Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Gallemore, Ron P.
Li, Jian-Dong
Govardovskii, Victor I.
and
Steinberg, Roy H.
1994.
Calcium gradients and light-evoked calcium changes outside rods in the intact cat retina.
Visual Neuroscience,
Vol. 11,
Issue. 4,
p.
753.
Karwoski, Chester J.
Xu, Xijing
and
Yu, Huaiyuan
1996.
Current-source density analysis of the electroretinogram of the frog: methodological issues and origin of components.
Journal of the Optical Society of America A,
Vol. 13,
Issue. 3,
p.
549.
Gallemore, Ron P.
Hughes, Bret A.
and
Miller, Sheldon S.
1997.
Retinal pigment epithelial transport mechanisms and their contributions to the electroretinogram.
Progress in Retinal and Eye Research,
Vol. 16,
Issue. 4,
p.
509.
Douglas Lee, M.
King, Landon S.
and
Agre, Peter
1997.
The Eye's Aqueous Humor - From Secretion to Glaucoma.
Vol. 45,
Issue. ,
p.
105.
MANGINI, NANCY J
HAUGH-SCHEIDT, LAURA
VALLE, JASON E
CRAGOE, JR, EDWARD J
RIPPS, HARRIS
and
KENNEDY, BRIAN G
1997.
Sodium-Calcium Exchanger in Cultured Human Retinal Pigment Epithelium.
Experimental Eye Research,
Vol. 65,
Issue. 6,
p.
821.
Lee, Douglas M.
King, Landon S.
and
Agre, Peter
1997.
The Aquaporin Family of Water Channel Proteins in Clinical Medicine.
Medicine,
Vol. 76,
Issue. 3,
p.
141.
Kim, In-Beom
Oh, Su-Ja
Nielsen, Søren
and
Chun, Myung-Hoon
1998.
Immunocytochemical localization of Aquaporin 1 in the rat retina.
Neuroscience Letters,
Vol. 244,
Issue. 1,
p.
52.
Nagelhus, Erlend A.
Veruki, Margaret L.
Torp, Reidun
Haug, Finn-M.
Laake, Jon H.
Nielsen, Søren
Agre, Peter
and
Ottersen, Ole P.
1998.
Aquaporin-4 Water Channel Protein in the Rat Retina and Optic Nerve: Polarized Expression in Müller Cells and Fibrous Astrocytes.
The Journal of Neuroscience,
Vol. 18,
Issue. 7,
p.
2506.
Piccolino, Marco
Pignatelli, Angela
and
Rakotobe, Liramalala A.
1999.
Calcium-independent release of neurotransmitter in the retina: a “Copernican” viewpoint change.
Progress in Retinal and Eye Research,
Vol. 18,
Issue. 1,
p.
1.
Nagelhus, Erlend A.
Horio, Yoshiyuki
Inanobe, Atsushi
Fujita, Akikazu
Haug, Finn-m.
Nielsen, S�ren
Kurachi, Yoshihisa
and
Ottersen, Ole Petter
1999.
Immunogold evidence suggests that coupling of K+ siphoning and water transport in rat retinal M�ller cells is mediated by a coenrichment of Kir4.1 and AQP4 in specific membrane domains.
Glia,
Vol. 26,
Issue. 1,
p.
47.
Bergersen, L
Jóhannsson, E
Veruki, M.L
Nagelhus, E.A
Halestrap, A
Sejersted, O.M
and
Ottersen, O.P
1999.
Cellular and subcellular expression of monocarboxylate transporters in the pigment epithelium and retina of the rat.
Neuroscience,
Vol. 90,
Issue. 1,
p.
319.
Wolfensberger, Thomas J.
Dmitriev, Andrey V.
and
Govardovskii, Victor I.
2000.
Macular Edema.
p.
57.
Venero, José L.
Vizuete, Marı́a L.
Machado, Alberto
and
Cano, Josefina
2001.
Aquaporins in the central nervous system.
Progress in Neurobiology,
Vol. 63,
Issue. 3,
p.
321.
Hibino, Hiroshi
Fujita, Akikazu
Iwai, Kaori
Yamada, Mitsuhiko
and
Kurachi, Yoshihisa
2004.
Differential Assembly of Inwardly Rectifying K+ Channel Subunits, Kir4.1 and Kir5.1, in Brain Astrocytes.
Journal of Biological Chemistry,
Vol. 279,
Issue. 42,
p.
44065.
Guadagno, Eric
and
Moukhles, Hakima
2004.
Laminin‐induced aggregation of the inwardly rectifying potassium channel, Kir4.1, and the water‐permeable channel, AQP4, via a dystroglycan‐containing complex in astrocytes.
Glia,
Vol. 47,
Issue. 2,
p.
138.
Tenckhoff, Solveig
Hollborn, Margrit
Kohen, Leon
Wolf, Sebastian
Wiedemann, Peter
and
Bringmann, Andreas
2005.
Diversity of aquaporin mRNA expressed by rat and human retinas.
NeuroReport,
Vol. 16,
Issue. 1,
p.
53.
Strauss, Olaf
2005.
The Retinal Pigment Epithelium in Visual Function.
Physiological Reviews,
Vol. 85,
Issue. 3,
p.
845.
Kang, Tae-Hoon
Choi, Yeon-Kyung
Kim, In-Beom
Oh, Su-Ja
and
Chun, Myung-Hoon
2005.
Identification and characterization of an aquaporin 1 immunoreactive amacrine-type cell of the mouse retina.
The Journal of Comparative Neurology,
Vol. 488,
Issue. 3,
p.
352.
RYMER, JODI
and
WILDSOET, CHRISTINE F.
2005.
The role of the retinal pigment epithelium in eye growth regulation
and myopia: A review.
Visual Neuroscience,
Vol. 22,
Issue. 3,
p.
251.
Iandiev, Ianors
Pannicke, Thomas
Reichel, Martin B.
Wiedemann, Peter
Reichenbach, Andreas
and
Bringmann, Andreas
2005.
Expression of aquaporin-1 immunoreactivity by photoreceptor cells in the mouse retina.
Neuroscience Letters,
Vol. 388,
Issue. 2,
p.
96.
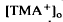 , which was maximal in amplitude in the most distal portion of the space surrounding photoreceptors, the subretinal space. The light-evoked decrease in
, which was maximal in amplitude in the most distal portion of the space surrounding photoreceptors, the subretinal space. The light-evoked decrease in 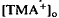 was considerably slower and of a different overall time course than the light-evoked decrease in
was considerably slower and of a different overall time course than the light-evoked decrease in 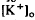 , also recorded in the subretinal space.
, also recorded in the subretinal space. 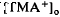 decreased to a peak at 38 s after the onset of illumination, then slowly recovered towards the baseline, and transiently increased following the offset of illumination. It resembled the light-evoked
decreased to a peak at 38 s after the onset of illumination, then slowly recovered towards the baseline, and transiently increased following the offset of illumination. It resembled the light-evoked 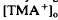 decreases previously recorded in the in vitro preparations of frog (Huang & Karwoski, 1990, 1992) and chick (Li et al., 1992, 1994) but was considerably larger in amplitude, 22% compared with 7%. As in frog, where it was first recorded, the light-evoked
decreases previously recorded in the in vitro preparations of frog (Huang & Karwoski, 1990, 1992) and chick (Li et al., 1992, 1994) but was considerably larger in amplitude, 22% compared with 7%. As in frog, where it was first recorded, the light-evoked 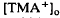 decrease is considered to originate from a light-evoked increase in the volume of the subretinal space (or subretinal hydration). A mathematical model accounting for
decrease is considered to originate from a light-evoked increase in the volume of the subretinal space (or subretinal hydration). A mathematical model accounting for 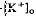 diffusion predicted that the volume increase underlying the response was 63% on average and could be as large as 95% and last for minutes. The estimated volume increase was then used to examine its effect on K+ concentration in the subretinal space. We conclude that a light-dependent hydration of the subretinal space represents a significant physiological event in the intact cat eye, which should affect the organization of the interphotoreceptor matrix, and the concentrations of all ions and metabolites located in the subretinal space.
diffusion predicted that the volume increase underlying the response was 63% on average and could be as large as 95% and last for minutes. The estimated volume increase was then used to examine its effect on K+ concentration in the subretinal space. We conclude that a light-dependent hydration of the subretinal space represents a significant physiological event in the intact cat eye, which should affect the organization of the interphotoreceptor matrix, and the concentrations of all ions and metabolites located in the subretinal space.

