Healthy cognitive functioning is of high value to all members of society because it is important for general wellbeing, and predicts work performance and successful career paths. To promote cognitive health, a major strategy is to reduce the impact of the ‘usual suspects’: physical inactivity, smoking, diabetes and high BP. However, for at least one of these risk factors, sustained high BP, the relationship to cognitive functioning is complex. Reviews on this relationship have yielded mixed conclusions (Novak & Hajjar, Reference Novak and Hajjar2010; Qiu et al., Reference Qiu, Winblad and Fratiglioni2005; Reitz & Luchsinger, Reference Reitz and Luchsinger2007), in keeping with the rather inconclusive findings in the primary studies (e.g., Harrington et al., Reference Harrington, Saxby, McKeith, Wesnes and Ford2000; Lande et al., Reference Lande, Kaczorowski, Auinger, Schwartz and Weitzman2003; Lyngdoh et al., Reference Lyngdoh, Viswanathan, Kobrosly, van Wijngaarden, Huber, Davidson and Bovet2013; Tzourio et al., Reference Tzourio, Dufouil, Ducimetiere and Alperovitch1999). Here, we address the heterogeneity in these results. To date, whether cognitive functioning actually benefits from the reduction of high BP remains an open question.
A limitation of the vast body of the studies done so far is that they mainly examine the association between BP and cognition in older samples. This is understandable in view of the relatively large number of hypertensives among the elderly (Harrington et al., Reference Harrington, Saxby, McKeith, Wesnes and Ford2000) and the large societal burden caused not just by neurodegenerative disease, but also by normal age-related cognitive decline. In the elderly, there are sensible mechanistic explanations for a negative association between high BP and cognitive functioning. A currently high BP level is the best predictor of prolonged past exposure to high BP. Sustained exposure to high BP increases the risk of atherosclerotic changes in intracerebrovascular vessels that result in a reduction of vessel number and diameter, leading to reduced vascular reserve and perfusion of the brain (Novak & Hajjar, Reference Novak and Hajjar2010; Pires et al., Reference Pires, Ramos, Matin and Dorrance2013). On the neurological level, this results in brain atrophy, white matter lesions, and possibly enhances Alzheimer-like pathology (Schmidt et al., Reference Schmidt, Fazekas, Koch, Kapeller, Augustin, Offenbacher and Lechner1995; Skoog, Reference Skoog1998; van Vliet et al., Reference van Vliet, Westendorp, van Heemst, de Craen and Oleksik2010; Waldstein, Reference Waldstein2003).
In spite of the face validity of high BP being detrimental for cognitive functioning, the actual association between BP and cognitive functioning in the elderly is more complex. First, high BP may be beneficial, possibly to compensate for loss of neural cell functioning (Anson & Paran, Reference Anson and Paran2005; Novak & Hajjar, Reference Novak and Hajjar2010). Second, in older people, BP may be lowered as a result of neurological disorders and brain lesions, which may have resulted from high BP in the first place (van Vliet et al., Reference van Vliet, Westendorp, van Heemst, de Craen and Oleksik2010). Third, apart from high BP, low BP has also been shown to have disadvantageous effects on cognitive functioning in elderly subjects (Hu et al., Reference Hu, Li, Colditz, Willett and Manson2003; Owen et al., Reference Owen, Healy, Matthews and Dunstan2010). Low BP may compromise autoregulatory processes such that elderly people are no longer capable of maintaining sufficient blood flow to neuronal tissue. This reduced perfusion causes reduced cortical activation, both during tonic states as well as when cognitive performance demands extra activation (Duschek & Schandry, Reference Duschek and Schandry2007). Taken together, this indicates that the association between BP and cognition is more complex, possibly because the relation is non-linear across the life span.
Despite the evidence for associations of BP and atherosclerotic processes with cognition, generalization of these results is difficult, because most studies have mainly involved elderly subjects. Much less is known about the association between BP and cognitive functioning in samples that are representative of the population at large, which should include adolescent, young adult, and middle-aged participants as well. Moreover, while general cognitive impairment (usually measured by the Mini-Mental State Examination, also known as MMSE) and memory loss have been the major focus of these studies in the elderly, other cognitive domains are relevant as well. Hypertension is related to brain function through reduced blood flow and metabolism, particularly in frontal, temporal, and subcortical parts of the brain (Waldstein, Reference Waldstein2003), suggesting possible different sensitivity of brain areas. This may explain why the majority of findings on specific cognitive domains find effects of BP on functions like memory, attention, and executive functions (Duschek & Schandry, Reference Duschek and Schandry2007; Gifford et al., Reference Gifford, Badaracco, Liu, Tripodis, Gentile, Lu and Jefferson2013; Mitchell et al., Reference Mitchell, van Buchem, Sigurdsson, Gotal, Jonsdottir, Kjartansson and Launer2011; Waldstein, Reference Waldstein2003), but that learning, verbal, spatial, and emotional abilities may be less affected. This means that different cognitive functions that depend on separate areas of the brain can be differentially susceptible to high BP, stressing the need to verify effects across cognitive domains (Birns et al., Reference Birns, Morris, Donaldson and Kalra2006).
A final complication to take into account when studying the association between cognition and BP is that both may be influenced by one or more ‘third’ factors, which could either have detrimental or beneficial effects on both, or have a detrimental effect on, for example, BP, but a beneficial effect on cognition. The latter could lead to the absence of a clear association even in the presence of true causal effects of BP on cognition. A clear example of the former ‘third’ factor influencing both cognition and BP in a detrimental way is age (Deary et al., Reference Deary, Corley, Gow, Harris, Houlihan, Marioni and Starr2009; Franklin, Reference Franklin1999). Hence, age effects need to be controlled for rigorously. An environmental third factor that could detrimentally impact on both cognitive functioning and BP is parental socio-economic status (SES). As compared to individuals from high SES backgrounds, individuals from low SES backgrounds tend to more often exhibit lifestyles (e.g., smoking) that may influence both cognitive functioning and BP negatively (Adler & Ostrove, Reference Adler and Ostrove1999). Third factors may also be of genetic origin: pleiotropic genetic variation may impact on common neurobiological pathways that influence both BP and cognitive functioning (Herrera et al., Reference Herrera, Pasion, Tan, Moran and Ruiz-Opazo2013; Obisesan, Reference Obisesan2009). This might reflect shared common neurobiological pathways, or may indicate there are independent effects on both BP and cognitive functioning (Waldstein, Reference Waldstein2003). A design that is optimally suited to address the influence of third factors is an MZ within-pair difference design. MZ twins have the same age and sex, share (nearly) 100% of their segregating genes and a large part of their childhood environment. If the MZ twin with a higher BP than the co-twin also has reduced cognitive functioning, this would argue in favor of a detrimental effect of high BP that is causal, as this design controls for the impact of age, shared environment, and genotype (e.g., de Moor et al., Reference de Moor, Boomsma, Stubbe, Willemsen and de Geus2008).
In the present study, we systematically investigate the association between current levels of SBP and DBP on age-dependent normal cognitive functioning while taking into account the sources that may have led to mixed results across previous studies. We allow the association between BP and cognitive functioning to be non-linear, such that both low and high BP constitute a risk. We further allow the sensitivity to the effects of BP to depend on the cognitive domains. Third, we test the relation between BP and cognition in a design that controls maximally for ‘third factors’ of genetic and/or environmental origin. To this end, we assessed BP and cognitive performance in participants from a large population-based twin-family sample ranging from children to elderly individuals across a wide range of well-defined neurocognitive domains. In a subsample of 123 MZ twin pairs, we ordered the twins within pairs according to BP levels and tested if the twin with the higher BP differed from the co-twin on any of the cognitive domains. Because MZ twins share both their genomic information and part of their environment, this analysis controls for genetic pleiotropy and the influence of shared environmental factors — for example, (parental) socio-economic status — on both traits.
Materials and Methods
Participants
In total, there were 1,140 participants, mainly (n = 1,110) recruited from 431 families from the Netherlands Twin Register (NTR; Boomsma et al., Reference Boomsma, de Geus, Vink, Stubbe, Distel, Hottenga and Willemsen2006; van Beijsterveldt et al., Reference van Beijsterveldt, Groen-Blokhuis, Hottenga, Franic, Hudziak, Lamb and Boomsma2013; Willemsen et al., Reference Willemsen, Vink, Abdellaoui, den, van Beek, Draisma and Boomsma2013). The other 30 participants were university students. Age of the participants ranged from 10 to 86 years old (668 female, 472 male).
All participants took part in one of two studies in which measurements of BP and cognitive performance were collected, referred to as the twin-family study and the twin-sibling study. The twin-family study sample consisted of 864 family members from 341 families. The majority of these participants were part of a twin pair (438) or siblings of twins (78). The rest were family of these twins and siblings, including parents (160 mothers, 126 fathers), children (9) and partners (53) of twins and siblings. In addition to this family sample, 30 university students participated in the pilot phase and were tested according to the twin-family protocol. The sample of the twin-sibling study consisted of 176 adolescent twins and 70 siblings from 89 families who participated in the third wave of assessment in a longitudinal study on development of brain and cognition (BrainScale study; van Soelen et al., Reference van Soelen, Brouwer, Peper, van Leeuwen, Koenis, van Beijsterveldt and Boomsma2012).
Procedure
Studies and procedures of the twin-family study were approved by the Medical Ethics Review Committee of the Vrije Universiteit Medical Center Amsterdam, and by the Central Committee on Research Involving Human Subjects for the twin-sibling study. First, participants were approached by mail. In a structured phone call interview, participants were asked if they were willing to participate; if necessary, additional information about the study was provided, and possible exclusion criteria were explored. After establishing an appointment, confirmation letters were sent, which included written informed consent forms and questionnaires. Consent forms were signed and returned at the appointment, after participants were given a full explanation of the procedure. Medication use and demographic data such as education were registered, and a reading test was administered.
In the twin-family study, testing took place at the Vrije Universiteit Amsterdam laboratory (n = 358) or at the participants’ home (n = 536). A baseline measurement of BP was taken for all participants during the first (n = 885) and second (n = 883) designated break point of the cognitive test battery, and for the more recent and largest part of the participants, an additional measurement was taken prior to the start of cognitive testing (n = 764).
In the twin-sibling study, testing took place in the University Medical Center Utrecht. As part of an extensive study that included MRI scans of the brain, the same cognitive test protocol was assessed as in the twin-family study, but only a single measurement of BP was taken.
The administration procedure of the Computerized Neuropsychological Battery (CNB) was kept similar across studies and locations. The battery was installed on Macbooks to enable offline administration during home visits. The test administrator would read the instructions to the participant (who could read them on the screen), and monitor as to whether the participant understood the instructions and practice trials. Test scores were later uploaded to the Pennsylvania servers for data storage and automated validation. Breakpoints were at specific moments; for example, after cognitively demanding tests (shown in Table 1). Test administration was not interrupted at other moments.
TABLE 1 Tests and Their Cognitive Measure and Domain, Given in Order of Assessment
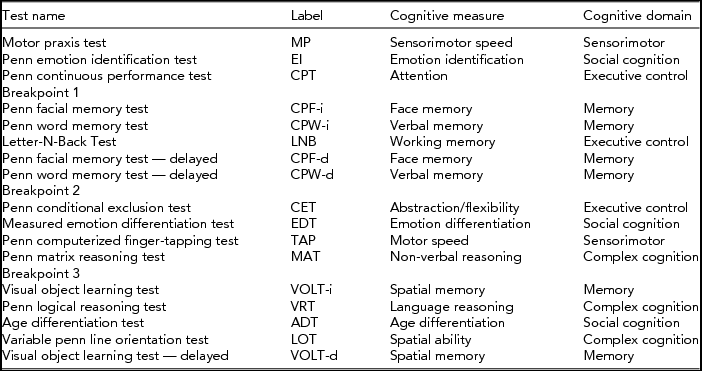
Materials
Blood pressure
BP was measured from the brachial artery in the arm by a digital BP monitor (Omron). Participants were in a sitting position. Both SBP and DBP were recorded. Depending on the study sample and study phase (see procedure), BP was measured one, two, or three times. The intercorrelations between DBP measurements ranged from 0.78 to 0.85 and between SBP measurements from 0.87 to 0.91, indicating the reliability of both BP measures.
Cognitive performance
Cognitive performance was assessed through the Dutch translation of the web-based CNB (Gur et al., Reference Gur, Richard, Hughett, Calkins, Macy, Bilker and Gur2010). In short, the CNB includes a total of 17 cognitive tests, generating 33 measures of cognitive of performance in five broad domains (Table 1): 16 measures of accuracy (the number or percentage of correct responses on the cognitive tests), 16 measures of reaction time (median response time in milliseconds for those correct responses), and one measure of motor speed (number of taps on the finger tapping test, TAP). Reaction times were multiplied by -1 so that high cognitive performance was always denoted by a high test score. We refer to Swagerman et al. (Reference Swagerman, de Geus, Koenis, Hulshoff Pol, Boomsma and Kan2015) for a more extensive description of the battery and full descriptives for the performance data.
Statistical Analyses
Invalid scores (~0.9%) and data from children under age 13 (n = 4) were excluded from the present analysis, which comprised two sets of statistical analyses: the first set involved data from the complete sample, the other the data from MZ twin pairs only. In both sets of analyses, DBP values were averaged over the (one, two, or three) measurements. The same held for SBP.
Whole sample analyses
For each of the 33 cognitive measures, the association of cognitive performance with BP was estimated by fitting the path model that is shown in Figure 1. That is, each cognitive performance measure was regressed on DBP or SBP and its mean centered square (DBP2 or SBP2), while regressing out the possible confounding effects of sex, age, and the (mean centered) square of age (age2). The coefficients of the direct paths from the BP variables to the cognitive measure were the parameters of interest. Hence, the entire set of analyses yielded 2 (linear and quadratic)*2 (systolic and diastolic)*33 (cognitive variables) = 132 path coefficient estimates, so 132 test statistics. In view of chance capitalization, while acknowledging Bonferroni correction for multiple testing may be too conservative, we corrected the original significance level (0.05) via Matrix Spectral Decomposition (Li & Ji, Reference Li and Ji2005), which indicated that the data could be described along 23 statistically independent dimensions. This yielded a corrected significance level of: α = 0.05/23 ≈ 0.002 (see also Swagerman et al., Reference Swagerman, de Geus, Koenis, Hulshoff Pol, Boomsma and Kan2015).
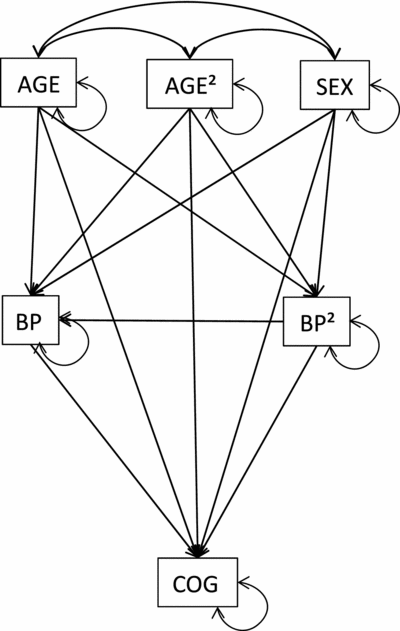
FIGURE 1 Graphical representation of the path model fitted to the data of the whole sample, showing that cognitive test performance (COG) depends on blood pressure (BP) and (mean centered) blood pressure squared (BP2), while regressing out the effects of sex, age, and the mean centered square of age (AGE2).
The path modeling was performed in R using packages lavaan (Rosseel, Reference Rosseel2012) and lavaan.survey (Oberski, Reference Oberski2014). We opted for robust sandwich estimation (in view of the data being skewed) and included family number as a cluster variable (in view of the data being family clustered). To estimate the sensitivity of the effects to antihypertensive medication, all analyses were rerun after excluding all individuals who take any type of antihypertensive medication (60 females, 58 males, mean age 69.08).
Monozygotic twin pairs difference analyses
To control for possible confounding effects other than those of age and sex, including shared environmental and genetic factors, additional analyses were performed using the data from the MZ twins only. Within twin pairs, the MZ twins were ranked on the basis of their BP, such that two groups were formed: a high BP group, consisting of the twins with the relatively high BP, and a low BP group, consisting of the twins with the relatively low BP. Next, we investigated the mean within-pair differences in cognitive performance using paired t tests. The rationale behind such analyses is as follows. As MZ twin pairs are not only of the same age and sex, but also matched on their genotype (since they are 100% genetically identical) and many environmental variables, the differences in cognitive performance between the MZ twins cannot be explained by the variables on which they are matched. Any significant mean group difference in cognitive performance between the groups can thus be attributed to the differences in BP (or non-shared causes thereof). If high BP has a negative effect on cognition, the high BP group is expected to display lower cognitive performance scores (on average) as compared to the low BP group; if BP has a positive effect, the high BP group is expected to score higher. If, despite differences in BP, within-pair differences between cognitive scores are insignificant, one is allowed to conclude that BP does not influence cognitive performance. The ranking, grouping, and subsequent analysis of mean scores was done for SBP and DBP separately.
Two-sided paired t tests were performed in SPSS version 21 (IBM Corp., 2011) separately for DBP and SBP on the 33 cognitive performance scores. In line with the whole sample analysis, we used a corrected significance level of 0.002 to account for the multiple testing in a set of correlated dependent variables. The 287 MZ twins in total included 132 pairs for whom data were complete for both twins. Ranks of mean SBP and DBP were consistent in 96 of these pairs. If both twins had exactly equal mean BP values (three twin pairs had equal mean DBP, one pair equal mean SBP), twins were assigned to the high and low BP groups randomly. If one or both twins of a pair used antihypertensive medication, both twins were excluded from the analyses (five pairs concordant, four pairs discordant for medication use). Ultimately, in total, the analyses included 123 complete twin pairs.
Results
Descriptive Statistics
The mean DBP was 75.49 (SD = 10.98) and the mean SBP 129.51 (SD = 17.02). Mean sex differences in DBP (males: M = 75.83, SD = 11.08; females: M = 75.25, SD = 10.91) were not significant (β = -0.04, SE = 0.03, p = 0.23). Mean sex differences in SBP (males: M = 134.87, SD = 15.87; females: M = 125.76, SD = 16.80) were, however, significant (β = 0.25, SE = 0.03, p < .001). Both DBP and SBP related significantly with age (β = 0.49, SE = 0.03, p < .001; β = 0.48, SE = 0.03, p < .001, respectively).
Whole sample analyses
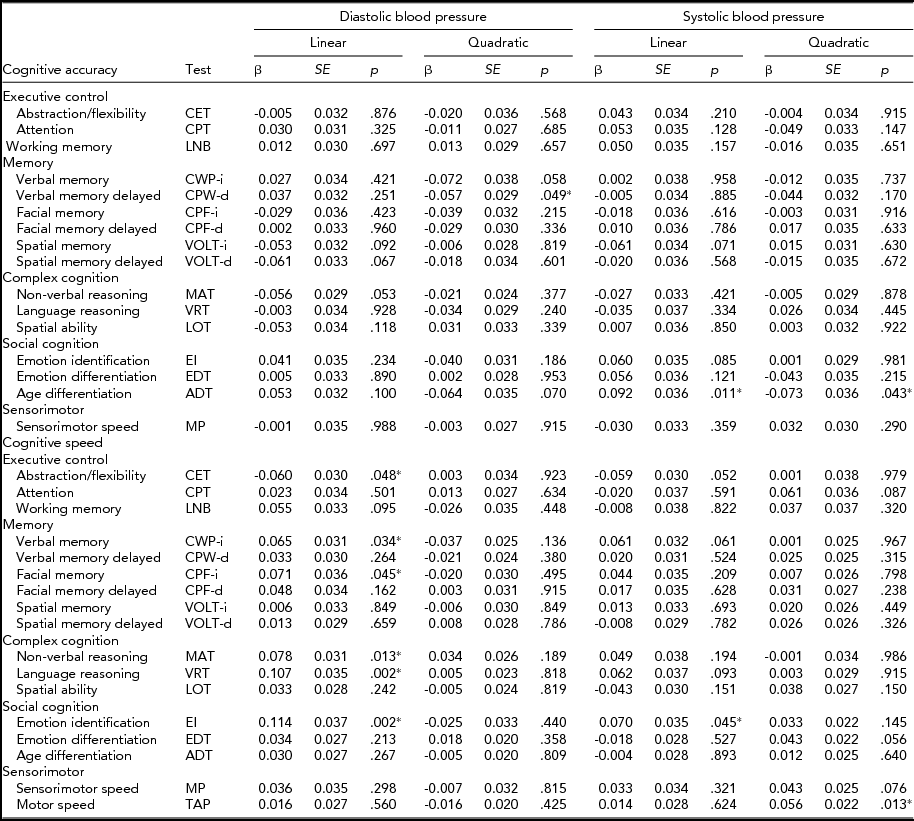
The standardized results of the path modeling, that is, the direct path coefficients of both the linear and quadratic terms of SBP and DBP on cognitive performance and their test statistics, are provided in Table 2 and summarized graphically in Figure 2. These coefficients can be interpreted as age- and sex-corrected effects of current BP on current cognitive performance.
TABLE 2 Age- and Sex-Corrected Linear and Quadratic Effects (β; Direct Path Coefficients in Figure 1) of Diastolic and Systolic Blood Pressure on Cognitive Accuracy and Speed Performance, Including Their Standard Errors (SE) and p Values (p)
Note: *Significant at α = 0.05.
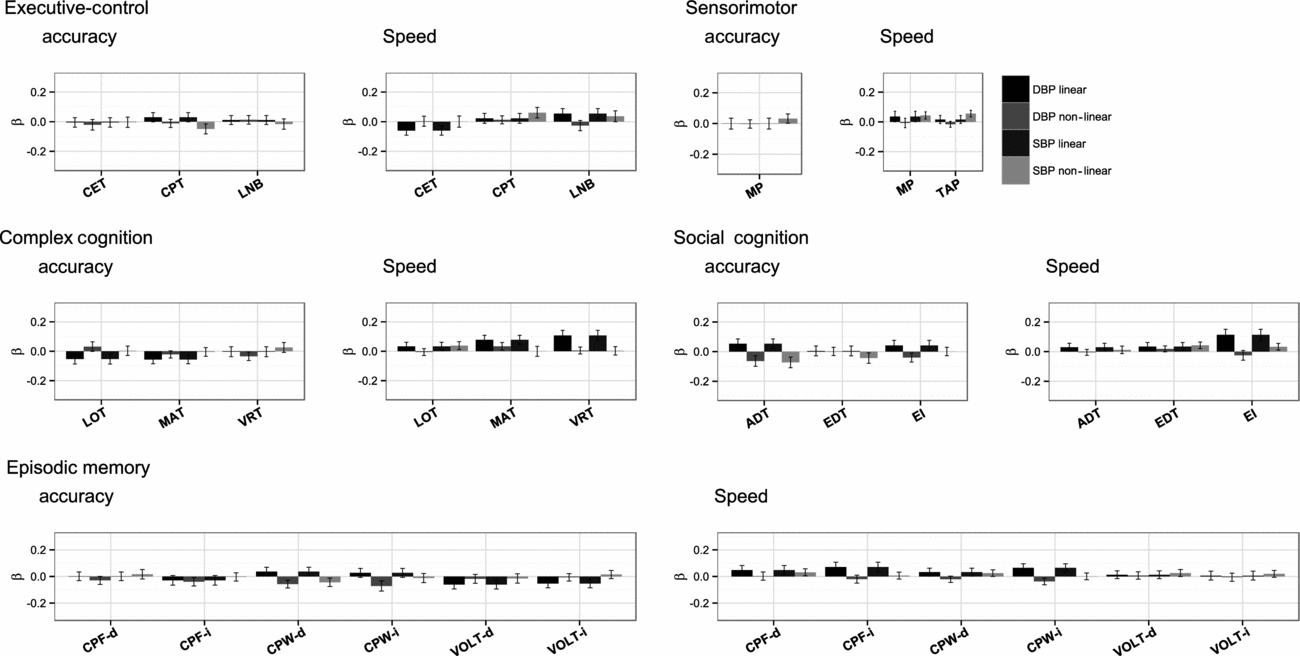
FIGURE 2 Standardized effect size of linear and non-linear effects of diastolic blood pressure and systolic blood pressure on cognitive accuracy and speed, ordered by cognitive domain.
Accuracy
The effects of BP on accuracy were small and distributed around zero. Linear effects of DBP ranged from -0.061 (spatial memory delayed test) to 0.053 (age differentiation test) with a median of 0.002, and those of SBP ranged from -0.061 (spatial memory test) to 0.092 (age differentiation test) with a median of 0.007. Quadratic effects of DBP ranged from -0.072 (verbal memory test) to 0.031 (spatial ability test) with a median of -0.02 and those of SBP ranged from -0.073 (age differentiation test) to 0.032 (sensorimotor test) with a median of -0.004. None of these effects reached the level of significance (α = 0.002). Medication status did not influence the results: nearly identical effect sizes were obtained when the analyses were repeated after removing the 118 individuals taking antihypertensive medication and are therefore not reported.
Speed
Like the effects of BP on accuracy, the effects of BP on speed were small. Linear effects of DBP ranged from -0.060 (abstraction and mental flexibility) to 0.114 (emotion identification test) and were mostly positive, with a median of 0.036. Yet, of those effects, only the linear effects on language reasoning and emotion identification were significant (α = 0.002). This was also true in the repeated analyses after removing individuals taking antihypertensive medication.
Linear effects of SBP were centered around zero again and ranged from -0.059 (abstraction and mental flexibility) to 0.070 (emotion identification test) with a median of 0.017. None of these coefficients were significant.
Quadratic effects of DBP ranged from -0.037 (verbal memory test) to 0.034 (non-verbal reasoning test) with a median of -0.005, and those of SBP ranged from -0.001 (non-verbal reasoning) to 0.061 (attention) with a median of 0.026. These were insignificant as well.
In conclusion, no systematic linear or quadratic association between BP and cognitive functioning was found. There is some evidence of a linear effect of DBP on the speed of language reasoning and emotion identification, but the DBP effects on cognitive speed were not replicated in other tasks that measure performance in the same domain.
Monozygotic twin pairs difference analyses
The results of the additional MZ twin pair analysis are provided in Table 3. The mean BP of the high DBP and SBP groups were significantly higher than mean BP in the group with their co-twins with low DBP and SBP. The mean within-pair differences in cognitive performance were small and for about half of all test scores, they were in the positive direction and for the other half in the negative direction. None of these intrapair MZ differences in cognitive performance remained significant after correction for multiple testing.
TABLE 3 Mean Blood Pressure and Cognitive Performance Scores of the High and Low Diastolic and Systolic Monozygotic Twin Groups, the Mean of the Within Twin Pair Differences (Δ) With Their Standard Deviations, and the Results of the Paired t Tests (t statistic, t; and p value, p)
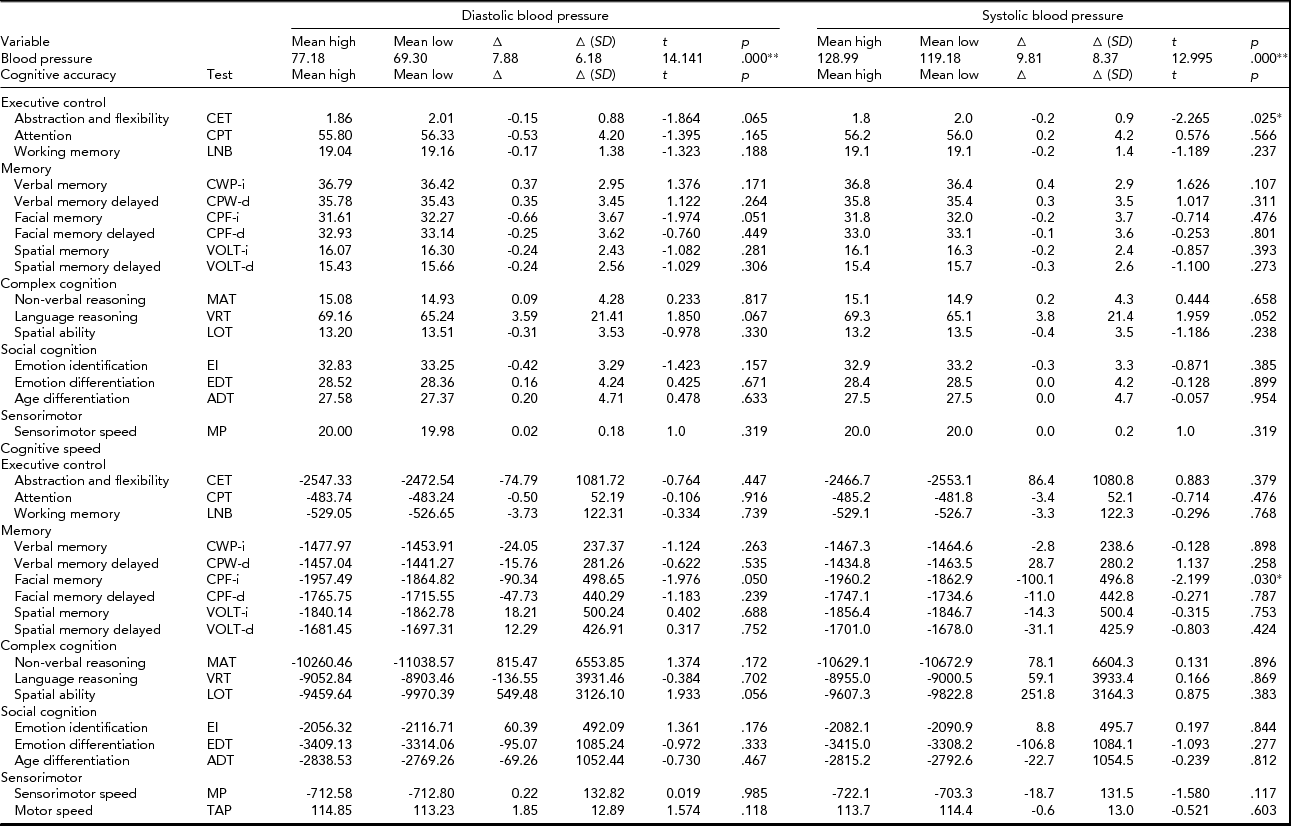
Note: *Significant at α = 0.05, **Significant after correction for multiple testing (α = 0.002).
From the combined sets of analyses, we may derive that in the population at large, associations between BP and cognitive performance are absent when effects of confounding factors like age, sex, genetic pleiotropic, and shared environmental factors are taken into account. We conclude that our analyses show no evidence for a causal relationship between (current) levels of BP on (current) cognitive functioning.
Discussion
We set out to explore the effects of DBP and SBP on cognitive functioning in a population-based sample with a wide age range. We attempted to explain the inconsistencies in studies thus far by allowing BP effects to be curvilinear and to be different across accuracy and speed measures of five cognitive domains. We accounted for confounding of any association by genetic factors and by many shared environmental factors, including parental SES. Our whole sample analyses showed that for the majority of the cognitive tests, very little indication was found that, after correction for substantial age-effects, BP was associated with cognitive test performance. The within-pair analyses in MZ twins also did not suggest any effect of DBP or SBP on cognitive performance.
When we look at the analyses in the whole sample in a liberal way, that is, without any correction for multiple testing, two instances of a possible adverse effect of BP on cognition were found, suggesting a detrimental effect of higher BP on the speed of emotion identification and verbal reasoning. The association with emotion identification is an isolated one as no other studies that we could identify have been conducted on BP and tests of social cognition. Previous findings for BP and language skills have been far from consistent, but a meta-analysis of 12 studies found a possible trend that was in keeping with our result (Gifford et al., Reference Gifford, Badaracco, Liu, Tripodis, Gentile, Lu and Jefferson2013). In view of the large number of cognitive domains tested, these two nominally significant associations most likely reflect false positives. They were not seen for other tasks within the relevant cognitive domains, and none were replicated for SBP.
Our findings were based on a large dataset of participants spanning a large age range, drawn from the general population. This differs from the bulk of the existing literature that focused on either children but mostly on the elderly. Studies in children are limited by the relatively few children showing hypertension, which is then often complicated in those children by comorbid disorders and congenital disease (Cha et al., Reference Cha, Patel, Hains and Mahan2012; Lande et al., Reference Lande, Kaczorowski, Auinger, Schwartz and Weitzman2003; Lyngdoh et al., Reference Lyngdoh, Viswanathan, Kobrosly, van Wijngaarden, Huber, Davidson and Bovet2013). Studies in the elderly are complicated by comorbidity of high BP with other atherosclerotic risk factors like cholesterol, diabetes, body mass index (BMI), immune parameters, and medical or psychiatric disorders, which can all assert an influence on cognitive functioning (Spauwen et al., Reference Spauwen, van Boxtel, Verhey, Kohler, Sep, Koster and Stehouwer2015; van Vliet et al., Reference van Vliet, Westendorp, van Heemst, de Craen and Oleksik2010; Waldstein, Reference Waldstein2003; Walther et al., Reference Walther, Birdsill, Glisky and Ryan2010). These factors can also cause cardiac pathology that itself reduces BP, as seen in heart failure patients, further complicating the association between BP and cognition because too low BP also has disadvantageous effects on cognitive functioning in elderly subjects (Hu et al., Reference Hu, Li, Colditz, Willett and Manson2003; Owen et al., Reference Owen, Healy, Matthews and Dunstan2010). Moreover, the effects of neurodegeneration and dementia are hard to separate from normal cognitive aging. The Institute of Medicine recently defined cognitive aging as a lifelong process of gradual changes in cognitive function that is highly variable across individuals and within individuals, and across cognitive domains (Blazer et al., Reference Blazer, Yaffe and Liverman2015). Animal models show that, in contrast to Alzheimer's disease or other neurodegenerative disorders, there is no loss of neurons with normal aging, but a gradual change in synaptic structure and function. The distinction is relevant because high BP can compensate the loss of neural cell functioning (Anson & Paran, Reference Anson and Paran2005; Novak & Hajjar, Reference Novak and Hajjar2010) and this may have a different impact on normal and neurodegenerative cognitive aging. In view of these complexities, it is perhaps not surprising that meta-analyses of large randomized controlled trials have not uniformly concluded that antihypertensive medication improves cognitive performance in the elderly (McGuinness et al., Reference McGuinness, Todd, Passmore and Bullock2009; Novak & Hajjar, Reference Novak and Hajjar2010).
As a large part of our study sample consisted of adolescents, or young and middle-aged adults, atherosclerotic or neurodegenerative damage will not have been a major confounder, which is a strength of the current study. Repeating analyses in age groups under 30 or over 50 showed virtually the same results (analyses not shown). However, our study also suffered some limitations. The absence of curvilinear effects of BP may mean that we overly complicated our analyses. We still recommend modeling them in future studies, since strong non-linear effects might be present in specific patient groups, as for example found in a subsample of diabetes patients (Spauwen et al., Reference Spauwen, van Boxtel, Verhey, Kohler, Sep, Koster and Stehouwer2015). The largest limitation is the use of only one to three measurements of BP in a sitting condition to characterize BP. This may not be sensitive to detect effects of more complex aspects of BP regulation. A more extensive assessment of BP — for example, also taken while lying down or while standing up to test for postural hypotension (e.g., Kuo et al., Reference Kuo, Sorond, Iloputaife, Gagnon, Milberg and Lipsitz2004) — might have revealed different results for cognition. Finally, our twin design capitalized on intrapair differences in BP in genetically identical individuals, which were modest in size due to the substantial heritability of BP (Hottenga et al., Reference Hottenga, Boomsma, Kupper, Posthuma, Snieder, Willemsen and de Geus2005).
In summary, associations between cognitive functioning and DBP and SBP were absent for the vast majority of the 33 cognitive accuracy or speed measures, most notably in a well-matched within-twin pair comparison of MZ twins. The few nominally significant associations between specific test scores and BP most likely reflect chance findings. We conclude that across a wide age range in the population at large, BP level is not associated with cognitive functioning in a clinically meaningful way. This does not detract from the possible value of treating BP to prevent atherosclerotic and neurodegenerative disease in older age, but it also suggests that there are no immediate cognitive benefits from lowering BP per se.
Acknowledgments
We thank all the participants. This work was supported by the Netherlands Consortium for Healthy Ageing (NCHA), the Netherlands Organization for Scientific Research (NWO, 433-09-220), European Research Council (ERC 230374), and the Neuroscience Campus Amsterdam (NCA).







