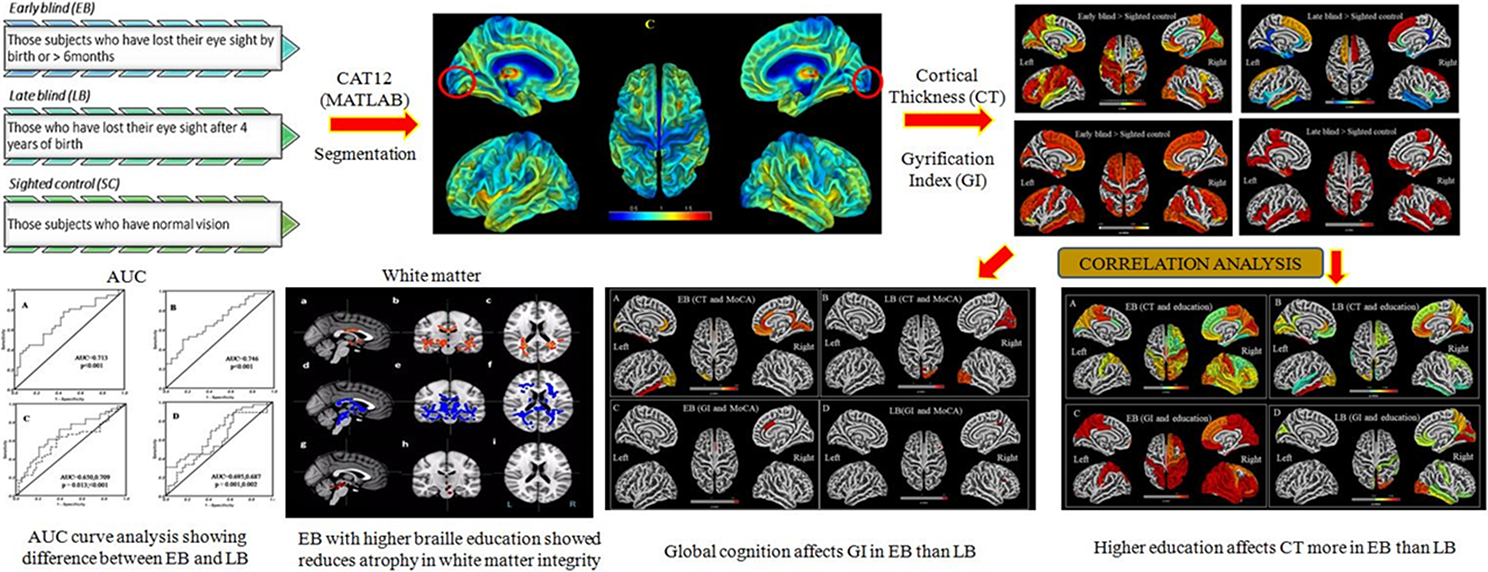
In early (EB) and late blind (LB) children, vision deprivation produces cross-modal plasticity in the visual cortex. The progression of structural- and tract-based spatial statistics changes in the visual cortex in EB and LB, as well as their impact on global cognition, have yet to be investigated. The purpose of this study was to determine the cortical thickness (CT), gyrification index (GI), and white matter (WM) integrity in EB and LB children, as well as their association to the duration of blindness and education. Structural and diffusion tensor imaging data were acquired in a 3T magnetic resonance imaging in EB and LB children (n = 40 each) and 30 sighted controls (SCs) and processed using CAT12 toolbox and FSL software. Two sample t-test was used for group analyses with P < 0.05 (false discovery rate-corrected). Increased CT in visual, sensory-motor, and auditory areas, and GI in bilateral visual cortex was observed in EB children. In LB children, the right visual cortex, anterior-cingulate, sensorimotor, and auditory areas showed increased GI. Structural- and tract-based spatial statistics changes were observed in anterior visual pathway, thalamo-cortical, and corticospinal tracts, and were correlated with education onset and global cognition in EB children. Reduced impairment in WM, increased CT and GI and its correlation with global cognitive functions in visually impaired children suggests cross-modal plasticity due to adaptive compensatory mechanism (as compared to SCs). Reduced CT and increased FA in thalamo-cortical areas in EB suggest synaptic pruning and alteration in WM integrity. In the visual cortical pathway, higher education and the development of blindness modify the morphology of brain areas and influence the probabilistic tractography in EB rather than LB.