Over the past decades, the incidence of twin pregnancies has increased mainly due to advanced maternal age and the broader use of assisted reproductive technologies (ART; Martin et al., Reference Martin, Hamilton and Osterman2012). As twins face higher risks of fetal chromosomal and structural anomalies than singletons (Chauhan et al., Reference Chauhan, Scardo, Hayes, Abuhamad and Berghella2010), prenatal screening and invasive diagnostic testing are essential for twin pregnancies. However, it is hard for patients to choose from prenatal screening or diagnostic testing. Prenatal screening has the advantage of being a noninvasive approach, but the accuracy is limited in twin gestations. One meta-analysis concluded that the performance of cell-free DNA (cfDNA) testing for trisomy 21 in twin pregnancies was comparable to that in singleton pregnancies, whereas the number of cases of trisomies 18 and 13 was too small for estimation (Gil et al., Reference Gil, Galeva, Jani, Konstantinidou, Akolekar, Plana and Nicolaides2019). The failure rate for cfDNA testing in twins was higher than that reported in singletons (Bevilacqua et al., Reference Bevilacqua, Gil, Nicolaides, Ordonez, Cirigliano, Dierickx, Willems and Jani2015; Sarno et al., Reference Sarno, Revello, Hanson, Akolekar and Nicolaides2016). On the other hand, invasive diagnostic testing remains the standard for determining fetal genetic outcomes, but procedure-related complications, especially the risk of pregnancy loss, are of keen concern.
Amniocentesis is the most common diagnostic procedure that implements percutaneous transabdominal puncture of the uterus to obtain amniotic fluid. In the Cochrane database, the spontaneous miscarriage rate after amniocentesis in singleton gestations spans between 0.13% and 0.33%, while there are no data for twin gestations (Alfirevic et al., Reference Alfirevic, Navaratnam and Mujezinovic2017). The risk of pregnancy loss in twin gestations reported in the last 20 years varies from 0% to 4.2% (Antsaklis et al., Reference Antsaklis, Souka, Daskalakis, Kavalakis and Michalas2002; Cahill et al., Reference Cahill, Macones, Stamilio, Dicke, Crane and Odibo2009; Chen et al., Reference Chen, Liu, Xia, He, Wang, Li, Lai, Liu and Zhang2020; Daskalakis et al., Reference Daskalakis, Anastasakis, Papantoniou, Mesogitis and Antsaklis2009; Dechnunthapiphat et al., Reference Dechnunthapiphat, Sekararithi, Tongsong, Wanapirak, Piyamongkol, Sirichotiyakul, Tongprasert, Srisupundit, Luewan, Jatavan and Traisrisilp2020; Kalogiannidis et al., Reference Kalogiannidis, Petousis, Prapa, Dagklis, Karkanaki, Prapas and Prapas2011; Kan et al., Reference Kan, Lee, Leung, Chan, Tang and Chan2012; Kim et al., Reference Kim, Moon, Kang, Jung, Chang, Ki, Kim and Ahn2019; Krispin et al., Reference Krispin, Wertheimer, Trigerman, Ben-Haroush, Meizner, Wiznitzer and Bardin2019; Lenis-Cordoba et al., Reference Lenis-Cordoba, Sanchez, Bello-Munoz, Sagala-Martinez, Campos, Carreras-Moratonas and Cabero-Roura2013; Millaire et al., Reference Millaire, Bujold, Morency and Gauthier2006; Simonazzi et al., Reference Simonazzi, Curti, Farina, Pilu, Bovicelli and Rizzo2010; Sperling et al., Reference Sperling, Zlatnik, Norton and Currier2019; Supadilokluck et al., Reference Supadilokluck, Tongprasert, Tongsong, Wanapirak, Piyamongkol, Sirichotiyakul and Srisupundit2009; Toth-Pal et al., Reference Toth-Pal, Papp, Beke, Ban and Papp2004; Yukobowich et al., Reference Yukobowich, Anteby, Cohen, Lavy, Granat and Yagel2001). One recent systematic review demonstrated that the pooled pregnancy loss rate before 24 weeks and within 4 weeks after the amniocentesis procedure in twin pregnancies were 2.1% and 2.1%, respectively, which were as high as the background risk in twin pregnancy without undergoing the procedure (Di Mascio et al., Reference Di Mascio, Khalil, Rizzo, Buca, Liberati, Martellucci, Flacco, Manzoli and D’Antonio2020).
However, there is still no consensus on the definition of pregnancy loss in published studies. It varies in the mechanism of loss (fetal death or miscarriage) and the number of fetuses involved (one or both). The intervals of pregnancy loss are recorded as loss before 24−28 weeks’ gestation, within 2−4 weeks after the procedure and overall loss rate at any gestational age. Some studies excluded fetuses with structural and chromosomal anomalies and defined outcomes as procedure-related pregnancy loss, while others included abnormal fetuses and estimated the pregnancy loss rate. In addition, some studies spanned a long time period and thus the amniocentesis technique was not conserved well. Sample sizes are also considered a contributing factor to this issue, ranging from 87 to 476, and are relatively small regarding the low chance of pregnancy loss. All those limitations make it hard for individual counseling.
This study aimed to assess the outcomes of twin pregnancies where amniocentesis was performed following the standard protocol in a tertiary referral center to give a more reliable pregnancy and procedure-related loss rate to help prenatal counseling.
Materials and Methods
This retrospective study was carried out at the Department of Fetal Medicine, Shanghai 1st Maternity and Infant Hospital of Tongji between December 2010 and December 2019. The study included patients with twin pregnancies who underwent amniocentesis between 15 and 24 weeks’ gestation and had two live fetuses at the time of the procedure. Exclusions were monoamniotic twin pregnancies, intrauterine procedures before amniocentesis (e.g. selective fetal reduction or CVS, amniocentesis in other hospitals) and puncture made only in a single sac.
In the current study, the indications for amniocentesis were advanced maternal age (≥35 years), abnormal ultrasound findings (including thickening of nuchal translucency [NT] ≥3.0 mm, soft markers, structural anomaly or fetal growth restriction), suspected TORCH infection, chromosomal aberration in parents or abnormal pregnancy history with congenital anomalies, genetic disease or recurrent miscarriage. All pregnant women with indications were referred to the twins’ clinic in the fetal medicine department and followed by a standard protocol. Serum analytes were assayed, including C reaction proteins, blood routine examinations and coagulation indexes. Patients also received detailed ultrasound assessments, including fetal biometry, sex, anatomy, position, placental location and cord insertion to help distinguish two fetuses. Cervical length was also measured before amniocentesis. Patients whose cervical lengths were below 25 mm were counseled with a higher risk of pregnancy loss (Conde-Agudelo et al., Reference Conde-Agudelo, Romero, Hassan and Yeo2010; Conde-Agudelo & Romero, Reference Conde-Agudelo and Romero2014). Genetic counseling was then offered regarding the process and possible risks of amniocentesis for twins, and the limitations and benefits of the diagnostic genetic testing. Patients who decided to perform amniocentesis were required to sign an informed consent sheet.
All amniocentesis procedures were performed between 15 and 24 weeks of gestation by three well-trained maternal–fetal medicine specialists at our center. The procedure was guided by continuous ultrasound and performed double insertion techniques using two disposable 22-gauge needles. Each amniotic sac was punctured separately regardless of chorionicity. The first 1–2 mL of amniotic fluid aspirated was discarded to avoid maternal cell contamination. The final amount of aspirated fluid was determined by the need for genetic tests. At our center, dyes were not used to confirm that separate sacs were sampled. After the procedure, fetal heart rates were assessed sonographically. Patients were asked to rest for 30 min to observe whether abdominal pain, severe uterine cramping, vaginal bleeding or fluid occurred.
The following baseline characteristics were obtained from the medical records: maternal age, body mass index (BMI), gravidity, parity, conception mode, chorionicity, cervical length and indications for amniocentesis. Pregnancy loss was defined as the loss of one or two fetuses prior to viability, and thus we only included amniocentesis cases performed until 24 weeks gestation. Pregnancy loss rates within 1, 2 or 4 weeks after amniocentesis were assessed. Cases that underwent selective feticide or termination of pregnancy within 1, 2 or 4 weeks after amniocentesis were excluded, respectively, when calculating. Pregnancy loss within 4 weeks after amniocentesis was stratified by advanced maternal age, conception mode, BMI, parity, chorionicity, placental position, cervical length and ultrasound results. We also estimated pregnancy loss rate before 24 weeks’ gestation that underwent amniocentesis before 22 weeks’ gestation as most published studies did (Antsaklis, Reference Antsaklis, Souka, Daskalakis, Kavalakis and Michalas2002; Cahill et al., Reference Cahill, Macones, Stamilio, Dicke, Crane and Odibo2009; Daskalakis et al., Reference Daskalakis, Anastasakis, Papantoniou, Mesogitis and Antsaklis2009; Dechnunthapiphat et al., Reference Dechnunthapiphat, Sekararithi, Tongsong, Wanapirak, Piyamongkol, Sirichotiyakul, Tongprasert, Srisupundit, Luewan, Jatavan and Traisrisilp2020; Kalogiannidis et al., Reference Kalogiannidis, Petousis, Prapa, Dagklis, Karkanaki, Prapas and Prapas2011; Kan et al., Reference Kan, Lee, Leung, Chan, Tang and Chan2012; Kim et al., Reference Kim, Moon, Kang, Jung, Chang, Ki, Kim and Ahn2019; Lenis-Cordoba et al., Reference Lenis-Cordoba, Sanchez, Bello-Munoz, Sagala-Martinez, Campos, Carreras-Moratonas and Cabero-Roura2013; Millaire et al., Reference Millaire, Bujold, Morency and Gauthier2006; Simonazzi et al., Reference Simonazzi, Curti, Farina, Pilu, Bovicelli and Rizzo2010; Toth-Pal et al., Reference Toth-Pal, Papp, Beke, Ban and Papp2004) to help compare results. Follow-ups of pregnancy outcomes were informed by electronic medical records or by phone calls.
Statistical analysis was performed using SPSS Statistics version 23.0 (IBM SPSS Statistics for Windows, IBM Corp., Armonk, NY, USA). Continuous variables were compared using the t test, and categorized variables were compared using chi-square or Fisher’s exact test, as appropriate. Two-sided p values ≤ .05 were considered statistically significant. This retrospective database study was considered exempt from ethical approval.
Results
Out of 838 twin pregnancies that underwent amniocentesis through double needle technique, 678 were performed between 15 and 24 weeks’ gestation. Complete follow-ups were achieved in 575/678 (84.8%) of patients, as presented in Figure 1. Baseline characteristics of our population are shown in Table 1. The average age of patients was 33.65 ± 4.50 years, and the proportion of advanced maternal age (≥35 years) was 40.8%; 495 (73.0%) patients were nulliparous and 388 (60.7%) were conceived after ART. Regarding chorionicity, 527 (77.7%) were dichorionic diamniotic (DCDA) and 151 (22.3%) were monochorionic diamnioti (MCDA). All patients underwent cervical length measurement just before the procedure, while 22 (1.9%) cases were not recorded. The mean cervical length was 38.19 ± 6.15 mm, ranging from 23 to 64 mm. The gestational age at procedure was 19.99 ± 1.69 weeks of gestation. Indications for performing the procedure are also illustrated in Table 1. Abnormal ultrasound findings were the most common indication.
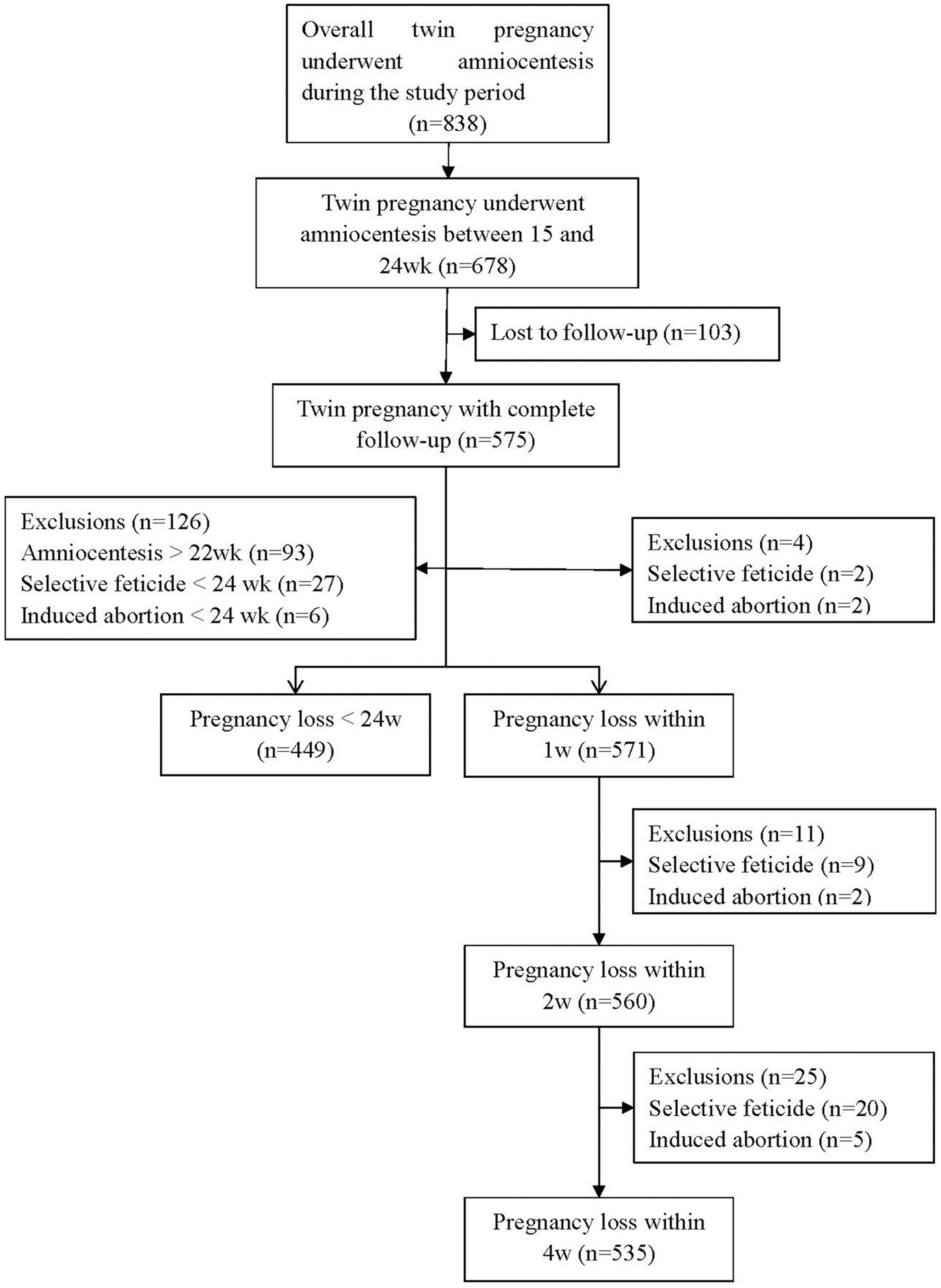
Fig. 1. Flow chart of subject eligibility.
Table 1. Baseline characteristics of women with twin pregnancies who underwent amniocentesis
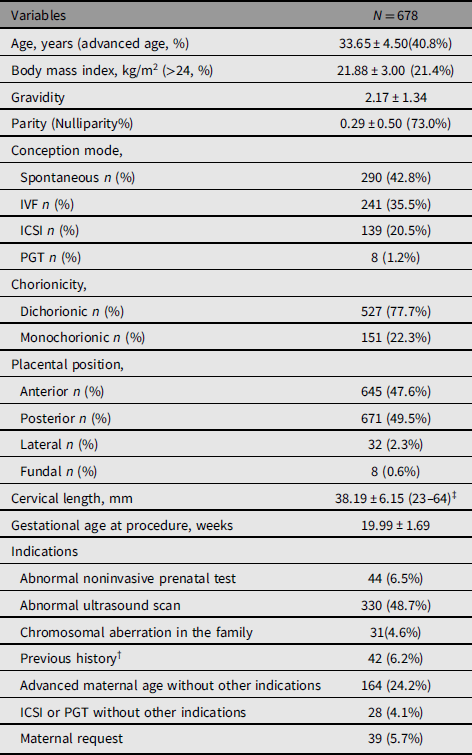
Note: †Previous child or fetal with chromosomal or congenital anomaly or RSA; ‡22 missing data. ICSI, intracytoplasmic sperm injection; PGT, preimplantation genetic testing.
After amniocentesis, 10 cases experienced intrauterine fetal death (IUFD) of one fetus within 4 weeks after the procedure, of which 7 cases were DCDA, including 3 cases complicated with severe FGR, 2 cases with structural anomalies, and 1 case with fetal genetic result of 21 trisomy. One case had preterm premature rupture of the membrane (PPROM) and subsequent IUFD of one fetus 3 days after amniocentesis at 19 weeks’ gestation, and the remaining twin was delivered at 37 weeks’ gestation. The patient’s cervical length before the procedure was 30 mm. Among the other three MCDA cases, two were complicated with selective intrauterine growth restriction and one was complicated with structural anomalies. As illustrated in Table 2, the pregnancy loss rate was 0.5%, 0.7% and 1.9% within 1, 2 and 4 weeks after the procedure, respectively. The pregnancy loss before 24 weeks’ gestation was 0.9%. Only one fetal loss case was regarded as merely related to the procedure due to PPROM.
Table 2. Procedure-Related pregnancy loss of amniocentesis
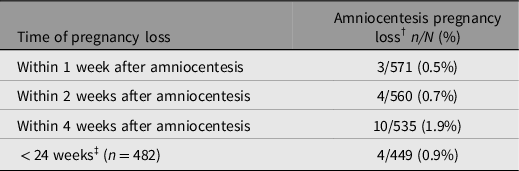
Note: †Pregnancy loss, defined as the loss of one or two fetuses; ‡<24 weeks, only included women who underwent amniocentesis before 22 weeks’ gestation.
When risk factors of pregnancy loss rate within 4 weeks after the procedure were investigated, twin pregnancies with abnormal ultrasound findings had a significantly higher rate of pregnancy loss with a relative risk of 4.81 (95% CI [1.03, 22.2], p = .049), whereas other risk factors, including maternal age, conception mode, chorionicity, placental position and cervical length, were not associated with the rate of pregnancy loss (see Table 3).
Table 3. Relative risk of pregnancy loss rates (within 4 weeks after amniocentesis) based on univariate analysis
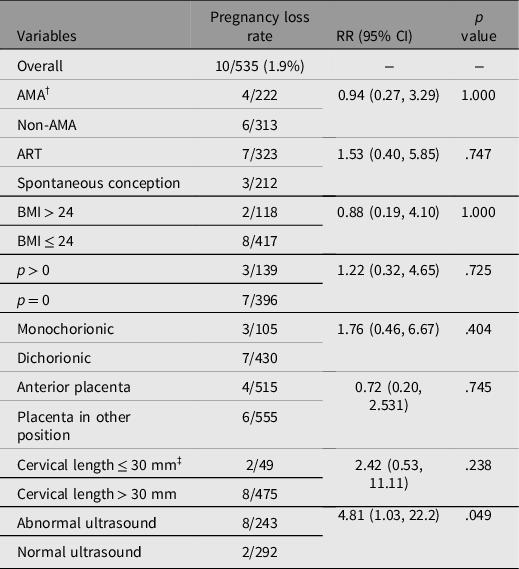
Note: †AMA, advanced maternal age; ‡11 missing data; RR, relative risk.
During the internal study period from 2014 to 2017, there were 1065 twin pregnancies without invasive testing and intrauterine interventions at our center. Twenty-three patients had miscarriage of both twins, and two patients had one fetus intrauterine death between 15 and 24 weeks of gestation. The background pregnancy loss rate (one or both fetuses) was 2.3% (25/1065).
Discussion
In the era of noninvasive prenatal testing, invasive diagnosis techniques have the advantage of obtaining precise genetic information of each fetus through karyotyping, chromosomal microarray analysis, or even whole exome sequencing. Since the fetal risk for genetic abnormalities must be carefully weighed against the risk of pregnancy loss associated with amniocentesis, it is essential to counsel pregnant women using procedure-related risks based on data from local centers. Our study reviewed data in the past 10 years, and the results suggested that the pregnancy loss rates within 4 weeks after amniocentesis and before 24 weeks’ gestation in twin pregnancies were 1.9% and 0.9%, respectively. The pregnancy loss rate only related to the procedure was 0.2%.
Our results are in accordance with or even lower than those reported in recent studies. In the recent meta-analysis, the pooled rates of fetal loss in twin pregnancies after amniocentesis within 4 weeks and before 24 weeks’ gestation were 45/1932 (2.1%, 95% CI [1.5, 2.9]) and 59/2439 (2.1%, 95% CI [1.4, 2.9]), respectively (Di Mascio et al., Reference Di Mascio, Khalil, Rizzo, Buca, Liberati, Martellucci, Flacco, Manzoli and D’Antonio2020). A more recent study of twin pregnancies not in the meta-analysis included 332 twin pregnancies undergoing amniocentesis, and the pregnancy loss rate before 24 weeks was 3.0% (Dechnunthapiphat et al., Reference Dechnunthapiphat, Sekararithi, Tongsong, Wanapirak, Piyamongkol, Sirichotiyakul, Tongprasert, Srisupundit, Luewan, Jatavan and Traisrisilp2020). Chen et al. (Reference Chen, Liu, Xia, He, Wang, Li, Lai, Liu and Zhang2020) reported that the pregnancy loss rate before 28 weeks in 418 twin pregnancies was 1.91%. The pregnancy loss rate only related to the procedure in our study was the lowest among all the studies, mainly due to sticking to the standard protocol that helped exclude potential infection and cervical incompetence.
Only a few studies introduced comparison groups of twin pregnancies without undergoing amniocentesis, and usually the maternal baseline of the control group did not match that of the amniocentesis group. Sperling et al. (Reference Sperling, Zlatnik, Norton and Currier2019) reported a multicenter cohort including 861 women with twin pregnancies screened positive. They were all offered amniocentesis, while 274 (31.8%) accepted and the rest declined. The rates in the accepted and declined group were 8.8% versus 6.8% with adjusted OR 1.32 (0.66−1.91), suggesting that amniocentesis did not appear to increase the risk of pregnancy loss further. Moreover, the recent meta-analysis reported that there was no significant difference between twin pregnancies who underwent amniocentesis and those who did not when focusing on either fetal loss before 24 weeks of gestation (OR 1.59, p = .06) or fetal loss within 4 weeks from the procedure (OR 1.38, p = .3), indicating that the risk of fetal loss following amniocentesis is lower than that previously reported (Di Mascio et al., Reference Di Mascio, Khalil, Rizzo, Buca, Liberati, Martellucci, Flacco, Manzoli and D’Antonio2020). Our study results were lower than the background pregnancy loss rate of 2.3% in the same center and that were published in other literature (range 0.6%−2.8%), suggesting that amniocentesis did not add to increased risk of pregnancy loss.
We also explored some potential risk factors, including maternal (advanced maternal age, ART conception mode, hither BMI, nulliparity, shorter cervical length) and fetal characteristics (monochorionicity, placental position and abnormal ultrasound findings) that might account for an increased risk of fetal loss in twin pregnancies. Only abnormal ultrasound findings were associated with increased risk, similar to that reported by Cahill et al. (Reference Cahill, Macones, Stamilio, Dicke, Crane and Odibo2009). Likewise, some other studies also reported that advanced maternal age, parity, conception mode and chorionicity did not influence pregnancy loss after amniocentesis (Cahill et al., Reference Cahill, Macones, Stamilio, Dicke, Crane and Odibo2009; Chen et al., Reference Chen, Liu, Xia, He, Wang, Li, Lai, Liu and Zhang2020; Daskalakis et al., Reference Daskalakis, Anastasakis, Papantoniou, Mesogitis and Antsaklis2009; Dechnunthapiphat et al., Reference Dechnunthapiphat, Sekararithi, Tongsong, Wanapirak, Piyamongkol, Sirichotiyakul, Tongprasert, Srisupundit, Luewan, Jatavan and Traisrisilp2020; Lenis-Cordoba et al., Reference Lenis-Cordoba, Sanchez, Bello-Munoz, Sagala-Martinez, Campos, Carreras-Moratonas and Cabero-Roura2013). To the authors’ knowledge, there are no earlier reports on cervical length as a risk factor in twin pregnancies after amniocentesis. However, in our cohort, only two patients’ cervical lengths were shorter than 25 mm, and these two patients did not have pregnancy loss after amniocentesis. Thus, more data are needed to answer whether amniocentesis will increase the risk of pregnancy loss in patients with shorter cervical lengths.
One strength of our study is a reliable estimation of the pregnancy loss rate after amniocentesis based on the largest sample size to date. Additionally, all procedures were mainly performed by two well-trained experts of double-needle insertions who followed the standard protocol. The comprehensive maternal baseline and the same performers’ skills through the study period enhanced the reliability of our estimation of pregnancy loss rate and the speculation of potential risk factors. Finally, our study carefully assessed the reasons for pregnancy loss and stratified the risks according to potential risk factors, which helped individual counseling.
There are some limitations in our study. The pregnancy loss rate might be underestimated by the incomplete follow-up (Halliday et al., Reference Halliday, Sheffield, Lumley, Robinson, Renou and Carlin1992). Second, our study lacked a well-designed and baseline-matched control group. Despite our large sample size, the relatively small number of final events limited the precision of stratified risk estimates.
In conclusion, our study showed a low pregnancy loss rate after amniocentesis in twin pregnancies, comparable with results in other international counterparts. The double-needle insertion technique of sampling in twins is a relatively safe procedure for prenatal diagnosis.
Acknowledgment
The authors thank Yixiang Zhang for his constructive comments and input to the manuscript.
Financial Support
This work was supported by the National Key Research and Development Program of China (grant number 2018YFC1002900); National Natural Science Foundation of China (grant number 82071656) and Clinical Research Plan of Shanghai Hospital Development Center (grant number SHDC2020CR6028-005).
Conflict of Interest
None.






