Adenomyosis is regarded as one of most common gynecological disorders, which has a morbidity of 8–27% in women with reproductive age (Huang et al., Reference Huang, Huang, Chen, Zhang, Lin and Zhang2015; Leyendecker et al., Reference Leyendecker, Wildt and Mall2009). The pathological change of adenomyosis is characterized by ectopic endometrial glands and stroma invasion into myometrium of uterus, which can also be either diffuse or focal lesion (Bird et al., Reference Bird, McElin and Manalo-Estrella1972). When the lesion in the myometrium exhibits limited nodules, the condition is then known as adenomyoma (Levy et al., Reference Levy, Dehaene, Laurent, Lernout, Collinet, Lucot, Lions and Poncelet2013). The patients with adenomyoma usually suffer from dysmenorrhea, subfertility, chronic pelvic pain and menorrhagia, leading to physical pain and quality of life reduction (Arnold et al., Reference Arnold, Ascher, Schruefer and Simon1995; Juang et al., Reference Juang, Chou, Yen, Twu, Horng and Hsu2007). Recent researches identified that estrogen could enhance the invasive and migratory ability of endometrial epithelial cells, which further results in the endometrium infiltrating the myometrial zone (Y.-J. Chen et al., Reference Chen, Li, Huang, Twu, Yen, Wang, Chou, Liu, Chao and Yang2010; Senturk & Imamoglu, Reference Senturk and Imamoglu2015). Therefore, hormone therapy has become the mainstream treatment for adenomyosis, but it can only partly control the symptoms of this disease, such as dysmenorrhea, and hysterectomy for severe adenomyosis is often required (Y.-J. Chen et al., Reference Chen, Li, Huang, Twu, Yen, Wang, Chou, Liu, Chao and Yang2010; Senturk & Imamoglu, Reference Senturk and Imamoglu2015). Thus, a detailed molecular mechanism of the above pathological process is urgently needed to provide novel and potential therapeutic strategies for adenomyosis.
MicroRNAs (miRNAs) are defined as noncoding RNAs and consist of 22−25 nucleotides that function as a posttranscriptional controller of their target genes via binding the 3’-untranslated regions (3′-UTRs; Simonson & Das, Reference Simonson and Das2015). Accumulating evidence confirms that multiple miRNAs are involved in the occurrence and development in adenomyosis; for instance, Hu et al. (Reference Hu, Li and He2017) indicated that miR-17 was significantly up-regulated in endometrial tissues of patients with adenomyosis, which further influenced proliferation and apoptosis of adenomyotic endometrial cell by directly targeting phosphatase and tensin homolog (PTEN); T.-X. Xu et al. (Reference Xu, Zhao, Dong and Yu2016) found that miR-210 could contribute to pathological progression of endometriosis by targeting Bcl2/Beclin-1 axis. The function of miR-30c has been extensively reported in numerous pathological processes such as lipid metabolism, cardiac remodeling, adipogenesis and progression of cancers (Irani & Hussain, Reference Irani and Hussain2015). At present, the latest research using microarray screen assay claims that miR-30c expression descends into ectopic endometrial lesions tissue instead of normal endometrium tissue, suggesting that miR-30c might be partly involved in pathologic change in adenomyosis (Guo et al., Reference Guo, Lang, Lu, Wang, Li, Liao, Jia, Zhao and Fang2015). However, no relevant studies have focused on the exact role of miR-30c-5p in adenomyosis until now.
Therefore, the aim of this study is to clarify the role of miR-30c-5p in adenomyotic progression and further investigate the potential downstream target of miR-30c-5p. Moreover, we intend to explore the interaction between miR-30c-5p and its target in regulating biological function of adenomyotic epithelial cells, providing a potential therapeutic method for adenomyosis.
Materials and Methods
Patient Samples
A total number of 23 patients (age range 29−45 years old) with adenomyosis who underwent hysterectomy at our hospital from April 2016 to September 2018 were enrolled in this study. Ectopic and eutopic endometrial tissues from adenomyotic patients were collected. Meanwhile, endometrial tissues obtained from 20 patients who underwent a hysterectomy (age range 34−48 years old) with benign gynecological diseases, such as uterine prolapse and subserousal leiomyoma, were regarded as normal controls. After surgery, the endometrial tissues were immediately collected and frozen in liquid nitrogen for further analysis. Before the hysterectomy, the clinicopathologic parameters of adenomyotic patients were recorded with a standard questionnaire using a visual analogue scale (VAS) and pictorial blood-loss assessment chart (PBAC). The Ethics Committee of our hospital reviewed and approved all protocols of this study, and all participants provided written informed consent.
Cell Culture
As previously described, ectopic and eutopic endometrial tissues and normal endometrium epithelial tissues were separated first (Zhang et al., Reference Zhang, Rees and Bicknell1995). Next, the isolated adenomyotic endometrial cells were cultured in DMEM (BD Biosciences, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, USA) in a humidified incubator with 5% CO2 at 37° C. The HEK 293T cells were purchased from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China) and cultured in the same condition.
Cell Transfection
miR-30c-5p mimics, miR-30c-5p inhibitor, pcDNA3.1-MAPK1 and a corresponding negative control (NC) were obtained from GenePharma (Shanghai, China). After cell growth reached approximately 60−80% confluence, the adenomyotic endometrial cells were then transfected with the above reagents using Lipofectamine 2000 (Thermo Fisher Scientific, USA) following the manufacturer’s instruction. After 48 h of transfection, the cells were collected for further analysis.
Cell Proliferation Assay
The CCK-8 assay and colony formation assay were used to detect cell proliferation rate. For CCK-8 assay, the transfected cells (1 × 103/per well) were seeded in 96-well plates and cultured for up to 72 h. Then, cell viability was proven using CCK-8 (Beyotime Biotechnology, China) at different time points (0, 24, 48 and 72 h) under a Multi-Detection Microplate Reader (Bio-Rad, USA).
Cell Migration Assay
Wound-healing assays were performed to assess cell migration. In brief, 5 × 104 cells were seeded in 6-well plates coated with fibronectin and cultured for 24 h until there was approximately 80% confluence. The scratches in each well were made by a 200-ul pipette tip, and then the cells were transfected with miR-30c-5p mimics, miR-30c-5p inhibitor and pcDNA3.1-MAPK1 and controls. The migratory cells were observed in a selected area at 0 and 48 h after initial scratch under a light microscope (Olympus, Japan).
Cell Invasion Assay
The invasive ability of endometrial epithelial cells was measured as previously described using the Transwell system (Dong et al., Reference Dong, Jia, Xu, Liu, Li and Feng2008). Briefly, the transfected cells were suspended in a serum-free medium at density of 2 × 105 cells/ml and then seeded into the upper chamber of the Transwell system, while the complete medium was added into the lower chamber. After incubation for 24 h, the invaded cells were fixed with 10% formaldehyde for 30 min and then stained with 0.5% crystal violet for 10 min and counted for five random fields using a light microscope (Olympus, Japan).
Luciferase Reporter Assay
Luciferase reporter plasmid containing wild MAPK1-3′-UTR (MAPK1-WT) or mutant MAPK1-3′-UTR (MAPK1-MUT) were synthesized by Promega (Madison, USA) and then subcloned into the pmiRGLO vector (Promega, USA). Next, the HEK293 cells were co-tranfected with MAPK1-WT or MAPK1-MUT reporter plasmid together with miR-30c-5p mimics and mimics-NC using Lipofectamine 2000 (Invitrogen, USA). After 48 h of transfection, the luciferase activity was measured by Dual Luciferase Assay System (Promega, USA) according to the manufacturer’s specification.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNAs were extracted from clinical tissues and cells using TRIZOL reagent (Thermo Fisher Scientific, USA). After that, cDNA was synthesized from RNAs using TaqMan MicroRNA Reverse Transcription Kit (Thermo Fisher Scientific, USA) according to the manufacturer’s specification. Then, quantitative real-time polymerase chain reaction (qRT-PCR) was conducted using SYBR Green PCR Master Mix (BioRad, USA) under a CFX96 Real-Time Thermocycler system (BioRad, USA). U6 was represent as internal controls for miR-30c-5p, whereas GAPDH was represented as an internal control for mitogen-activated protein kinase 1 (MAPK1). The relative expression of each mRNA or miRNA was analyzed using 2−ΔΔCT method. All primer sequences are listed in Table 1.
Table 1. List of primers used for real-time polymerase chain reaction

Western Blot
Total proteins were extracted using RIPA lysis buffer (Beyotime, China) and qualified by a BCA detecting kit (Beyotime, China) following the manufacturer’s specifications. Subsequently, the extracted protein was separated on a 10% SDS-PAGE and then transferred onto PVDF membrane (Millipore, USA) blocked with 5% nonfat milk for 2 h at room temperature and incubated overnight with primary antibodies against MAPK1 (1: 1000, Abcam, UK) and GAPDH (1: 5000, Abcam, UK) at 37° C. Finally, the membrane was incubated with HRP-conjugated secondary antibodies (1:5000, Abcam, UK) at room temperature for 1 h. The blots were visualized using enhanced chemiluminescence (Bio-Rad, USA) and quantitative calculated using image J (National Institutes of Health, USA).
Statistical Analysis
All experiments were carried out in triplicate. Data are presented as the mean ± standard deviation (SD), and the error bars represent the SD from three independent experiments. The statistical analysis was conducted using SPSS 21.0 (SPSS Inc, USA) or GraphPad Prism 7.1 software (National Institutes of Health, USA). Student’s t test or one-way analysis of variance (ANOVA) was used for comparisons between groups. The correlation between miR-30c-5p and clinical-pathological parameters of adenomyotic patients were measured by Pearson’s correlation methods. Statistical significance was determined as a p value less than .05.
Results
MiR-30c-5p Expression Is Down-Regulated Both in Human Adenomyosis Tissues and Adenomyotic Epithelial Cells
To explore whether miR-30c-5p served an essential role in human adenomyosis, we first evaluated the expression of miR-30c-5p in adenomyosis tissues using qRT-PCR. Our results found that miR-30c-5p expression was significantly reduced in eutopic and ectopic tissue of adenomyosis compared with normal tissues (Figure 1A). As shown in Figure 1B, the level of miR-30c-5p was conspicuously down-regulated in isolated adenomyotic epithelial cells in comparison with normal endometrial cells, suggesting that miR-30c-5p might be involved in pathological process of adenomyosis.

Fig. 1. MiR-30c-5p was significantly down-regulated both in human adenomyosis tissues and adenomyotic epithelial cells. (A) The expression levels of miR-30c-5p in adenomyotic tissue samples were down-regulated compared with normal tissues. (B) The expression levels of miR-30c-5p in adenomyotic cells were down-regulated compared with normal cells. Data were represented as mean ± SD.
The Level of miR-30c-5p is Correlated with the Clinicopathological Parameters of Adenomyotic Patients
On account of the above results, we tried to find the correlation between the expression level of miR-30c-5p with the clinicopathological parameters of 23 adenomyotic patients, such as age, clinical symptoms (dysmenorrhea or menometrorrhagia or both), duration of symptoms, VAS score for dysmenorrhea, as well as menstrual bleeding (PBAC score). As shown in Table 1, the lower expression level of miR-30c-5p was tightly correlated with dysmenorrhea (p = .021), longer duration of symptoms (p = .000) and more menstrual bleeding (p = .001); however, the results showed that there was no significant relationship between age or VAS score for dysmenorrhea and miR-30c-5p expression in adenomyotic patients (p > .05; Table 2).
Table 2. The correlation between relative miR-30c-5p expression and clinical features of patients with adenomyosis

MiR-30c-5p Regulates Proliferation, Invasion and Migration of Adenomyotic Epithelial Cells
From previous findings, we were conscious that the expression of miR-30c-5p might act as a pivotal role in development of adenomyosis; hence, the relevant deep mechanism was further explored in adenomyotic epithelial cells. First, miR-30c-5p mimics and miR-30c-5p inhibitor were transfected into adenomyotic epithelial cells to verify their overexpression or down-expression efficiency. As shown in Figure 2A, miR-30c-5p mimics significantly up-regulated the miR-30c-5p expression, whereas miR-30c-5p inhibitor could knock down miR-30c-5p expression. Then, the proliferation of adenomyotic epithelial cells were measured by CCK-8 assay. Our results found that overexpression of miR-30c-5p could distinctly inhibit the cell viability of adenomyotic epithelial cells, where miR-30c-5p inhibitor showed an enhanced effect on cell viability (Figure 2B and C). Moreover, the transwell invasion assay and wound-healing assay were conducted to assess the invasive and migratory ability of adenomyotic epithelial cells transfected with miR-30c-5p mimics or miR-30c-5p inhibitor. The data revealed that the cell invasion and migration were remarkably suppressed in the miR-30c-5p mimics group, which were dramatically expedited by transfection of miR-30c-5p inhibitor (Figure 2D and E). Taken together, our results identified that miR-30c-5p could regulate the proliferation and invasion, as well as migration of adenomyotic epithelial cells, and might play an important role in progression of adenomyosis.

Fig. 2. The level of miR-30c-5p is correlated with the clinicopathological parameters of adenomyotic patients. (A) Endometrial epithelial cells isolated from ectopic endometrial tissues of adenomyosis were transiently transfected with miR-30c-5p mimics or miR-30c-5p inhibitor. (B–C) CCK-8 assay was performed to measure the effect of miR-30c-5p on cell viability. (D) Transwell invasion assay was performed to evaluate the influence of miR-30c-5p on cell invasion capacity. (E) Wound-healing assay was employed to determine the effect of miR-30c-5p on cell migration capacity. Data were represented as mean ± SD.
MAPK1 is Negatively Correlated with miR-30c-5p
In order to determine the deeper mechanism, we first searched for the potential genes that are targeted by miR-30c-5p using bioinformatics tools. We found that MAPK1 is represented in miR-30c-5p recognition sites in its 3’-UTRs (Figure 3A). As shown in Figure 3B, luciferase reporter assays showed that luciferase activity was significantly inhibited in cells cotransfected with miR-30c-5p and MAPK1-3′UTR (WT), while the luciferase activity of MAPK1-3′UTR (MUT) remained unchanged. Next, we estimated the suppressive efficiency of miR-30c-5p mimics on endogenous MAPK1 expression in adenomyotic epithelial cells using RT-PCR and western blot. Our results found that expression of MAPK1 was significantly decreased in miR-30c-5p mimics group, while the expression of MAPK1 was significantly up-regulated in miR-30c-5p inhibitor group (Figure 3C and D). To sum up, our results proved that MAPK1 was considered as a directly target of miR-30c-5p.

Fig. 3. MAPK1 is a direct target of miR-30c-5p. (A) MAPK1 was predicted as a potential target of miR-30c-5p by bioinformatical tools. (B) The relative luciferase activity in cells co-transfected MAPK1-3′UTR(WT) or MAPK1-3′UTR(MUT) with miR-30c-5p mimics or miR-NC. (C–D) The relative mRNA and protein expression of MAPK1 in adenomyotic epithelial cells transfected with miR-30c-5p mimics, miR-30c-5p inhibitor or NC. Data were represented as mean ± SD.
MAPK1 is Involved in miR-30c-5p-Mediated Suppression in Proliferation, Invasion and Migration
To better understand the correlation between miR-30c-5p and MAPK1, we designed and conducted a series rescue assay. First, we verified the overexpression ability of pcDNA-MAPK1 in adenomyotic epithelial cells (Figure 4A). Accordingly, we detected that biological functions of cells cotransfected miR-30c-5p mimics or mimic-NC with pcDNA3.1-MAPK1 or empty vector. For cell viability, we could clearly find that MAPK1 overexpression remarkably alleviated miR-30c-5p-mediated facilitation, which was measured by CCK-8 assay (Figure 4B). In addition, our results validated that cells transfected with pcDNA3.1-MAPK1 also attenuated miR-30c-5p-mediated invasive and migratory auxo-action (Figure 4C and D). Taken together, our data clarified that MAPK1 involved in miR-30c-5p triggered antiproliferative, anti-invasive and anti-migration roles in adenomyotic epithelial cells.
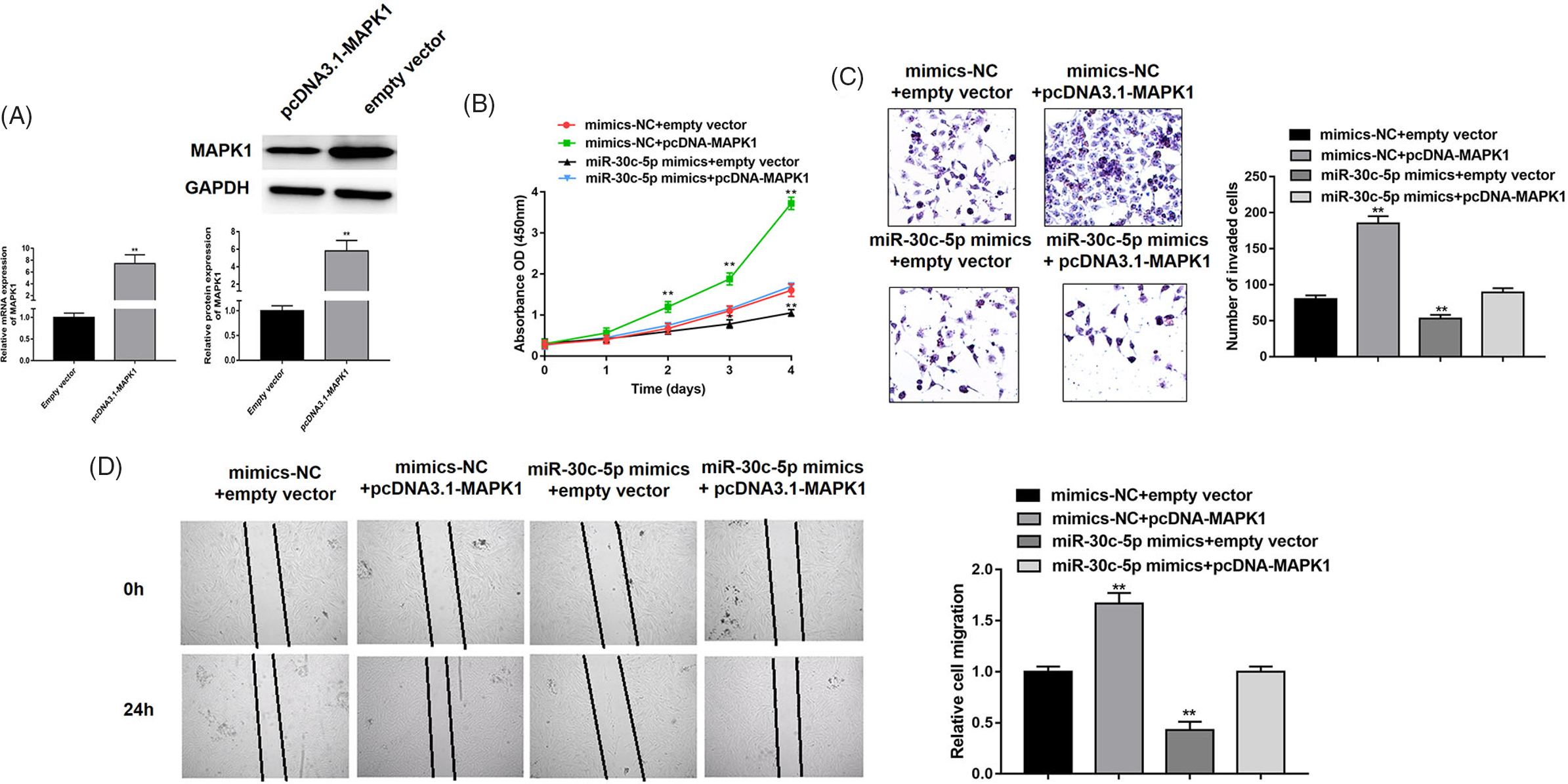
Fig. 4. MAPK1 is involved in miR-30c-5p-mediated suppression of cell proliferation, migration and invasion. (A) The overexpression efficiency of pcDNA3.1-MAPK1 was verified by RT-PCR and western blot. (B) The cell proliferation of adenomyotic epithelial cells co-transfected miR-30c-5p mimics or mimics-NC with pcDNA3.1-MAPK1 or empty vector, which measured by CCK-8 assay. (C–D) The transwell invasion assay and wound-healing assay were performed to detect invasion and migration abilities of denomyotic epithelial cells co-transfected miR-30c-5p mimics or mimics-NC with pcDNA3.1-MAPK1 or empty vector. Data were represented as mean ± SD.
Discussion
Recently, accumulating evidence suggests that numerous miRNAs are involved in the occurrence and development of endometriotic lesion development (Dai & Di, Reference Dai and Di2011), as well as the formation of adenomyosis (Guo et al., Reference Guo, Lang, Lu, Wang, Li, Liao, Jia, Zhao and Fang2015), such as miR-10, miR-142-3p, miR-17, miR-191 and miR-29c and miR-210 (Guo et al., Reference Guo, Lang, Lu, Wang, Li, Liao, Jia, Zhao and Fang2015; Hu et al., Reference Hu, Li and He2017; Ohlsson Teague et al., Reference Ohlsson Teague, Van der Hoek, Van der Hoek, Perry, Wagaarachchi, Robertson, Print and Hull2009; Tian et al., Reference Tian, Xu and Wang2015). According to current knowledge, miRNAs serve as regulatory roles in the biological function of endometrial cells, including proliferation, invasion, inflammation response, apoptosis and angiogenesis (Bjorkman & Taylor, Reference Bjorkman and Taylor2019; Lin et al., Reference Lin, Wang, Wu, Yang, Li and Tsai2012), which are considered to be the main causes of adenomyosis (Erkilinc et al., Reference Erkilinç, Taylan, Gülseren, Erkilinç, Karadeniz, Bağci, Temel, Solmaz, Gökçü and Sanci2018; Ibrahim et al., Reference Ibrahim, Chiantera, Frangini, Younes, Köhler, Taube, Plendl and Mechsner2015; Vannuccini et al., Reference Vannuccini, Tosti, Carmona, Huang, Chapron, Guo and Petraglia2017); for example, Hu et al. (Reference Hu, Li and He2017) declared that miR-17 dramatically promoted the proliferation but suppressed the apoptosis of adenomyotic endometrial cells; Guo et al. (Reference Guo, Lang, Lu, Wang, Li, Liao, Jia, Zhao and Fang2015) showed that miR-10b was significantly reduced in both adenomyotic epithelial tissues and cells, overexpression of which could inhibit the migration and invasion ability of adenomyotic epithelial cells by targeting ZEB1 and PIK3CA.
MiR-30c-5p has previously been seen as participating in endometriotic-related diseases; for instance, Hu et al. (Reference Hu, Li and He2017) first reported that miR-30c played a role as a tumor suppressor in regulating migration and proliferation of human endometrial cancer cells by directly targeting the metastasis-associated gene-1 (MTA-1), which might be modulated by the AKT/mTOR/4E-BP1 pathway (X. Xu et al., Reference Xu, Kong, Liu, Zhou, Wu, Fu, Wang, Zhu, Yao, Ding, Ding, Li, Zhu, Tang, Zhang, Yang, Ling and Zhou2019; Zhou et al., Reference Zhou, Xu, Xun, Yu, Ling, Guo, Yan, Shi and Hu2012); X. Chen et al. (Reference Chen, Jiang and Pan2017) also confirmed that miR-30c could inhibit cells proliferation, invasion and migration and induce apoptosis in endometrial cancer cells by negatively regulating plasminogen activator inhibitor type 1 (PAI-1); in our study, we found that miR-30c-5p was remarkably down-regulated in adenomyotic tissues and isolated adenomyotic epithelial cells compared with normal controls. Moreover, the expression level of miR-30c-5p was associated with the severity of clinical symptoms of adenomyosis as dysmenorrhea and menometrorrhagia. Furthermore, we explored the functional role of miR-30c-5p in regulating adenomyotic epithelial cells and indicated that overexpression of miR-30c-5p suppressed the cell proliferation, invasion and migration, while down-expression of miR-30c-5p showed opposite effects. Therefore, the present study was the first to investigate whether miR-30c-5p is involved in the development of adenomyosis and altering the biological functions of adenomyotic epithelial cells.
As one of the most well-known members of the MAP kinase family, MAPK1 might act as a binding site for multiple biochemical signals, which has been reported in a wide range of cellular processes, including cell proliferation, differentiation, migration and transcription development (Hoefen & Berk, Reference Hoefen and Berk2002; Li et al., Reference Li, Chen, Liu, Yang, Yang and He2014; Sun et al., Reference Sun, Liu, Liu, Feng, Yang and Zhou2015; Wainstein & Seger, Reference Wainstein and Seger2016). Current studies have claimed that MAPK1 is involved in endometrial cell-related disease via targeting different miRNAs, such as miR-381 (Tu et al., Reference Tu, Wang and Wan2018), miR-143 (Chang et al., Reference Chang, Zhang, Shi, Bian and Guo2017) and miR-93 (Gao et al., Reference Gao, Deng, Liu, Dai, Chen, Chen, Chen, Huang, Dai and Chen2019). More interestingly, we validated that miR-30c-5p directly targeted the 3'-UTRs of MAPK1 using luciferase reporter assay, RT-PCR and western blot. Using a series of rescue experiments, we further found that MAPK1 participated in miR-30c-5p-mediated suppression of proliferation, migration and invasion in adenomyotic epithelial cells. Hence, we held the opinion that miR-30c-5p could limit bioactivity of aberrant epithelial cells via suppression of MAPK1 expression, which acted as an inhibitor of adenomyotic progression.
Conclusion
In summary, our study demonstrated that miR-30c-5p was down-regulated in adenomyotic tissues and cells, which correlated with some clinical symptoms of patients. In addition, miR-30c-5p could play a suppressor role on adenomyotic epithelial cells in proliferation, migration and invasion by directly targeting MAPK1. Therefore, our results suggested that miR-30c-5p might be a potential therapeutic target for adenomyosis.
Data
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions
Guarantor of integrity of the entire study: Airong Zhang; Study concepts: Juan Wang; Study design: Xiujuan Li; Definition of intellectual content: Kui Deng; Literature research: Kui Deng; Clinical studies: Airong Zhang; Experimental studies: Airong Zhang; Data acquisition: Jianghua Wang; Data analysis: Airong Zhang; Statistical analysis: Kui Deng; Manuscript preparation: Xiujuan Li; Manuscript editing: Kui Deng; Manuscript review: Juan Wang.
Financial support
This study was supported by Quality Engineering Project of Anhui Province in 2019 and Academic Funding Project for Top-notch Talents in disciplines (Majors) of Universities of Anhui Province in 2020, (gxbjZD2020043).
Conflicts of interest
None.
Ethical standards
Ethics Committee of Anqing Medical College reviewed and approved all protocols of this study, and all participants had written the informed consents. Informed consent was obtained from all individual participants included in the study.








