Multiple pregnancy is the result of complex interactions between genetic and environmental factors. Apart from hereditary factors, its unequivocal risk factors are the use of assisted reproductive technology (ART), older maternal age, and advanced parity (Bortolus et al., Reference Bortolus, Parazzini, Chatenoud, Benzi, Bianchi and Marini1999). Monozygotic twinning appears to be constant (approximately 4/1,000 births) but dizygotic twinning varies significantly across races, and demonstrates geographical and temporal trends (Bortolus et al., Reference Bortolus, Parazzini, Chatenoud, Benzi, Bianchi and Marini1999; Piontelli, Reference Piontelli2002).
Multiple gestation is considered at high risk as it is linked with various obstetric complications, including spontaneous abortion, hypertensive disorders, placenta previa, abruption, mal-presentation, malformations, and cerebral palsy (Choi et al., Reference Choi, Park, Shim, Choi, Chang, Hahn and Bae2010). Further, multiple births have increased risk of prematurity and low birth weight, and suffer from the consequences (Choi et al., Reference Choi, Park, Shim, Choi, Chang, Hahn and Bae2010; Pharoah et al., Reference Pharoah, Glinianaia and Rankin2010). Multiple births have substantially higher risk of perinatal mortality and globally account for 14% of all infant deaths (Collins, Reference Collins2007).
Multiple births account for 3% of all births worldwide (Pharoah et al., Reference Pharoah, Glinianaia and Rankin2010). Globally, recent decades have seen a major increase in multiple births rates (Martin et al., Reference Martin, Hamilton and Osterman2012; Pison & D’Addato, Reference Pison and D’Addato2006). From 1980 to 2009 in the United States, the number of twins has doubled and the twinning rate has risen by more than 75% (Collins, Reference Collins2007). Similar increasing trends have also been observed in Western Europe and other countries (Pison & D’Addato, Reference Pison and D’Addato2006). Older maternal age accounts for about one third of the growth in the twinning rate, and the increased use of infertility treatments is likely to explain much of the remainder of the rise (Martin et al., Reference Martin, Hamilton and Osterman2012).
Spontaneous multiple birth (i.e., multiple birth not related to ART) is high in Africa. Studies conducted as early as 1960s witnessed the high incidence of multiple births in Western Africa, especially in Nigeria (Cox, Reference Cox1963; Nylander, Reference Nylander1971). A study concluded that in 1999, out of 2.8 million twins born worldwide, nearly 1.1 million (41%) were born in Africa as compared to 39% in Asia, 13% in America and 6% in Europe (Pison, Reference Pison2000). Another study found very high twinning rates (above 18/1,000 births) in many Central, Western, and South-Eastern Africa countries (Smits & Monden, Reference Smits and Monden2011). Studies conducted in Nigeria (Akinboro et al., 2010), Ghana (Mosuro et al., Reference Mosuro, Agyapong, Opoku-Fofie and Deen2001), and Kenya (Musili & Karanja, Reference Musili and Karanja2009) concluded the same. Nevertheless, population-based data regarding the epidemiology, postnatal survival, and growth pattern of multiple births in the continent are still limited.
Accordingly, the present study — an analysis of 25 demographic and health surveys (DHS) conducted in SSA since 2008 — is intended to assess the magnitude, risk factors, postnatal survival, and growth pattern of multiple births in the subcontinent.
Methods and Materials
Study Setting
SSA consists of 49 of Africa's 55 states that are fully or partially located south of the Sahara Desert. The subcontinent covers an area of 24.2 million km2. According to the 2010 World Bank estimate, the region is the most underdeveloped part of the world, with GDP per capita of 2,235 USD. The population nears 900 million and the annual population growth rate is as high as 2.5%. Life expectancy at birth is 56 years (World Bank, 2014).
Study Design
A cross-sectional study was conducted based on the secondary data of 25 DHS surveys in the subcontinent since 2008.
Inclusion and Exclusion DHS Surveys
All DHS survey types, including Standard DHS, Continuous DHS, Malaria Indicator Survey (MIS), and Standard AIDS Indicator Survey (AIS), conducted since 2008 in the region were considered for the analysis. Survey datasets that were yet to be released have not been used. In the presence of multiple surveys for a country, the most recent standard DHS was used (Table 1).
TABLE 1 List of 25 Demographic and Health Surveys Included in the Study
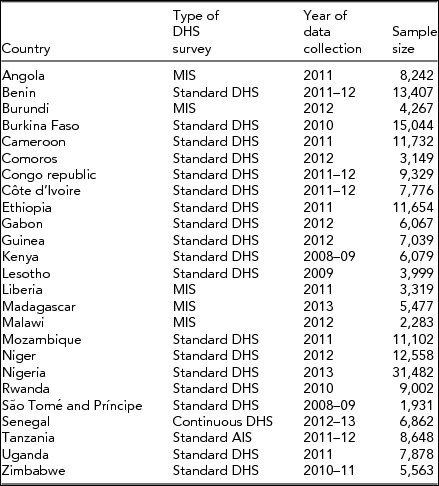
Note: DHS = demographic health surveys.
Data Extraction
For all of the surveys, the ‘child record’ dataset, which contains information about every alive birth that happened to the selected mothers in the preceding 5 years of the surveys, was used. From the datasets, variables related to maternal socio-demographic characteristics, utilization of maternity services, type of birth (singleton or multiple), survival status of the babies, basic characteristics of the babies who were alive at the time of the surveys, and anthropometric status of the mothers and the babies were extracted. All the DHS survey types had complete information regarding the aforementioned variables; nevertheless, MIS and AIS lacked anthropometric measurement and maternity service utilization related variables.
Sample Size
The adequacy of the available sample size in each country for estimating the multiple birth rate was evaluated using single proportion formula. The sample was found to be sufficient to estimate the rate at the level of 99% confidence level, 0.2% margin of error, expected rate of 18/1,000 births (Smits & Monden, Reference Smits and Monden2011) and design effect of 2. The sample was also adequate to compare the survival pattern of multiple and singleton births with effect size of 0.5 and power of 99%.
Sampling Technique Used in DHS
The DHS surveys utilized a stratified two-stage cluster sampling procedure designed to generate a representative sample at national, place of residence (urban–rural) and regional/state levels. At the first stage, a stratified sample — based on place of residence and region/state — of enumeration areas (EAs) were drawn from recent national census files using a probability proportional to size procedure. In each stratum, a sample of a predetermined number of EAs was selected; consecutively, in every selected EA, a household listing was conducted. At the second stage, fixed numbers of households were selected using a systematic random sampling technique. All eligible women (15–49 years) in the selected households were included in the study (ICF International, 2012a, 2012b).
DHS Data Collection
Data were collected by trained and experienced interviewers and supervisors. Field editors were also deployed to check the consistency of the completed questionnaires. In all of the surveys an intensive training lasting for 3–4 weeks was provided. Data were gathered using standard questionnaires that have been translated into the major local languages in which interviews were conducted. Prior to the surveys, pretesting had been conducted in a small number of EAs that have not been selected for the main surveys. Reproduction history was gathered from all eligible women (15–49 years) in the selected households. For this specific study, records of births in the preceding five years of the surveys were used.
Data Management and Analysis
Data management and analysis were conducted using SPSS and STATA statistical packages, respectively. From all of the surveys, the ‘child record’ dataset was downloaded from the DHS program website in SPSS format. Then the unnecessary variables were dropped and the datasets were merged.
All the statistical indicators were computed using weighted analysis. The ‘sampling weight’ variable found in the datasets were further corrected for the population size of the countries and used as the ultimate weight. Multiple birth rate was computed as number of multiple confinements per 1,000 births. The monozygotic and dizygotic twining rates were estimated using the Weinberg rule (Fellman & Eriksson, Reference Fellman and Eriksson2006). Factors associated with multiple births were identified using binary logistic regression model. The survival pattern in multiple and singleton births were compared using the Kaplan–Meier survival curves and Cox proportional hazard model. The assumptions of the models were tested.
Ethical Considerations
The datasets were accessed after receiving permission from the DHS program. The primary data were collected in line with international ethical guidelines. For all the surveys, ethical clearance was provided by local institutional review boards and ICF Macro. Informed consent was taken from study participants following standard procedure.
Results
Socio-Demographic Information
The records of 213,889 children born in the preceding 59 months of the surveys were included in the analysis. The proportion of males (50.6%) was slightly higher than that of females (49.6%). The median birth order was 3, with an inter-quartile range of 3. About 3.6% of all the babies were born as multiple births. Nearly three quarters of the children (71.6%) were sampled from rural areas. The mean (±SD) maternal age during the delivery was 26.9 (±17.1) years and nearly half (45.0%) of mothers had no formal education. Among 93.1% of the babies who were alive at the time of the surveys, the mean age (±SD) was 28.5 (±17.1) months (Table 2).
TABLE 2 Socio-Demographic Characteristics of the Children Included in the Analysis, Sub-Saharan Africa, 2008–2013

Magnitude of Multiple Birth Rate
The multiple birth rate was estimated based on 209,943 deliveries reported in the reference period. The weighted rate was 17.1 (95% CI: 17.7–16.6) per 1,000 births. Nearly all (99.0%) of the multiple births were twins while the remaining 1.0% were higher order births. The weighted twinning rate was 17.0 (95% CI: 17.5–16.4). The monozygotic and dizygotic twinning rates were 2.9 and 14.9/1,000 births, respectively. The multiple birth rate showed considerable variation across the 25 countries. The highest and lowest were reported in Benin and Ethiopia (Table 3).
TABLE 3 Multiple Birth Rate in 25 Sub-Saharan Africa Countries, 2008–2013

Factors Associated With Occurrence of Multiple Births
The relationship between the occurrence of multiple births and selected characteristics (maternal height, age during the first birth, age and parity during the delivery, and household wealth index), was appraised. In the multivariate logistic model adjusted for the aforementioned variables and country of residence, all characteristics except age at first birth and wealth index were significantly associated with the outcome.
Regarding maternal age, the rate sharply rose from the lowest of 3.2/1,000 births in girls younger than 16 years to 20.1/1,000 births among women in their late twenties. Later, it plateaued throughout the thirties and subsequently declined in older age groups. In the multivariate logistic model, compared to the youngest age group, the odds of multiple births in the age groups 20–24 and 25–29 were significantly raised by 1.63 and 2.08 folds. Likewise, women in the age groups 30–34, 35–39, and 40–44 years had 2.41, 2.32, and 2.05 times increased odds, respectively.
The multiple birth rate showed an approximate inverse relationship with parity. The highest rate (26/1,000 births) was reported among women with parity of 4. As compared to primiparas, women with parity of 1, 2, and 3 had 1.37, 1.51, and 1.54 times increased odds of multiple births, respectively. Women with parity of 4 and 5 or more also had 1.85 and 1.64 times increased odds.
Maternal height demonstrated a direct relationship with multiple birth rate. Compared to women from the first height quartile, those in the second, third, and fourth had 1.24, 1.21, and 1.52 times increased odds, respectively (Table 4).
TABLE 4 Association of Multiple Births with Selected Maternal Characteristics Sub-Saharan Africa, 2009–2013

With the intention of assessing seasonal variation in the occurrence of multiple birth, the multiple birth rates were compared across 12 months using a bivariate logistic regression model. However, no significant variation was found.
Survival of Multiple Birth Babies
The survival pattern among multiple births was compared with that of singletons. The cumulative survival of multiple births was significantly lower (log rank χ2 = 3,004.195, p < 0.000) and the risk of under-five years’ mortality was increased four-fold, hazard ratio = 3.92 (95% CI: 3.73–4.12). Especially, survival probability declined sharply in multiple births soon after birth and reached 0.85 at the end of the neonatal period. At the end of the fourth year of age, the cumulative survival probability was as low as 0.77 in multiple births compared to 0.93 in their counterparts (Figure 1).
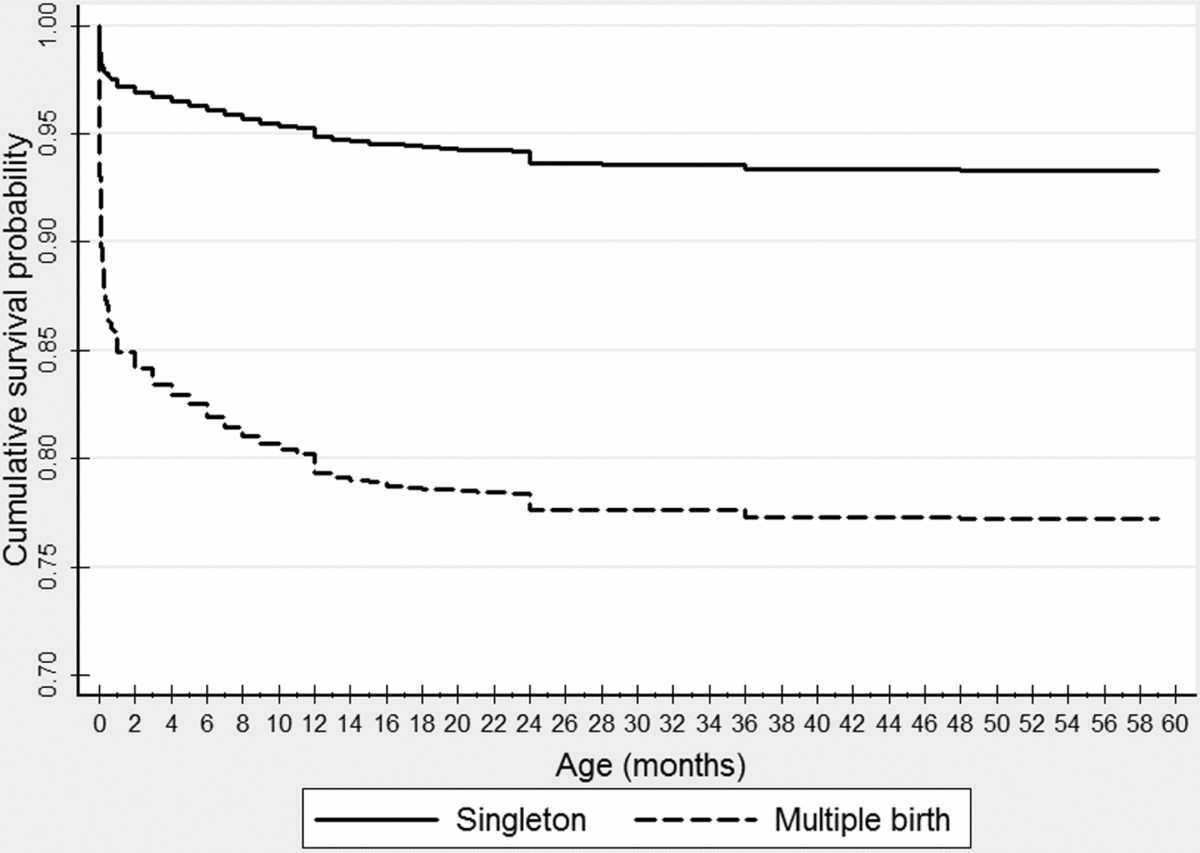
FIGURE 1 Cumulative survival probability of singleton and multiple birth babies in Sub-Saharan Africa, 2009–2014.
Among all 213,889 children included in the study, the level of neonatal (NMR), infant (IMR), and under-5 (U5MR) mortality rates were 33, 56, and 81 per 1,000 live births, respectively. Among singleton births, the rates were 29, 52, and 75, respectively. The corresponding mortality rates in multiple births were extremely high. NMR was as high as 141. IMR and U5MR were 193 and 233 per 1,000 live births, respectively. Compared to singletons, the odds of neonatal, infant, and under-5 mortality were 5.55 (95% CI: 4.26–7.23), 4.39 (95% CI: 3.49–5.52), and 3.72 (95% CI: 3.01–4.60) times increased, respectively. The odds of post-neonatal infant and child mortality rates were over twice (2.63 (95% CI: 2.37–2.90) and 2.07 (95% CI: 1.85–2.32), respectively) as high in multiple births as in singletons.
Among all neonatal, infant, and under-5 deaths that occurred in the preceding five years of the surveys, 14.6%, 11.5%, and 9.7% happened in multiple birth babies, respectively. Among all deaths of multiple birth babies, more than one in three (34.5%) occurred in the early neonatal period (0–7 days of age) and an additional 7.1% happened in late neonatal period (8–28 days). The remaining 30.3% and 28.1% took place between 1–11 months and 12–59 months, respectively.
Delivery at a Health Institution as a Strategy for Reducing Neonatal Mortality in Multiple Births
The association between place of delivery and neonatal death was assessed separately for multiple and singleton births, with the intention of evaluating whether health institution delivery had an additional benefit for multiple births as compared to singletons.
In the multivariate model adjusted for place of residence (urban or rural), maternal education and wealth index, health institution delivery reduced the odds of neonatal mortality by a higher proportion of 44%, OR = 0.56 (95% CI: 0.45–0.69), in multiple births as compared to 19%, OR = 0.81 (95% CI: 0.76–0.87), in singletons.
Nutritional Status and Growth Pattern of Multiple Births
Among children who were alive during the survey, 54.9% had comprehensive anthropometric data. The prevalence of wasting (15.5%), underweight (32.9%), and stunting (49.5%) in multiple births were significantly higher than the corresponding figures in singletons (12.4%, 21.5%, and 36.9%, respectively). In the logistic model adjusted for household wealth index, maternal age, education status, and parity; multiple births had 1.31 (95% CI: 1.20–1.45), 1.83 (95% CI: 1.70–1.97), and 1.73 (95% CI: 1.62–1.85) times increased odds of wasting, stunting, and underweight, respectively.
As compared to singletons, the mean standardized WFA and HFA scores for multiple births remains inferior throughout early childhood. However, especially from the 18th and 30th months of age onwards respectively, the WFA and HFA deficits start to be abridged and by the end of the fourth year, the differences reach a statistically insignificant level (p < 0.05). The difference in the standardized WFH score remains insignificant beyond the 24th month of age.
Discussion
The multiple birth rate in SSA (17/1,000 births) appears to be higher compared to the level in other developing countries where the magnitude is unlikely to be affected by ART. A study reported that in many South and South-East Asian countries, including China, India, Indonesia, and Pakistan, the twinning rates remains below 10/1,000 births; likewise, the incidence in Latin American countries is similarly low (less than 9/1,000 births; Smits & Monden, Reference Smits and Monden2011). The rate is also higher than the 1980s pre-ART multiple birth incidence in England and Wales (9.6/1,000 births) and several other West European countries (less than 10/1,000 births; Pison & D’Addato, Reference Pison and D’Addato2006).
Multiple birth rates appear to vary substantially across the 25 countries included in the study. In general, the rate was higher in Central and West Africa countries and lower in Eastern and Southern Africa countries. The lowest (12/1,000 births) and highest (25/1,000 births) figures were reported in Ethiopia and Benin, respectively. The disparity can be likely due to genetic differences as variations in risk factors of multiple pregnancy (e.g., age and parity) across the countries are unlikely to be substantial.
In the current study, multiple birth rates increased with advancing age, remained constant between 30–39 years, and declined thereafter. This is roughly consistent with the pattern witnessed in other studies. This has been attributed to the rise in the level of gonadotrophins with age, with maximum follicular stimulation occurring in late thirties and subsequent decline in ovarian function taking place at older ages (Bortolus et al., Reference Bortolus, Parazzini, Chatenoud, Benzi, Bianchi and Marini1999).
Increasing birth order is an unequivocal risk factor of multiple births and the same is witnessed here. In the present study, the highest multiple birth rate was observed in a parity group of 4. Two studies in India also reported higher risks in high parities and the rate picked at parity of 4 (Rao, Reference Rao1978; Sharma, Reference Sharma1997). Another study in India found a twinning rate of 6/1,000 deliveries among mothers with pregnancy order of 1 and the rate increased significantly to reach 19/1,000 in women with birth order of 4 or more (Satija et al., Reference Satija, Sharma, Soni, Sachar and Singh2008).
A positive relationship was witnessed between maternal height and occurrence of multiple pregnancy. Previous studies also reported the same. Analysis of the Danish national birth registry showed that the odds of dizygotic twining was 1.36 times higher in women taller than 173 cm compared to those shorter than 165 cm (Basso et al., Reference Basso, Nohr, Christensen and Olsen2004). A hospital-based study conducted in United States also concluded that the odds of dizygotic twinning was increased by 1.66 times in women taller than 165 cm compared to women shorter than 157.5 cm (Reddy et al., Reference Reddy, Branum and Klebanoff2005). A study based on the Netherland twin registry concluded likewise (Hoekstra et al., Reference Hoekstra, Willemsen, van Beijsterveldt, Lambalk, Montgomery and Boomsma2010). The biological mechanism by which maternal height affects incidence of multiple pregnancy has not been described well.
Information regarding the seasonality variation of multiple birth is equivocal (Bortolus et al., Reference Bortolus, Parazzini, Chatenoud, Benzi, Bianchi and Marini1999). In Japan, higher twinning rates were reported in April (Imaizumi et al., Reference Imaizumi, Asaka and Inouye1980) whereas in Denmark peak rates were observed in May, June, and December (Bonnelykke et al., Reference Bonnelykke, Sogaard and Nielsen1987). A study in the United States also reported a surge in triplet births in April and May (Elster & Bleyl, Reference Elster and Bleyl1991). However, in the current study, parallel to a comparative study conducted in Nigeria and Scotland (Nylander, Reference Nylander1981), no significant seasonal variation had been observed.
The study found that the rate of mortality of multiple birth babies was extremely high. Compared to singletons, the mortality remains significantly higher not only during neonatal period, but also in infancy and subsequent childhood ages. In the current study, the odds of neonatal and infant mortality were 5.55 and 4.39 times increased in multiple births. Studies conducted in developing countries reported comparable figures. A study in Nepal found that the risk of neonatal mortally was 7.32 times increased (Katz et al., Reference Katz, West, Khatry, LeClerq, Christian, Pradhan and Shrestha2001), whereas a study in Nigeria reported that children born from multiple births were more than twice as likely to die during infancy as infants born as singletons (Uthman et al., Reference Uthman, Uthman and Yahaya2008).
The reduced survival probability in multiple births, especially in the early stage of life, is likely to be due to the immediate complications of prematurity and low birth weight, including respiratory and immunologic problems. The reduced survival during infancy and childhood can be due to consequences of low birth weight and a higher prevalence of under-nutrition. Under-nutrition is known to contribute to more than half of all deaths in children in the developing world (Caulfield et al., Reference Caulfield, de Onis, Blössner and Black2004).
The study showed that multiple births tend to catch up their WFA and HFA deficits and become comparable to singletons at the end of the fourth year of age. Many studies concur on the existence of compensatory growth in twins but contradict on the age at which the full recovery is achieved. A longitudinal study conducted in the United States concluded that compared to singletons, twins were substantially smaller and shorter at birth, but later showed a recovery until they ultimately achieve the singleton norms at eight years (Wilson, Reference Wilson1979). A study in Japan also found the size deficit was fully recovered over the first six years of age (Ooki & Asaka, Reference Ooki and Asaka1993). A Budapest longitudinal twin study reported the weight and height deficit of twins was recouped by the age of two and three years, respectively (Bodzsár et al., Reference Bodzsár, Zsákai and Susanne2014).
The findings of the analysis should be interpreted with considerations of the strengths and limitations of the study. The typical strength emanates from the use of large and nationally representative data collected across 25 countries. Further, the article discussed various aspects of multiple birth including the epidemiology, survival, and growth pattern. In contrast, the risk factors have not been disaggregated based on zygosity. Further, the survival rates were not separately estimated for the number of multiple births as the sample size for triplet and higher order births were too small for detailed analysis.
Conclusion
Based on the 25 SSA countries studied, the multiple birth rate was 17.1 (95% CI: 17.7–16.6) per 1,000 births. The rate showed considerable variation across the countries. The odds of multiple births were significantly increased with advanced maternal age, parity, and maternal height but not with wealth index, age at first birth, and month of birth. As compared to singletons, the survival probability of multiple births was lower throughout the first five years of age. Likewise, multiple birth babies tend to be more malnourished than singletons although they catch up their growth deficit by the end of their fourth year of age.
Counseling mothers with multiple gestation pregnancy to give birth at health institution and proving close medical follow-up during and beyond neonatal period can improve the survival of multiple births.
Acknowledgment
The author acknowledges the Measure DHS Program for granting access to the data.







