The adequate treatment of psychiatric disorders is burdened by their complex etiology. The joint action of multiple genetic and environmental factors leads to onset of illness, which usually starts with non-specific symptoms in adolescence (Lesch, Reference Lesch2004; Van Os et al., Reference Van Os, Rutten and Poulton2008). Thus, the identification of biological and psychological markers that underlie the development and progression of illness and link to relevant pathophysiology is of crucial importance to understanding the mechanisms leading to psychiatric disorders and hence to their prevention and treatment.
Chronic stress and an impaired ability to cope with it, both at the psychological and biological level, are thought to play a key role in the development and maintenance of psychiatric disorders (de Kloet et al., Reference de Kloet, Joels and Holsboer2005). The most common biological marker to measure stress response is cortisol, which is released by the hypothalamic-pituitary-adrenal (HPA) axis. The HPA axis is the main endocrine mediator of the stress response, and it is modulated by and exerts feedback on brain regions involved in psychiatric disorders; for example, the amygdala, the hippocampus, and the prefrontal cortex (Dedovic et al., Reference Dedovic, Duchesne, Andrews, Engert and Pruessner2009; de Kloet et al., Reference de Kloet, Joels and Holsboer2005; Herman et al., Reference Herman, Figueiredo, Mueller, Ulrich-Lai, Ostrander, Choi and Cullinan2003).
Studies in healthy population samples show an increase in cortisol in blood, urine, and saliva during and after exposure to short-term stressors and a decrease once the stressor disappears (Miller et al., Reference Miller, Chen and Zhou2007). The regulation of the HPA axis has been reported to be altered in patients suffering from depression, schizophrenia, and anxiety disorders, supporting the hypothesis of impaired ability to cope with stress in psychiatric disorders (Bradley & Dinan, Reference Bradley and Dinan2010; Faravelli et al., Reference Faravelli, Lo Sauro, Lelli, Pietrini, Lazzeretti, Godini and Ricca2012; Herbert, Reference Herbert2013; Jones & Moller, Reference Jones and Moller2011). The measurement of cortisol in blood, saliva, or urine is a sensitive method to assess acute stress responses and reflects short-term cortisol release. However, single cross-sectional samples may not provide a valid measure of chronic stress and long-term alterations of HPA functioning as they are sensitive to daily circumstances such as circadian rhythm, nutrition, and so forth.
A relatively new method that enables the efficient assessment of cortisol release over a longer period is its measurement in hair of the scalp (Stalder et al., Reference Stalder, Steudte, Miller, Skoluda, Dettenborn and Kirschbaum2012). Scalp hair grows at an average rate of 1 cm per month, so cortisol assayed from a 3 cm hair segment closest to the scalp likely reflects the average release over the past 3 months and is therefore a potential biomarker of long-term altered HPA functioning. Initial studies showed altered hair cortisol concentrations (HCC) in unaffected adults with high levels of chronic stress and in cases suffering from psychiatric disorders (including bipolar disorders, depression, and anxiety disorder) compared with controls (reviewed in Herane Vives et al., Reference Herane Vives, De Angel, Papadopoulos, Strawbridge, Wise, Young and Cleare2015). More recent studies found similar associations in children and adolescents for objective stress and HCC (Rippe et al., Reference Rippe, Noppe, Windhorst, Tiemeier, van Rossum, Jaddoe and van den Akker2016; Simmons et al., Reference Simmons, Badcock, Whittle, Byrne, Mundy, Patton and Allen2016; Vliegenthart et al., Reference Vliegenthart, Noppe, van Rossum, Koper, Raat and van den Akker2016).
These alterations in HCC may reflect causal mechanisms such as genetic determinants that jointly influence the level of HCC, and moderate affective symptoms and perceived stress (pleiotropy), or may be a direct consequence of the pathogenesis such that affective symptoms and perceived stress increase HCC; or both mechanisms may operate. However, few studies have assessed the association between HCC, affective symptoms, and perceived stress. Furthermore, to our knowledge, no study has assessed the association between HCC and the personality traits of neuroticism and extraversion, where the first is a risk factor for the development of psychiatric disorders and the latter is not (Kendler et al., Reference Kendler, Gatz, Gardner and Pedersen2006; Van Os & Jones, Reference Van Os and Jones2001).
Many previous studies assessing the relationship between perceived stress and HCC found no significant or low correlations (Heinze et al., Reference Heinze, Lin, Reniers and Wood2016; Staufenbiel et al., Reference Staufenbiel, Penninx, Spijker, Elzinga and van Rossum2013; Streit et al., Reference Streit, Memic, Hasandedić, Rietschel, Frank, Lang and Rietschel2016). This so-called lack of psychoendocrine covariance, with endocrine response not coinciding with psychological stress, might be due to most studies assuming a linear association between hair cortisol and psychological markers. It is possible though that a pathological threshold exists for cortisol as it does for other metabolite biomarkers such as creatinine, where only higher values are associated with renal damage and lower levels have no clinical implications (Singhal & Saha, Reference Singhal and Saha2014).
The primary aim of our pilot study was to assess the association of HCC with psychological risk factors for psychiatric disorders — perceived stress, symptoms of depression, and neuroticism, and also the personality trait extraversion, for which there is no expected relation with cortisol. Our secondary aim was to explore the genetic and environmental causation of these associations by making use of the MZ and DZ twins in our sample.
Materials and Methods
Sample
Our sample comprised 135 adolescent or young adult twins (95 females) who had taken part in either the Brisbane Longitudinal Twin Study (BLTS; Wright & Martin, Reference Wright and Martin2004) or the Queensland Twin Imaging Study (QTIM study; Blokland et al., Reference Blokland, McMahon, Hoffman, Zhu, Meredith, Martin and Wright2008) between 2009 and 2012. In the BLTS, psychiatric symptoms are assessed at ages 12, 14 and 16 years (Hansell et al., Reference Hansell, Wright, Medland, Davenport, Wray, Martin and Hickie2012). The QTIMS is a follow-up study of the BLTS cohort, which involves investigation of twins aged 21–28 years. Zygosity was initially determined by a combination of standard questions (Martin & Martin, Reference Martin and Martin1975) and a photograph of the twin pairs, and subsequently confirmed by extensive genotyping. The sample consisted of 8 monozygotic (MZ) and 24 dizygotic (DZ) complete twin pairs, and 66 individual twins and 5 siblings. In both the MZ and DZ groups, female twin pairs were predominant.
Data of those subjects with a weight of <7.5mg of hair cortisol were excluded (n = 26 which left 8 MZ pairs, 21 DZ pairs, and 51 unpaired twins or sibs for further analysis; for details see section below titled Hair Cortisol Analysis). The mean age of the sample was 15.1 years (SD = 1.99; range 12–21 years).
We are well aware that this is too small a sample for reliable heritability estimation, but our principal interest is in the relation between HCC and our psychological variables, and the genetic and environmental causes of any such correlation for which the power of analysis is greater given the multivariate nature of the data.
Self-Report Measures
For participants aged 12, 14, and 16 years, perceived stress in the past month was measured using the 30-item Daily Life and Stressors Scale (DLSS; Kearney et al., Reference Kearney, Drabman and Beasley1993). The trait variables neuroticism and extraversion were measured using the Junior Eysenck Personality Questionnaire (JEPQ; Eysenck, Reference Eysenck1972) with 20 items for neuroticism and 24 items for extraversion. For participants aged 16 years or older, perceived stress was measured using the 10-item Perceived Stress Scale (PSS; Cohen et al., Reference Cohen, Kamarck and Mermelstein1983), and neuroticism and extraversion were measured using the NEO-Five Factor Inventory revised version (Neo-FFI-R; McCrae & Costa Jr., Reference McCrae and Costa2004), each with 12 items for neuroticism and extraversion. Depressive symptoms at all ages were assessed using the 34-item Somatic and Psychological Health Report (SPHERE; Hansell et al., Reference Hansell, Wright, Medland, Davenport, Wray, Martin and Hickie2012; Hickie et al., Reference Hickie, Davenport, Hadzi-Pavlovic, Koschera, Naismith, Scott and Wilhelm2001).
Hair Cortisol Analysis
Sample collection and preparation were performed as described elsewhere (Kirschbaum et al., Reference Kirschbaum, Tietze, Skoluda and Dettenborn2009). In brief, a swatch of hair about 3 mm in cross-section from the back of the head (posterior vertex position) was cut with fine scissors as close as possible to the scalp. As hair grows at an average speed of 1 cm per month, the 3-cm segment proximal to the scalp can be assumed to reflect cortisol secretion during the preceding 3 months (LeBeau et al., Reference LeBeau, Montgomery and Brewer2011), and our sample was trimmed from the distal end to this length. Hair washing and steroid extraction were performed as described in Davenport et al. (Reference Davenport, Tiefenbacher, Lutz, Novak and Meyer2006). The hair samples were then micro-centrifuged at 10,000 rpm for 2 min. A 1 ml volume of the clear supernatant was then transferred into a new cryo vial in order to allow the alcohol to evaporate at 60 °C under a constant stream of nitrogen until the samples were completely dry. Finally, 0.4 ml of phosphate buffer was added and the tube was vortexed for 15 s before cortisol was assayed using a commercial immunoassay kit as described by Kirschbaum et al. (Reference Kirschbaum, Tietze, Skoluda and Dettenborn2009).
Ideally, the HCC assays should be performed on a hair sample weighing at least 10 mg. However, for some individuals (n = 46, mainly males) this amount of hair had not been collected. Preliminary analysis of the entire sample of observations revealed a strong negative correlation (r = -0.49) between the weight of the hair sample being analyzed and the HCC. We have no explanation for this effect, which was observed in men and women and both did not differ in average cortisol levels (t 133 = 0.24, p = .81). On inspection, this relation appeared to apply only to the smallest samples and to asymptote above a sample weight of 7.5 mg so we used this as a lower limit for inclusion in the study.
Data Analysis
Due to skewness, hair cortisol concentration (mean = 14.07 pg/mg, SD = 6.48, range 1.61–41.33) was log transformed for all analyses.
To compare neuroticism scores between participants, the neuroticism, and extraversion sum-scores of the NEO-FFI and the JEPQ-scores were separately z-transformed and then combined. For the assessment of perceived stress measured by the PSS (Cohen et al., Reference Cohen, Kamarck and Mermelstein1983) and the DLSS (Kearney et al., Reference Kearney, Drabman and Beasley1993), and depressive symptoms measured by the SPHERE (Hickie et al., Reference Hickie, Davenport, Hadzi-Pavlovic, Koschera, Naismith, Scott and Wilhelm2001), item response theory (IRT) analyses were performed (Wray et al., Reference Wray, Coventry, James, Montgomery, Eaves and Martin2008) to harmonize data. The details and advantages of IRT analysis are described elsewhere (Rietschel et al., Reference Rietschel, Zhu, Kirschbaum, Strohmaier, Wüst, Rietschel and Martin2014).
Preliminary analysis of the association between hair cortisol and the different psychological measures (e.g., ratings of perceived stress and neuroticism) was complicated by the fact that our twin study contained two children from the same family, leading to non-independent observations. As such, we randomly split the data so that no family member would be in the same group; Spearman correlations were then performed to examine the relationship between neuroticism, extraversion, symptoms of depression, perceived stress, and hair cortisol, using pairwise deletion of missing data. Afterwards, correlations were meta-analytically combined using Hedges method, which adjusts estimated standard errors and sample size. To distinguish subjects with high versus low hair cortisol, a median split was carried out.
Model Fitting
To allow use of data from all individual twins, including those without co-twins, and from participants with missing outcome measures, structural equation models (SEM) were fitted using the full information maximum likelihood (FIML) method implemented in Mx (Neale et al., Reference Neale, Boker, Xie and Maes2004). Details of the twin design and analytical methods, including assumption testing and multivariate modeling (below), are described elsewhere (Neale & Cardon, Reference Neale and Cardon1992). For multivariate analysis, a Cholesky decomposition for the ACE model was compared with Cholesky decompositions for simpler AE, CE, and E models. Despite evidence for a non-linear relationship between HCC and the affect variables, we carried out the variance components analysis, assuming only linear relations given our small sample size. The fit of each model was assessed by the differences in log likelihood between the sub and the full models. For all models, sex and age were fitted as fixed effects.
Results
Relation Between HCC and Our Affect Measures
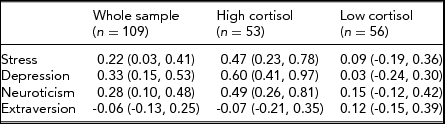
Preliminary inspection of scatterplots of HCC with behavioral measures suggested non-linear relationships. We therefore performed a median split at HCC = 11.36 pg/mg and estimated correlations for the high and low halves of the sample as well as the whole. Table 1 reveals the following: (a) correlations between HCC and the three risk factors — perceived stress, symptoms of depression, and neuroticism — are modest and significant in the whole sample but high and significant for the group of high HCC (HCC > 11.36 pg/mg); (b) there are no significant associations between HCC and the three risk factors in the low-cortisol group (HCC ≤ 11.36 pg/mg); (c) there is no significant association between extraversion and HCC.
TABLE 1 Correlations (95% C I) Between Psychological Variables and HCC in the Whole Sample (n = 109), and after Median Split (HCC >\≤11.36 pg/mg)
Twin Correlations
The twin- correlations between the psychological variables of perceived stress, neuroticism, extraversion, and HCC are displayed in Table 2. As expected, correlations between MZ twins are higher than for DZ twins for neuroticism, perceived stress, and extraversion. However, unexpectedly, the correlation for HCC is higher in the DZ (r = 0.63) than in the MZ (r = 0.20) group, though not significantly so, given these small sample sizes.
TABLE 2 Pearson Correlations Between All Variables in DZ (21 Pairs) and MZ (8 Pairs) Twins (MZ in lower, DZ in Upper Triangle) Corrected for Age and Sex

Genetic Covariance Between HCC and Affect Variables
Rather than focus on the zero estimate of heritability for HCC (which is likely a stochastic result due to low numbers), we are more interested in evidence for genetic covariance between HCC and the psychological variables. To investigate this, we fitted a series of Cholesky decomposition models, including variously A, C, and E, as sources of covariation. The aim of the analyses was to determine whether genetic influences specific to HCC exist after perceived stress, neuroticism, and depressive symptoms have been accounted for. Therefore, perceived stress was used as the first, depressive symptoms as the second, neuroticism as the third, and HCC as the fourth latent factor. We began by fitting an ACE Cholesky model, but no significant worsening of the fit was observed when either the C matrix was fixed to zero (χ2 10 = 4.51) or the A matrix was fixed to zero (χ2 10 = 4.07). In fact, both A and C matrices could be fixed to zero without fatally affecting fit (χ2 20 = 18.22) underlining the fundamental lack of power of our experiment. Nevertheless, the point estimates from the ACE Cholesky decomposition are of great interest. Table 3 shows that the estimates of factor loadings for the A matrix are substantial, those for the C matrix are negligible except for C (4,4), the specific loading on HCC, reflecting the low r mz for HCC noted above. Indeed, even the cross-loadings for the E matrix are considerably smaller than their corresponding elements in the A matrix. Notwithstanding the deficiencies in power of our study, our results point strongly to the predominant importance of genetic factors in shaping the correlations observed between HCC and the psychological variables.
TABLE 3 Cholesky Decomposition for ACE Model
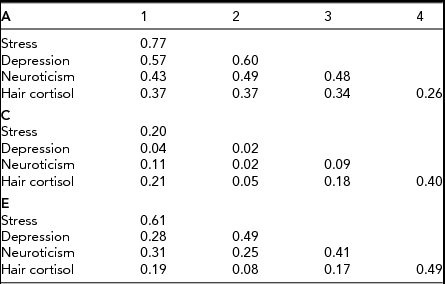
Note: Latent factor loadings are standardized to unit variance and must be squared to obtain standardized variance components.
Nevertheless, because A and C estimates tend to be intimately confounded in the twin model, and because the point estimates from Figure 1 suggest that the dominant source by far is A, for simplicity, in Figure 1 we show the standardized path coefficients for the AE Cholesky decomposition (these must be squared to obtain variance components), remembering that, to a small extent, the A estimates are confounded with some shared environmental effects. Under this simplified model, genetic factors would account for more than half the total variance for all four variables. Further, the first genetic factor (A1), which loads primarily on perceived stress and accounts for 63.3% of its variance, also accounts for 32.2% of the variance in depressive symptoms, 20% of the variance for neuroticism, and 11.4% of the variance for HC. The second genetic factor (A2), which loads primarily on depressive symptoms (35.7%), also accounts for 24.2% of the variance in neuroticism and 15.4% in HCC. The third genetic factor (A3), which loads primarily on neuroticism (23.2%), also accounts for 11.6% of the variance in HC. This leaves a specific genetic contribution (A4) to HCC that accounts for 24.9% of its variance. Decomposition of the non-shared environmental covariance shows that the first factor (E1) accounts for 36.7% of the variance for neuroticism and also accounts for 7.6% of the variance in depressive symptoms, 9.6% of the variance in perceived stress, and 4% of the variance for HC. The second factor (E2), which loads on depressive symptoms (24.6%), also accounts for 6% of the variance in perceived stress and 0.3% of the variance for HCC. The third factor (E3) loads on neuroticism and accounts for 17% of its variance and 3% on HCC. The specific non-shared environmental contribution to HCC (E4) accounts for 29.9 % of its variance.

FIGURE 1 Cholesky decomposition for AE model. Latent factor loadings are standardized to unit variance and must be squared to obtain standardized variance components. A1–A4 additive genetic factors, E1–E4 unique environmental factors.
Genetic Correlation Between HCC and Affect Variables
Also for simplicity, the genetic and environmental correlations between the four variables, derived from the AE Cholesky analysis, are shown in Table 4.
TABLE 4 Additive Genetic and Unshared Environmental Correlations Between Psychological Variables and HCC Corrected for Age and Sex

We can see that the correlations between the three psychological variables largely derive from genetic sources. The correlations between these three and HCC are also much more strongly genetically than environmentally influenced.
Discussion
The aim of this exploratory study was to assess the association between HCC and three affect variables we might reasonably expect to be associated with it: perceived stress, symptoms of depression and neuroticism. We also included extraversion as a control variable with no a priori expectation of association. Our sample included a small number of complete MZ and DZ twin pairs, and these enabled us to explore the genetic and environmental contributions to covariation between our variables. In particular, we were interested in the causes of covariation between HCC and the affect variables. Finally, we were interested in whether relationships between HCC and the affect variables are linear, and we found some evidence that the relationship is much stronger in those with high HCC, suggesting there might be some threshold effect.
Our results show moderate but significant associations between HCC and neuroticism, perceived stress, and depressive symptoms, respectively (r = 0.22–0.33). Interestingly, splitting the sample into low versus high HCC revealed that lower levels of HCC seem to be unrelated to these psychological variables whereas the associations in the high-HCC group are substantial and significant (r = 0.47–0.60). Furthermore, our study suggests strongly that these associations are genetic in origin. As expected, however, extraversion is not associated with HCC in either the high- or the low-HCC group, and this accords with previous studies of other cortisol markers (Munafò, Reference Munafò, Lee, Ayres, Flint, Goodwin and Harmer2006; Wilson et al., Reference Wilson, Zilioli, Ponzi, Henry, Kubicki, Nickels and Maestripieri2015).
Positive correlations between perceived stress and cortisol markers have been reported previously, even though the reports are not consistent (Staufenbiel et al., Reference Staufenbiel, Penninx, Spijker, Elzinga and van Rossum2013; Vanaelst et al., Reference Vanaelst, De Vriendt, Huybrechts, Rinaldi and De Henauw2012). These inconsistencies, together with our finding that the association is stronger in the high-HCC group, might indicate a potential pathological threshold for HCC; although, mindful of our small sample size, we have to regard this finding most tentatively, and clearly it needs to be replicated in a much larger dataset. To our knowledge, no previous study has suggested the existence of such a threshold, although others have suggested a more complex non-linear relationship (Wells et al., Reference Wells, Tremblay, Flynn, Russell, Kennedy, Rehm and Graham2014).
In monkeys, the heritability for HCC has been estimated at 30% (Fairbanks et al., Reference Fairbanks, Jorgensen, Bailey, Breidenthal, Grzywa and Laudenslager2011), while other twin studies in humans suggest that genetic factors account for 62% of the variance of saliva and serum cortisol (Bartels et al., Reference Bartels, Van den Berg, Sluyter, Boomsma and de Geus2003). Due to the small sample size, we were not able to reliably assess the heritability of HCC; the DZ correlation for HCC is high (r = 0.63) and most likely the very low MZ correlation (r = 0.20) reflects our having only eight MZ twin pairs; we are currently collecting a much larger sample that will give us a more reliable estimate.
Most pertinent to the main aim of our study, our results suggest that the association between HCC and the affect variables seems to be largely driven by genetic factors. This supports the hypothesis of a common genetic association between all four measures, whereas environmental factors do not seem to contribute much to covariation. This is consistent with our results from a much larger twin sample (of which the present study uses a subsample) in which we estimated high genetic covariances between neuroticism, depression, and perceived stress (Rietschel et al., Reference Rietschel, Zhu, Kirschbaum, Strohmaier, Wüst, Rietschel and Martin2014).
The most obvious limitation of our study is the small sample size; this will be rectified in our forthcoming study with a sample six times larger than here. The much greater limitation, however, is that even if the relationships we observe here are replicated with increased significance, it will not elucidate the direction of causation in the correlation between these variables. While it is possible that perceived stress is environmentally induced and in turn induces high HCC, the reverse could also be true. Another suggestion from our present results though is compatible with a pleiotropic explanation, in which the same genes simultaneously influence both HCC and the psychological variables. Elucidation of this conundrum might be advanced by sensitive longitudinal studies, or as a byproduct of large-scale GWAS studies that enable construction of powerful instrumental variables for use in Mendelian randomization analyses (Richmond et al., Reference Richmond, Sharp, Ward, Fraser, Lyttleton, McArdle and Relton2016). Larger samples of MZ twins with varying degrees of discordance for HCC and the other measures here may also shed light on the nature and direction of causation between these variables.
In conclusion, our pilot study provides new evidence that HCC is related to psychological variables that elevate the risk for psychiatric disorders but not extraversion. Further, these correlations are seen most strongly in the higher range of HCC values, suggesting some sort of threshold effect. Most interestingly, analysis of these associations in MZ and DZ twins suggest that they are largely driven by genetic rather than either familial or idiosyncratic environmental influences. However, the direction of causation between these variables remains unclear.
Acknowledgments
We thank twins and their family members who participated in the BLTS and provided hair and blood samples, as well as nurses Natalie Garden and Reshika Chand, who made categorical ratings of hair curliness; and we thank Kerrie McAloney for coordination.
Financial Support
Funding for collection of hair samples from twins was provided by Australian NHMRC grants to NGM. Funding for measurement of hair samples (by Mark Brims, BSC Electronics, Perth) and for salary for Yvonne Ho was provided by an Australian Research Council Linkage Grant (LP 110100121: P.I. Dennis McNevin, University of Canberra) with contributing funding from Identitas Inc (principals Mike Fitzgerald (Monaco), Laurence Rubin (Toronto) via the Visigen Consortium convened by Tim Spector (Kings College London); further contributions were from the Victorian Police Forensic Services Department (Runa Daniels, Roland van Oorschot), the Australian Federal Police Forensics (Simon Walsh, Paul Kirkbride), and Unisys Australia (David Chadwick). Hair cortisol assays were funded by a grant to MR from the German Federal Ministry of Education and Research (BMBF) through the Integrated Network IntegraMent (Integrated Understanding of Causes and Mechanisms in Mental Disorders, grant BMBF01ZX1314G), under the auspices of the e:Med Programme.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The project was approved by the QIMR Human Research Ethics Committee.







