Thought to represent a special category of stunted fetal growth, twins have a higher risk of diseases associated with lower birth weight and major incidence of morbidity and mortality (Blickstein & Kalish, Reference Blickstein and Kalish2003). In particular, monochorionicity has been associated with an increased rate of adverse perinatal outcomes when compared with dichorionicity. This is in part due to a higher incidence of intrauterine growth restriction (IUGR), abnormal placental vascular anastomoses, fetal anomalies, and stillbirth. Compared with singletons, the growth rate of twin fetuses is often characterized by a higher incidence of IUGR in one of the fetuses, varying from 15% to 25% in all monochorionic (MC) pregnancies, according to the definition of the American College of Obstetricians and Gynecologists (ACOG), and frequently associated with Doppler velocimetry alterations (Amaru et al., Reference Amaru, Bush, Berkowitz, Lapinski and Gaddipati2004; Committee on Practice Bulletins — Gynecology, 2001; Gratacos et al., Reference Gratacos, Carreras, Becker, Lewi, Enriquez, Perapoch and Deprest2004).
It is now well known that IUGR is a condition associated with an increased risk of adult onset disease. The fetal origin hypothesis in fact proposes that cardiovascular diseases and type 2 diabetes mellitus in adult life are associated with low birth weight, through several adaptation mechanisms of the undernourished fetus in the structure and function of the body (Barker, Reference Barker2000, Reference Barker2006; Irving et al., Reference Irving, Belton, Elton and Walker2000; Osmond & Barker, Reference Osmond and Barker2000).
Early endothelial dysfunction and aortic wall intima–media thickening (aIMT) occurring in utero may play an important role in premature in utero stiffening of the aortic vessels (Skilton et al., Reference Skilton, Evans, Griffiths, Harmer and Celermajer2005). Previous studies have demonstrated an association between low birth weight and abdominal aorta intima–media thickness in utero and at 18-month follow-up, and high blood pressure, and microalbuminuria in childhood (Cosmi et al., Reference Cosmi, Visentin, Fanelli, Mautone and Zanardo2009; Koklu et al., Reference Koklu, Ozturk, Gunes, Akcakus and Kurtoglu2007; Zanardo et al., Reference Zanardo, Fanelli, Weiner, Fanos, Zaninotto, Visentin and Cosmi2011).
Twins present a distribution of birth weights shifted to the left of the normal singleton distribution; it would be expected that twins may be at increased risk for the long-term outcomes, but the relationship between birth weight and the onset of adult diseases in those born as a twin is unclear, with only scarce evidence in the literature (Joseph et al., Reference Joseph, Liu, Demissie, Wen, Platt, Ananth and Kramer2003; Lopriore et al., Reference Lopriore, Sluimers, Pasman, Middeldorp, Oepkes and Walther2012; McMillen & Robinson, Reference McMillen and Robinson2005). In this perspective, research on twin fetuses provides a unique opportunity to mimic a scientific experiment to study IUGR and, reflecting nutritional stresses within a similar genetic fetal background, to distinguish between genetic and environmental causes of phenotypic variations in the human population (Muhlhusler et al., Reference Muhlhusler, Hancock, Bloomflied and Harding2011). The cause of discordant growth in MC twin pregnancies probably originates from a variety of conditions: limited maternal uterine environment, intertwin distribution of blood and nutrients along vascular placental anastomosis, unequal placental sharing, abnormal cord insertion, or impaired transport of amino acids through the placenta (Bajoria et al., Reference Bajoria, Sooranna, Ward, D'Souza and Hancock2001; Ijzerman et al., Reference Ijzerman, Boomsma and Stehouwer2005; Ropacka-Lesiak et al., Reference Ropacka-Lesiak, Bręborowicz and Dera2012; Valsky et al., Reference Valsky, Eixarch, Martinez, Crispi and Gratacós2010).
Metabolomics, a relatively new approach used for characterizing the metabolic phenotype of individuals by integrating genetic, epigenetic, and post-transcriptional data, has recently been applied in the field of nutritional fetal and pediatric research (Alexandre-Gouabau et al., Reference Alexandre-Gouabau, Courant, Le Gall, Moyon, Darmaun, Parnet and Antignac2011; Dessì et al., Reference Dessì, Ottonello and Fanos2012). Utilizing this technique, our previous studies detected significant differences in the distribution of essential amino acids (Favretto et al., Reference Favretto, Cosmi, Ragazzi, Visentin, Tucci, Fais and Ferrara2012) and in the expression of proteins (Cecconi et al., Reference Cecconi, Lonardoni, Favretto, Cosmi, Tucci, Visentin and Ferrara2011) between IUGR and appropriate for gestational age (AGA) single fetuses.
The aim of this study was (1) to compare, in a selected sample, the abdominal aorta–intima thickness (aIT) among selective intrauterine growth restricted (sIUGR) twin fetuses with and without umbilical artery (UA) Doppler vasculopathy and AGA twin fetuses in utero; (2) to compare metabolomic profiles of MC sIUGR twins with UA Doppler abnormalities to controls; and (3) to test the hypothesis of an association between aIT and metabolic profiles in IUGR twins with UA Doppler alterations.
Materials and Methods
Patients
This study was carried out in 24 monochorionic diamniotic (MCDA) twins from 12 pregnancies with and without IUGR at the Center for Prenatal Diagnosis of the University of Padova. The institutional review board and the ethics committee for clinical studies of the University Hospital approved the study protocol, and all patients or their guardians provided informed consent. Patients were selected from a larger cohort included in a recently published study on mochorionic and dichorionic twin pregnancies (Visentin et al., Reference Visentin, Grisan, Zanardo, Bertin, Veronese, Cavallin and Cosmi2013).
The diagnosis of monochorionicity was performed at 10–14 weeks of gestation in the presence of a single placental mass and the ‘T’ sign (Carroll et al., Reference Carroll, Soothill, Abdel-Fattah, Porter, Montaque and Kyle2002), and was confirmed at birth by histology. Gestational age was calculated on the basis of the first day of the last menstrual period and confirmed by the ultrasound measurement of the fetal crown-rump length (CRL; Drumm, Reference Drumm1977).
sIUGR in MCDA pregnancies was defined as an estimated fetal weight (EFW) below the 10th percentile in the smaller twin and above the 10th percentile in the larger one (Ananth et al., Reference Ananth, Vintzileos, Shen-Schwarz, Smulian and Lai1998; Fox et al., Reference Fox, Rebarber, Klauser, Roman and Saltzman2011). sIUGR twins were further divided into two groups based on UA Doppler waveforms: Group 1 included sIUGR twins with abnormal UA Doppler (UA Pulsatility Index [PI] > 2 standard deviation [SD], absent or reverse end-diastolic flow); Group 2 included sIUGR twins with normal UA Doppler and with normal end-diastolic flow. MC twins with an EFW above the 10th percentile, confirmed after birth, and normal UA Doppler constituted the AGA control group (Group 3). Table 1 presents a summary of the studied cases.
TABLE 1 Characteristics of the Cases

Bold type indicates the pairs of twins (each one independently considered) that were analyzed by a multivariate approach (see Results section). Group 1 = sIUGR with abnormal UA Doppler waveforms; Group 2 = sIUGR with normal UA Doppler waveforms; Group 3 = AGA twins.
Exclusion criteria were as follows: unknown last menstrual period and chorionicity; triplet pregnancy; twin-to-twin transfusion syndrome (TTTS) or related conditions; MC monoamniotic twin pregnancies; first trimester discrepancy of CRL among the pair of twins >5 days; structural or chromosomal abnormalities; single intrauterine death or selective feticide; maternal history of cardiovascular disease or endocrine disorders such as diabetes, hypercholesterolemia, pre-eclampsia, thyroid and adrenal problems; clinical chorioamnionitis; as well as maternal consumption of alcohol, drugs of abuse or nicotine. TTTS was defined according to the Quintero classification (Quintero et al., Reference Quintero, Morales, Allen, Bornick, Johnson and Kruger1999).
aIT and diameter measurements were determined for each twin at a median gestational age of 32 weeks (range 28–36 weeks) in a coronal or sagittal view of the fetus at the arterial wall of the most distal 15 mm of the abdominal aorta, sampled below the renal and above the iliac arteries. Abdominal aIT was measured placing the calipers at the leading edge of the blood–intima interface and at the end of the inner portion of the intima vessel. We chose this measurement instead of aIMT because a recent study of our group on fetal aorta sampled after intrauterine fetal demise showed an increase of the intima of the aorta in the IUGR stillbirth (Lo Vasco et al., Reference Lo Vasco, Salmaso, Zanardo, Businaro, Visentin, Trevisanuto and Cosmi2011). All images were stored digitally for off-line analysis. All measurements were performed in high resolution with the same ultrasound scan with a 3.5- to 5-MHz linear array transducer (Antares, Siemens Medical Solutions, Mountain View, CA).
Three aIT measurements were taken and the median was considered. All images were captured during the last phase of the cardiac cycle to minimize variability. The aortic diameter was measured at the same level of aIT, from the inner wall to the wall edges. End diastole was determined as the maximal expansion of the vessel using the cine loop capability of the ultrasound machine once the images of the entire cardiac cycle were frozen. Each measurement was taken during fetal apnea after three consecutive, similar waveforms were obtained. Pulsatility index and time-averaged velocities (defined as the area under the velocity spectral envelope) were measured using the machine software.
Before starting the main research, the intraobserver and interobserver agreements were evaluated in the measurement of aIT, as reported elsewhere (Visentin et al., Reference Visentin, Grisan, Zanardo, Bertin, Veronese, Cavallin and Cosmi2013).
All MC pregnancies were also monitored by routine ultrasound examination, including a complete morphological examination, estimation of fetal weight, amniotic fluid index calculations, and Doppler assessment of the UA, middle cerebral artery (MCA), and ductus venosus (DV).
Sample Collection
Fetal blood samples (0.5 mL) were obtained from the umbilical vein from a doubly clamped segment of each cord immediately after fetal extraction, and were collected in a Vacutainer SST II Advance (BP Diagnostic, Belliver Industrial Estate, Plymouth, UK) tube and centrifuged at 1,500 × g for 10 minutes at 4 °C. In none of the cases did the time from birth to sample collection exceed 10 minutes. Time was recorded with a stopwatch. Additional umbilical arterial and venous samples from a segment of cord that was clamped at both ends were also obtained for the determination of acid–base status as routine clinical practice.
The samples were collected at the Department of Health of the Woman and Child and were analyzed at the Department of Molecular Medicine, Forensic Toxicology and Antidoping Unit, University of Padova.
Sample Preparation
Serum samples (200 μL) were deproteinized by mixing with methanol (600 μL) at room temperature, vortex mixed for 20 seconds, and incubated at -20 °C overnight. Samples were then centrifuged (13,845 × g, 15 minutes, 4 °C). Supernatants were transferred to Eppendorf tubes, lyophilized, and kept at 4 °C. Samples were reconstituted in 100 μL of water prior to analysis.
Chemicals
Twenty essential amino acids (Gly, Ala, Ser, Pro, Val, Thr, Cys, Leu, Ile, Asn, Asp, Gln, Lys, Glu, Met, His, Phe, Arg, Tyr, and Trp) were purchased from Sigma (Saint Quentin Fallavier, France). Standards were dissolved in water to obtain solutions of 50 μmol/L before liquid chromatography (LC) tandem mass spectrometry analysis (MS/MS).
The standard mixtures used for the external calibration of the MS instrument (caffeine, L-Ultramark 1621, sodium dodecyl sulfate, and sodium taurocholate) were from Thermo Fisher Scientific (Les Ulis, France). Methanol, formic acid, and acetonitrile were from Merck (Briare-le-canal, France).
All the chemicals used were of analytical or reagent grade.
Metabolomic LC-HRMS Analysis
Liquid chromatography–high-resolution mass spectrometry (LC-HRMS) analysis was obtained using an LTQ-Orbitrap mass spectrometry system (Thermo Fisher Scientific, Bremen, Germany) connected to a Surveyor Plus LC. High performance LC separation was performed with a Luna C18 (1.0 × 150-mm) column (Phenomenex, Torrance, CA) with a constant flow rate of 100 μL/min. The LC eluents were water, 0.1% formic acid (phase A) and methanol, and 0.1% formic acid (phase B). A chromatographic gradient was used. After an isocratic step of 1 minute at 90% phase A and 10% phase B, a linear gradient from 10% to 100% B was run over the next 35 minutes, with a mobile phase flow of 100 μL/min. A 10-μL sample volume was injected into the column. An electrospray source was used, working in positive ion mode. For the positive ion detection mode, the sheath gas was set at 13 (arbitrary units) and the auxiliary gas at 29 (arbitrary units), and capillary temperature was 275 °C. The spray voltage and tube lens voltage were 4,500 and 85 V, respectively. Lens parameters were optimized with a tune solution containing 14 metabolites (cystine, arginine hydrochloride, arginine, tryptophan, lysine monohydrochloride, lysine, tyrosine, serine, valine, leucine, asparagine, glutamic acid, methionine, and histidine) at a concentration of 1–3 mmol/L and infused at 5 μL/min. Gas flows were then optimized with the same compounds but with a 0.1-mL/min LC flow.
Orbitrap calibration was performed in the positive ion mode. Full-scan mass spectra were acquired over the m/z range of 50–1,000 at a speed of 0.45 s/scan (2 μscans) using the Orbitrap mass analyzer operating with a target mass resolving power of approximately 30,000 (full-width at half-maximum of peak).
To account for any analytical variability, measurements were performed in triplicate (three injections from the same sample).
All data were processed with Qualbrowser (Thermo Fisher) and its chemical formula generator was used to provide elemental compositions with a relative error lower than 5 ppm.
Raw data from analysis were transformed to peak tables by the XCMS software (http://massspec.scripps.edu/xcms/xcms.php), including statistical tools (see below).
Metabolomic Data Analysis and Statistics
Data preprocessing
Raw LC-HRMS data were preprocessed for untargeted metabolite profiling with the open-source package XCMS (Smith et al., Reference Smith, Want, O'Maille, Abagyan and Siuzdak2006), written in R statistical programming language, available at Metlin Metabolite Database and the Bioconductor Bioinformatics Project (Gentleman et al., Reference Gentleman, Carey, Bates, Bolstad, Dettling, Dudoit and Zhang2004). Peak matching and single-step retention time alignment of all samples were obtained automatically, and a matrix of aligned peaks was produced in .tsv format. Values close to zero due to baseline noise were filtered and excluded, since these values can interfere with computational algorithms in further data analysis. Peak-matching algorithm considered a bandwidth (SD or half-width at half-maximum) of 30 for the Gaussian smoothing kernel and fixed-interval bins 0.25 m/z wide. Retention time alignment algorithm was the Loess method with an automatized outlier detection and removal. The abundance of each ionic species was normalized according to the total ion current (TIC) measured for each sample and expressed as percentage.
Statistics
To identify the most relevant differences between groups, a univariate statistical analysis was used on spectral data, considering each neonate as an independent unit. Filtering procedures, such as fold-change analysis and t tests (for paired and unpaired data) were applied, as provided by MetaboAnalyst web-based software (Xia et al., Reference Xia, Psychogios, Young and Wishart2009; Xia & Wishart, Reference Xia and Wishart2011). Significantly different data at the probability level of p < .05 were used for further procedures of the multivariate analysis, to obtain the identification of relevant biomarkers. One-way ANOVA followed by post hoc tests was used to detect differences among groups. Differences were judged statistically significant at p < .05. Tentatively, the entire metabolomic dataset was submitted to the multivariate analysis (with MetaboAnalyst; Xia et al., Reference Xia, Psychogios, Young and Wishart2009; Xia & Wishart, Reference Xia and Wishart2011). The procedure is explained in the supplementary material.
Metabolite identification
The putative masses responsible for the metabolic differentiation among the three groups were used to make queries in the METLIN (http://metlin.scripps.edu/), Mass Bank Database (http://www.massbank.jp/en/database.html), and Human Metabolome (http://www.hmdb.ca/) online databases in order to obtain the corresponding chemical structures. The identities of metabolites of particular interest were confirmed by a comparison of their retention times, accurate mass spectra, and HRMS information with those of authentic chemical standards. The KEGGs Database (http://www.genome.jp/kegg/) was used to identify metabolic pathways of interest.
Results
The study was conducted on a total of 12 consecutive MCDA twin pairs: four sIUGR neonates in Group 1, four sIUGR neonates in Group 2, and 16 AGA in Group 3. For eight neonates, the pair consisted of an sIUGR twin (four with UA Doppler alterations and four without) and the corresponding AGA co-twin (Table 1).
Maternal and neonatal data are shown in Table 2. There were no differences in maternal age and ethnicity among the groups. All the patients were subjected to cesarean section for fetal reasons or in a planned way as per the internal protocol of the hospital. Birth weight and length were significantly diminished in the sIUGR Group 1, as well as pH of cord blood. Instead, there were no differences among the groups in Apgar score 5 minutes after birth and in base excess.
TABLE 2 Maternal and Neonatal Data

Values for continuous variables are given as median and range. Group 1 = sIUGR with abnormal UA Doppler waveforms; Group 2 = sIUGR with normal UA Doppler waveforms; Group 3 = AGA twins.
aIT = aorta–intima thickness; PI = Pulsatility Index; AEDF/ARED = absent and reverse diastolic flow; UA = umbilical artery; SD = standard deviation; DV = ductus venosus; BE = base excess.
For continuous variables, difference among groups was evaluated by using the Kruskal–Wallis test followed by a non-parametric Wilcoxon test to assess the difference between pairs. For frequency data, difference was evaluated with the Fisher exact probability test.
**p < .002; *p < .01, comparisons versus Group 3; †p < .05, comparison Group 1 versus Group 2.
Both Groups 1 and 2 twins showed an aIT value significantly greater than that of Group 3, but the difference was not significant when comparing Groups 1 and 2. As previously demonstrated, aIT correlated with Doppler UA abnormalities (Cosmi et al., Reference Cosmi, Visentin, Fanelli, Mautone and Zanardo2009; Visentin et al., Reference Visentin, Grisan, Zanardo, Bertin, Veronese, Cavallin and Cosmi2013).
The metabolomic analysis was carried out comparing the LC-HRMS data obtained from the three different groups of twin pregnancies, according to their status. In a first set of multivariate statistical analysis, the three groups of twins were compared; PCA analysis and cluster analysis (Ward's method) did not allow a clear distinction between the three groups; in some cases the pattern of ionic species identified appeared consistent among paired twins, but not always belonging to same pathology classes (Figures S1–S2 in the supplementary material).
Another approach was then tested: only pairs of twins of which one was normal and the other affected by sIUGR with abnormal UA Doppler waveforms were considered (i.e., four sIUGR vs. four AGA neonates). Due to the rarity of this condition, only four paired cases were available (cases from Groups 1 and 3 shown in bold type in Table 1).
In considering the four pairs of twins, a multivariate approach was tried first with paired test option (MetaboAnalyst; Xia et al., Reference Xia, Psychogios, Young and Wishart2009; Xia & Wishart, Reference Xia and Wishart2011). The most relevant ionic species that resulted from a preliminary multivariate analysis were selected, and a further PCA and cluster analysis were performed. Although PCA did not allow a satisfactory separation of the groups (Figure S3 in the supplementary material), an appreciable clustering of data was obtained following cluster analysis (Figure S4). The multivariate approach permitted the identification of the most relevant variations by using the fold-change value between the two groups, controls (Group 3, AGA) and sIUGR (Group 1). Figure 1 indicates the ionic species that were the most different between the pairs of AGA (Group 3) and sIUGR (Group 1) co-twins. The low sample size and the large dispersion of data did not reach a statistically significant difference (Student's t test for paired data did not reveal a significant difference between the AGA Group 3/sIUGR Group 1); however, a peculiar trend was observed, with some ionic species being more abundant in AGA (positive δ) and others more abundant in sIUGR Group 1 twins (negative δ).

FIGURE 1 Species presenting major changes between the respective AGA neonates (Group 3) and sIUGR neonates with abnormal UA Doppler waveforms (Group 1); data are the mean difference (± SEM) in the abundance of the metabolites determined in four twin pairs, of which one baby was normal (AGA) and the other affected by sIUGR with abnormal UA Doppler waveforms (Group 1). The identified species are indicated in the labels (Table 3). GPC = gycerophosphocholine.
A search using the Metlin database identified several of these ionic species (Table 3). As shown in Table 3 and Figure 1, some of the species identified were amino acids. Concentrations of the essential amino acids, valine and isoleucine, and non-essential amino acids, tryptophan and proline, were lower in Group 1 than in AGA co-twins; on the contrary, phenylalanine appeared upregulated in sIUGR Group 1 twins compared with AGA ones.
TABLE 3 Ionic Species Identified in the LC-HRMS Spectra

Figure 2 presents a comparison of cumulative absolute abundance (in arbitrary units; a.u.) of total ionic currents (TIC) for the amino acids in the four pairs of twins. As outlined in Figure 2(a), when the sum of TIC for the amino acids valine, tryptophan, proline, isoleucine is plotted, the control twins always show an upregulation. On the contrary, as shown in Figure 2(b), when the sum of TIC for the amino acid phenylalanine is plotted, the trend is reversed, with Group 1 twins overexpressing this aromatic, essential amino acid. In this case, however, the overexpression is not markedly evident.

FIGURE 2 A comparison of the sera levels of amino acids valine, tryptophan, proline, isoleucine (a), and phenylalanine (b) between four sIUGR neonates with abnormal Doppler (Group 1, dotted bars) and four AGA controls (Group 3, striped bars). Each subject was considered as an independent statistical unit. As indicated in the text, a statistical test was performed (Student's t test for paired data), but due to the low sample size and the large dispersion of data it did not reach a statistically significant difference.
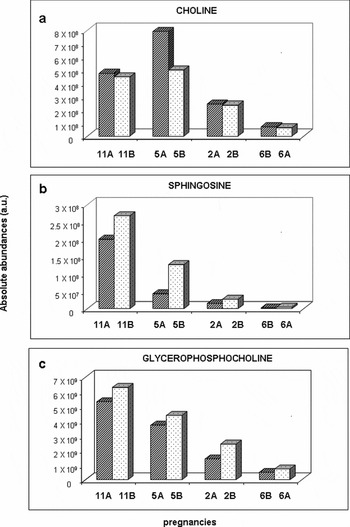
Figure 3 reports the comparison of absolute abundance (a.a.) of TIC for the other ionic species identified: choline (Figure 3a), sphingosine (Figure 3b), and the different isomers of glicerophosphocoline (Figure 3c); choline is downregulated in sIUGR Group 1 twins, whereas the other species are upregulated.

FIGURE 3 A comparison of the sera levels of choline (a), sphingosine (b), and glycerophosphocholine (c) between four sIUGR neonates with abnormal Doppler (Group 1, dotted bars) and four controls (Group 3, striped bars). Each subject was considered as an independent statistical unit. As indicated in the text, a statistical test was performed (Student's t test for paired data), but due to the low sample size and the large dispersion of data it did not reach a statistically significant difference.
Discussion
To the best of our knowledge, this study is the first to investigate endothelial structure in a selected group of MCDA twins, measuring the abdominal fetal aIT and performing metabolomic analyses on cord blood collected immediately after birth. The data collected evidenced a higher aIT in sIUGR with abnormal UA Doppler waveforms, with respect to AGA. Additionally, the metabolomic investigation showed several differences in the concentration of essential and non-essential amino acids in sIUGR neonates (four belonging to Group 1) compared with their MCDA co-twins (four AGA neonates), although, due to the small number of cases, the measured differences did not reach statistical significance.
Although recent twin studies have taken into account the effect of intrauterine environment on fetal development trajectory using regression analyses and a between-twin coefficient (Carlin et al., Reference Carlin, Gurrin, Sterne, Morley and Dwyer2005), few studies have compared the long-term health outcomes within twin pairs, and it is still not clear whether the heavier twin in the pair has better health outcomes than the lower one (Phillips et al., Reference Phillips, Davies and Robinson2001). In the literature, there are conflicting data concerning the relationship between IUGR, postnatal growth, and long-term outcomes (Bleker et al., Reference Bleker, Wolf and Oosting1995; De Matteo et al., Reference De Matteo, Stacy, Probyn, Desai, Ross and Harding2008; McMillen & Robinson, Reference McMillen and Robinson2005; Ross et al., Reference Ross, Desai, Guerra and Wang2005; Westwood et al., Reference Westwood, Gibson, Sooranna, Ward, Neilson and Bajoria2001).
Intima–media thickness is currently considered to be one of the earliest morphological markers of plaque formation and atherosclerosis in IUGR fetuses and neonates (Cosmi et al., Reference Cosmi, Visentin, Fanelli, Mautone and Zanardo2009; Järvisalo et al., Reference Järvisalo, Jartti, Näntö-Salonen, Irjala, Rönnemaa, Hartiala and Raitakari2001; Koklu et al., Reference Koklu, Ozturk, Gunes, Akcakus and Kurtoglu2007; Litwin & Niemirska, Reference Litwin and Niemirska2008; McGill et al., Reference McGill, McMahan, Herderick, Tracy, Malcom, Zieske and Strong2000; Skilton, Reference Skilton2008; Zanardo et al., Reference Zanardo, Fanelli, Weiner, Fanos, Zaninotto, Visentin and Cosmi2011, Reference Zanardo, Visentin, Trevisanuto, Bertin, Cavallin and Cosmi2013).
We have previously described the ultrasound-based measurement of abdominal aIMT in utero in IUGR single fetuses characterized by an EFW below the 10th percentile and PI > 2 SD, presenting persisting aortic wall thickening and microalbuminuria at the 18-month follow-up. Moreover, a microscopic observation of abdominal aortic walls of stillbirth IUGR fetuses confirmed that there is intima thickening (aIT) and the presence of inflammatory elements, such as macrophages and activated endothelial cells, even in amniotic fluid, indicating that intrauterine growth restriction is a potential marker of atherosclerosis development and may be associated with an increased risk of adult onset diseases (Cosmi et al., Reference Cosmi, Visentin, Fanelli, Mautone and Zanardo2009; Lo Vasco et al., Reference Lo Vasco, Salmaso, Zanardo, Businaro, Visentin, Trevisanuto and Cosmi2011, Reference Lo Vasco, Cosmi, Visentin, Di Raimo, Salmaso, Zanardo and Businaro2012; Zanardo et al., Reference Zanardo, Fanelli, Weiner, Fanos, Zaninotto, Visentin and Cosmi2011).
Although the effect of IUGR on vascular function, assessed using indicators of endothelial damage such as the aIT, has previously been studied in dichorionic and monochorionic twin pregnancies (Dwyer et al., Reference Dwyer, Blizzard, Morley and Ponsonby1999; Poulter et al., Reference Poulter, Chang, MacGregor, Snieder and Spector1999), this is the first study conducted in a population of sIUGR monochorionic twins with and without Doppler alterations (Visentin et al., Reference Visentin, Grisan, Zanardo, Bertin, Veronese, Cavallin and Cosmi2013). Our data show that low birth weight and Doppler umbilical artery vasculopathy in MDCA twins are accompanied by a higher aIT, compared with AGA co-twins and sIUGR neonates without Doppler alterations. This evidence suggests that a grading of endothelial alterations might exist, and that low birth weight and UA Doppler modifications could be used as predictors of the severity of the vasculopathy.
In light of the above, we have analyzed cord blood metabolomic profiles in sIUGR twins with and without UA Doppler abnormalities in order to deepen the investigation of the molecular mechanisms behind endothelial alterations and IUGR pathogenesis.
In previous studies conducted on singleton pregnancies, an upregulation of the amino acids phenylalanine, tryptophan, and glutamate was found in IUGR samples compared with AGA, suggesting a role of placental transport and metabolism in IUGR pathogenesis (Favretto et al., Reference Favretto, Cosmi, Ragazzi, Visentin, Tucci, Fais and Ferrara2012; Matthews et al., Reference Matthews, Beveridge, Malandro, Rothstein, Campbell-Thompson, Verlander and Novak1998; Morris et al., Reference Morris, Burston, Ramsay and Sooranna1995; Paolini et al., Reference Paolini, Marconi, Ronzoni, Di Noio, Fennessey, Pardi and Battaglia2001).
In this investigation, a trend for increase of phenylalanine and a trend for downregulation of valine, isoleucine, tryptophan, and proline were found in sIUGR twin fetuses with UA Doppler abnormalities compared with control co-twins (no statistical significance could be reached due to the small number of subjects). Bajoria et al. (Reference Bajoria, Sooranna, Ward, D'Souza and Hancock2001) described the selective reduction of phenylalanine in cord blood of MC twin pregnancies such as of other essential (valine, isoleucine, leucine, and arginine) and non-essential (taurine, serine, glycine, tyrosine, and aspartic acid) amino acids by HPLC. The data of Bajoria and those of our investigation support the hypothesis that IUGR affecting one of the MC twins could be caused by an impaired placental transport of amino acids rather than by an intertwin transfusion (Bajoria et al., Reference Bajoria, Sooranna, Ward, D'Souza and Hancock2001).
Additionally, a trend for increase of sphingosine was found in sIUGR twin neonates with UA Doppler alterations compared with AGA co-twins. Sphingosine is an aliphatic amino alcohol component of all sphingoglycolipids, most of which are localized on the cell surface and are involved as second messengers in a variety of cellular signaling pathways such as for cellular growth, differentiation, and migration. Sphingosine 1-phosphate, generated from sphingomyelin, is also expressed in the cardiovascular system and may be involved in the pathophysiology of diseases associated with endothelial dysfunction (Alewijnse & Peters, Reference Alewijnse and Peters2008; Mulders et al., Reference Mulders, Mathy, Meyer, Heringdorf, Ter Braak, Hajji and Peters2009; Spijkers et al., Reference Spijkers, Alewijnse and Peters2010). Because of their vasoactive properties, sphingolipids could play a pivotal role in hypertension, exemplified by the fact that the pharmacological modulation of vascular sphingoglycolipid composition in animal models induces pronounced transient contractile responses in several vessels; in particular, an elevation of ceramide, precursor of sphingosine, might induce a marked endothelium-dependent release of thromboxane A2, involved in hypertension, and exocytosis of coagulation, pro-inflammatory and vasoactive substances (Chatterjee, Reference Chatterjee1998; Daum et al., Reference Daum, Grabski and Reidy2009; Fenger et al., Reference Fenger, Linneberg, Jørgensen, Madsbad, Søbye, Eugen-Olsen and Jeppesen2011; Spijkers et al., Reference Spijkers, Van den Akker, Janssen, Debets, De Mey, Stroes and Peters2011).
In this study, increased glycerophosphocholine (GPC) sera concentrations were found in sIUGR Group 1 twins, compared with AGA co-twins. This finding could be explained by the need for the sIUGR fetus to use energy reserves due an insufficient supply. It is well known, indeed, that when energy requirements exceed energy availability, body fat is mobilized as a physiological mammalian process. Excessive mobilization leads to metabolic stress, which is thought to play a major role in the increased occurrence of food intake (Easter et al., Reference Easter, Patton and McCarthy1971).
Although our study is limited by the very small sample size (partially explained by the rarity of the targeted condition in MDCA twins), and by the lack of cross-sectional observations that did not permit a longitudinal monitoring of the affected neonates, we believe that both echocardiography and metabolomic data could provide a good starting point for future more in-depth investigations of sIUGR in MDCA fetuses.
Conclusions
This study showed that aortic wall thickening is higher in sIUGR twins with UA Doppler velocimetry alterations than in sIUGR twins without UA Doppler anomalies or AGA co-twins. Moreover, an upregulation of serum phenylalanine, sphingosine, and GPC levels in sIUGR with UA Doppler waveforms was observed compared with AGA co-twins. Although no statistical significance was reached for metabolomic data due to the small number of investigated cases, we believe that our pilot study represents a valid starting point for future in-depth metabolomic and proteomic investigations of sIUGR in MDCA fetuses.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/thg.2013.33.