History of the Guangzhou Twin Eye Study
The Guangzhou Twin Registry is a population-based registry of twins in Guangzhou, China. In early 2005, with the assistance of the Guangzhou City Bureau of Statistics, those who shared the same birthday, home address and household owner were selected from the Official Resident Registry as possible twins. After double-checking the address and parents’ names, a total of 9709 pairs of twins born between 1987 and 2000 were identified. Door-to-door visits were undertaken by trained interviewers from the Guangzhou City Bureau of Statistics between January and March 2006.
Following this, all twins aged 7–15 years, living in two neighboring districts of the Zhongshan Ophthalmic Center, were invited to attend an annual eye examination. A total of 705 pairs of eligible twins were invited, and 559 were examined in 2006. The sample size was further expanded by enrolling twins from other districts in Guangzhou. These additional recruitment phases to increase the sample size took place in 2008, 2009, 2010 and 2012. At present, there are 1291 sets of twins enrolled, and 11 year’s follow-up visits were completed in 2018 (Table 1). Biological parents of these twins were also invited to attend the baseline examinations.
Table 1. Sample size enrolled in each year in Guangzhou Twin Eye Study
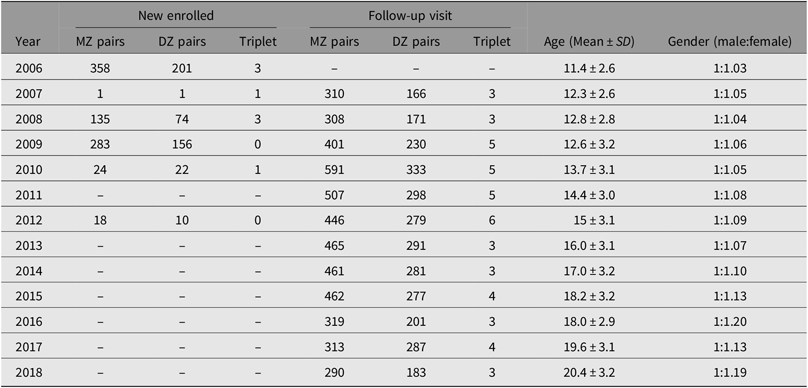
Note: MZ = monozygotic, DZ = dizygotic.
The major aim of this young twin study is to explore the etiology of common eye diseases, particularly myopia and glaucoma. Ocular phenotypes related to myopia progression, such as cycloplegic refraction, axial length (AL), height and weight were collected annually. Phenotypes not related to myopia, such as parental refraction, central corneal thickness, fundus appearance and intraocular pressure, were only collected at baseline. The systemic and ocular data collected during the study are summarized in Table 2.
Table 2. Data collection in the Guangzhou Twin Study
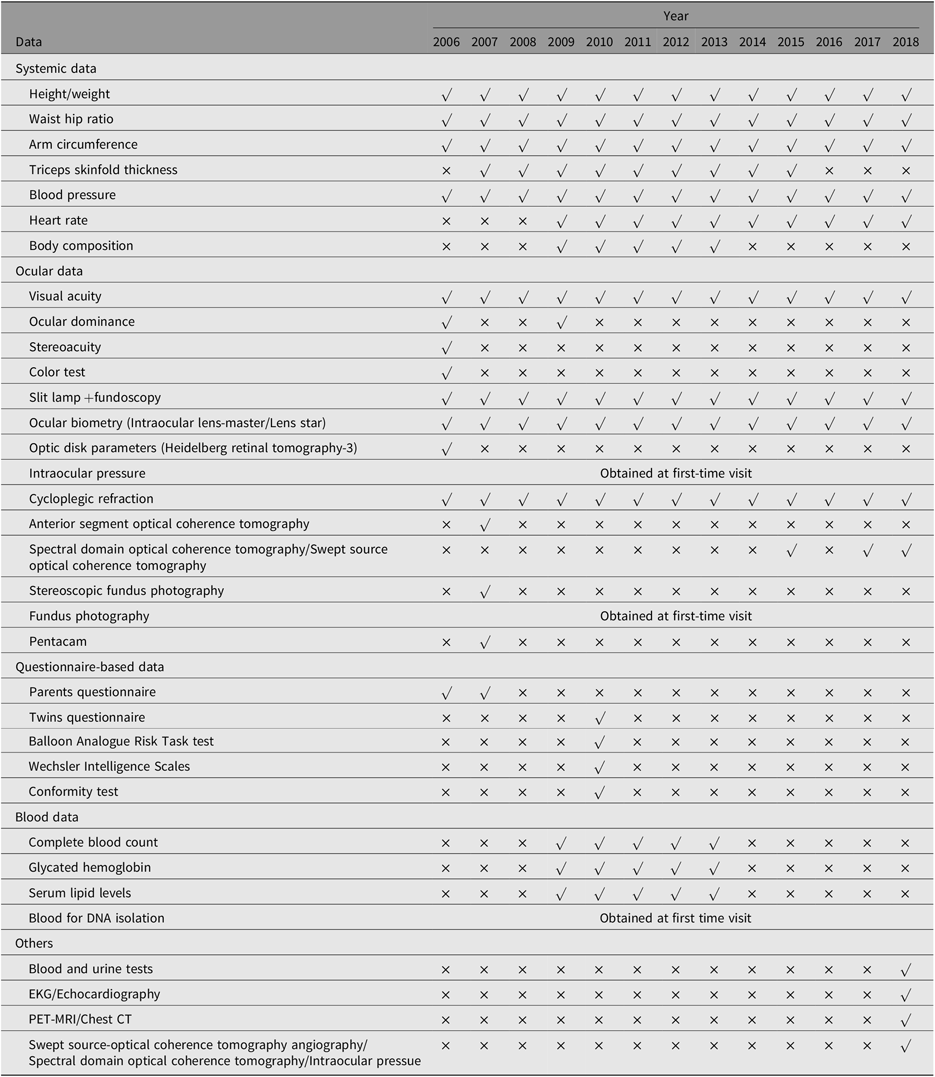
Note: Collected demographic data, zygosity, birth weight, gestational age, parental lifestyles, parental education level, parental economic status, ocular and medical history of parents and twins, indoor and outdoor activities, near works.
IOL, Intraocular lens; HRT-3, Heidelberg Retinal Tomography-3; AS-OCT, anterior segment optical coherence tomography; SS-OCT, Swept Source optical coherence tomography; SD-OCT, spectral domain optical coherence tomography; IOP, intraocular pressure.
Approximately 10 ml of blood was taken from all the twins at their baseline visit for DNA isolation and zygosity testing if required. All DNA were isolated by Fuji Film Quick Gene kit and stored in a −80 °C refrigerator. Zygosity of all same-sex twin-pairs was determined by conducting 16 multiplex short tandem repeats (PowerPlex 16 System; Promega, Madison, WI, USA) at the Forensic Medicine Department of Sun Yat-sen University. Opposite-sex twin-pairs were deemed dizygotic (DZ); therefore, they did not require genotyping.
This study was conducted in accordance with the tenets of the World Medical Association’s Declaration of Helsinki. Ethical approval was obtained from the Ethics Committee of Zhongshan Ophthalmic Center. Prior to examination, written informed consent was obtained from parents or guardians of all the twins after careful explanation of the project objectives and examination procedures. This included the risks and benefits of participation, and the need to analyze DNA information for further etiology exploration.
Major Findings of the GTES
Phenotypic Heritability Study and New Statistical Methods
A principle goal of twin studies is to estimate heritability, as well as the proportion of individual phenotypic variation that can be explained by genetic variations among individuals at a given time and in a given population. Phenotypic variance encompasses several genetic effects: additive (A) or dominant (D) genetic variance; and environmental effects: shared (C) or unique (E) environmental variance. The E component also contains measurement error. In the design of classic twin studies, as the C and D components confound each other when pairs of twins are reared together, only one parameter (either C or D) is allowed to be included in a single model. If the pairwise correlation in dizygotic twins is less than half of that in monozygotic (MZ) twins, it suggests that genetic dominance is a major contributor. In this case, the saturated model is fitted with an ADE model; otherwise, the saturated model is fitted with an ACE model. Using this traditional theory, we are able to estimate myopic ocular traits such as refraction, AL, central corneal thickness, intraocular pressure, optic disk parameters, peripheral refraction and peripheral eye length, which were similarly high, ranging from 0.6 to 0.9 (Table 3) (Ding et al., Reference Ding, Lin, Huang, Zheng, Congdon and He2012, Reference Ding, Chen and He2018; He, Ge et al., Reference He, Ge, Wang, Zhang, Hewitt, Hur and Foster2008; He, Liu et al., Reference He, Liu, Huang, Zhang, Yin, Zheng and Ge2008; He et al., Reference He, Wang, Console, Zhang, Zheng and Huang2009; Shen et al., Reference Shen, Ding, Zheng, Congdon and He2012; Zheng, Ge et al., Reference Zheng, Ge, Huang, Zhang, Liu, Hur and He2008; Zheng, Huang et al., Reference Zheng, Huang, Huang and He2008; Zheng et al., Reference Zheng, Xiang, Huang, Huang, Yin and He2009). Our results concur with previous heritability data from the Australia (Dirani et al., Reference Dirani, Chamberlain, Shekar, Islam, Garoufalis, Chen and Baird2006) and the UK twin registry (Lopes et al., Reference Lopes, Andrew, Carbonaro, Spector and Hammond2009), which suggests there is consistency in the level of heritability across different ethnic groups and environments.
Table 3. Heritability estimates using cross-sectional data from the Guangzhou Twin Study
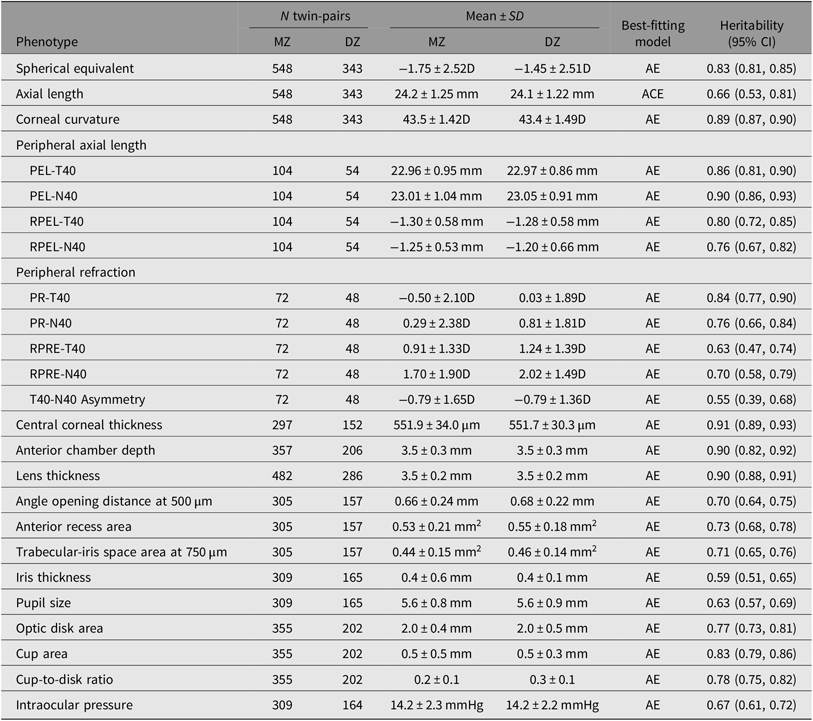
A = additive genetic effect; C = common environmental effect; E = unique environmental effect; D = diopter; PR = peripheral refraction; RPRE = relative peripheral refractive error; PEL = peripheral eye length; RPEL = relative peripheral eye length; T40 = temporal 40°; N40 = nasal 40°.
Nonetheless, it has been argued that ACE and ADE models can overestimate the phenotypic heritability, as described above. Adding parental phenotypic information into the model (extended twin family design study) allows us to distinguish the effects of C and D from each other (Neale & Cardon, Reference Neale and Cardon1992). This shows that the ACDE model is less biased when estimating heritability than the ADE or ACE model (Keller & Coventry, Reference Keller and Coventry2005). However, if we use this ACDE model when estimating the phenotypical heritability of myopia, a significant difference may exist between parents and children due to changes in environmental exposure in China over the last three decades.
We can assume there is a heterogeneous environmental effect between two generations. Therefore, we built a statistical model to test whether this heterogeneity (H) effect existed in our myopia-related phenotypes, named as the ACDE-H model (Guo et al., Reference Guo, Liu, Wen, He and Wang2013). Three myopia-related biometrics — spherical equivalence (SE), AL and corneal curvature (CC) — were evaluated using three models: ACE or ADE model, using only twins’ information; traditional ACDE model, using parents and twins’ data together; and our ACDE-H model, using the same data as the ACDE model. We found that compared to the classic twin study (ACE model), the extended twin study (ACDE model) significantly decreases the phenotypic heritability of AL and SE, but not CC. Furthermore, differences in environmental exposures between parents and children were only significant for SE and AL, which explained about 9.6% of the variation for SE, and about 17.1% of AL variation (Ding et al., Reference Ding, Guo, Morgan and He2013). This heterogeneity effect of SE and AL also supports the idea that environmental change has been a significant contributor to the myopia boom in recent decades (Dolgin, Reference Dolgin2015).
Shared Genetic Determinants of Ocular and Systemic Phenotypes
Many genetically related systemic traits coexist with ocular traits, suggesting that these phenotypes or diseases may have shared genetic pathways. In the past 12 years, a broad range of phenotypes have been collected through the GTES, which has allowed us to investigate whether correlations between phenotypes result from shared genetic factors. Using the Cholesky model, we quantified the shared genetic effects among angle opening distance (AOD), ACD and AL. We found that 23% of genetic factors are shared between AOD and ACD, 13% between AOD and AL, and 25% between ACD and AL (He, Hur et al., Reference He, Hur, Zhang, Ding, Huang and Wang2008). This finding confirms the strong, shared additive genetic effect of traits related to angle closure and myopia, and reveals that the pleiotropic actions of genes probably contribute to the associations between angle closure and myopia-related traits. Moreover, a significantly positive relationship was found between AL and height, with 89% of this phenotypic correlation due to shared genetic factors (Wang et al., Reference Wang, Ding, Liu, Zhang and He2011; Zhang et al., Reference Zhang, Hur, Huang, Ding, Feng and He2011). In addition, we have identified a significant association between cardiovascular risk factors (such as blood pressure and BMI) and retinal vascular caliber (an early marker of microvascular damage) and, more importantly, the shared genetic components for these phenotypic correlations (Xiao et al., Reference Xiao, Gong, Chen, Ding, Chang and He2015; Zheng et al., Reference Zheng, Huang, Zhang and He2013). For example, 83.3% of the phenotypic correlation between mean arterial pressure and retinal arteriolar caliber was attributable to shared genetic factors, while the phenotypic correlation between BMI and retinal arteriolar caliber was nearly 100%. These genetic findings indicate the important influence that shared genetic factors have on ocular and systemic diseases. There is also the potential to identify genes unique to each phenotype, which may help to unravel the underlying pathogenesis of its correlated phenotypes.
Environmental Factor Estimation on Myopia
School-age myopia is a complex eye condition. It involves genes, the environment and gene–environment interactions. Near work and outdoor activity are considered two important environmental factors. However, previous environmental estimations cannot adjust for the genetic background from environmental exposure or be adjusted by including parental refraction in the regression model, which is a relatively crude and contestable method. MZ twins share age, gender, genetic background and family culture. So, it is reasonable to assume that differences in environmental exposures are responsible for the discordance among MZ twins.
We used this MZ twin control methodology to explore the effect of near work and outdoor time on myopia. A standard questionnaire was used to collect data on near work and time spent outdoors (He et al., Reference He, Xiang, Zeng, Mai, Chen, Zhang and Morgan2015). Using mixed-model analysis, we found that difference in the amount of near work was a risk factor for discordance in myopic SE. Furthermore, the interaction between time spent outdoors and age was a protective factor for discordance in myopic SE, but the overall association between difference in the amount of time spent outdoors and SE discordance was not significant. Furthermore, the difference in near work and time spent outdoors explained only about 1.8% and 2.5% of the variation in SE discordance, respectively.
Collaborative Findings with Domestic and Overseas Researchers
In 2013, we received an invitation to COllaborate with the Development of Anthropometrical measures in Twins (CODA Twins) consortium, which aims to enroll a large sample of twins from birth to old age to explore the effects of genetic and environmental factors on height, weight and BMI (Jelenkovic et al., Reference Jelenkovic, Yokoyama, Sund, Honda, Bogl, Aaltonen and Silventoinen2015; Silventoinen et al., Reference Silventoinen, Jelenkovic, Sund, Honda, Aaltonen, Yokoyama and Kaprio2015, Reference Silventoinen, Jelenkovic, Sund, Hur, Yokoyama, Honda and Kaprio2016). We also collaborated with psychologists to explore factors influencing risk-taking propensity and intellectual quotient (IQ) of twins using the Balloon Analogue Risk Task and the Wechsler Intelligence Scale (WISC-IV-Chinese version). The multivariate regression model revealed that risk-taking propensity increased significantly with increasing age. Furthermore, higher IQ was significantly associated with lower SNP. Our team developed two behavior tests — word memorization and price estimation — to examine the conformity of twins. We found that social conformity was inheritable, with a heritability estimation of 0.25 to 0.37, providing a basis for further exploration of the molecular mechanism of conformity. In addition, we identified several inherited susceptibility genes related to social conformity, including NAV3, PTPRD, ARL10 and CTNND2 using genomewide association study analysis (Chen, Zhu et al., Reference Chen, Zhu, Wang, Ding, Guo, He and Rao2018).
Current Works and Future Plan
The previous focus of the GTES was to establish a longitudinal cohort of ocular disease of twins from childhood to adulthood. The available 13 year’s annual data are particularly valuable for identifying genetic factors and the effects of early environmental exposure on ocular and systemic diseases. After completing the 13 year’s follow-up, most participants had entered adulthood. Therefore, one of our current efforts is to analyze longitudinal data to quantify the longitudinal phenotype changes and potential predictors. In addition, we plan to extend our study to the following areas to maximize the scientific potential of the project:
1. Quantitative analysis of longitudinal phenotype changes and influence of genetic and environmental factors. The initial goals of the study were to identify and quantify the genetic components that account for the variation in common ocular phenotypes such as SE and AL, and these have been fulfilled. However, the longitudinal change of ocular phenotypes in twins is unclear. In addition, the genetic effects of many novel phenotypes, including in-vivo imaging metrics, were obtained by advanced imaging devices (such as vessel density and foveal avascular zone from optical coherence tomography angiography) and new molecular biomarkers in blood and urine (such as levels of noncoding RNA expression, methylation levels at CpG sites and serum metabolite). Finally, we are working on the quantification of dynamic heritability in this longitudinal cohort.
2. Identifying the key structural and functional differences from full-body multimodality imaging between MZ twins. Each individual is unique, including identical MZ twins (Brodin et al., Reference Brodin, Jojic, Gao, Bhattacharya, Angel, Furman and Davis2015; van Dongen et al., Reference van Dongen, Slagboom, Draisma, Martin and Boomsma2012). Researchers are keen to understand intratwin differences and the mechanism of monozygosity. Various studies have identified the structural difference between MZ twins; for example, morphological analysis of three-dimensional magnetic resonance imaging (MRI) revealed that brain volume measures were highly correlated within MZ twins but surface measures of the brain were influenced by environmental factors (de Manzano & Ullen, Reference de Manzano and Ullen2018; Oppenheim et al., Reference Oppenheim, Skerry, Tramo and Gazzaniga1989; Steinmetz et al., Reference Steinmetz, Herzog, Huang and Hacklander1994; White et al., Reference White, Andreasen and Nopoulos2002). However, whether the key differences resulted from genetic factors, environmental factors or at random are poorly understood due to the limitations of the imaging modality. With the development of more sophisticated in-vivo devices, total body imaging will be able to better characterize the structure and molecular processes in humans. Among these, the combined positron emission tomography (PET) and MRI imaging enables simultaneous imaging, providing molecular, morphological and functional information (Cherry et al., Reference Cherry, Jones, Karp, Qi, Moses and Badawi2018; Sauter et al., Reference Sauter, Wehrl, Kolb, Judenhofer and Pichler2010). Multimodality imaging data of a subgroup of 20 pairs of MZ twins from the GTES were acquired using total body PET/MRI scanning, chest computed tomography (CT) scanning, echocardiography, vascular ultrasound and ocular imaging. This subgroup of MZ twins also underwent wealth assays, including regular blood and urine tests, serum electrolyte, complete lipid and metabolic panels, serum viscosity, immune system biomarkers, metabolic system, endocrinal system, kidney and liver. In addition, participants completed various surveys in relation to medical history, diet, physical activity, sleep, cognition and stress. Wearable devices were also equipped to obtain information on activity levels, sleep, physiology and continuous glucose monitoring. In-depth analysis is ongoing to detect key differences in image-derived phenotypes within MZ twins, which will pave the way for a better understanding of the biological difference of MZ twins.
3. Continuous collection of multidimensional, dense, dynamic data clouds of MZ twins from a longitudinal big data approach. The precision medicine (personalized, P4 or stratified medicine) outcomes highlighted the medical decision based on personal characteristics, including molecular and behavioral biomarkers, rather than the average level of population. The recent advances in multiomics technology and wearable devices have enabled deep molecular analysis and physiological monitoring, providing a vital source of big data for precision medicine (Chen et al., Reference Chen, Mias, Li-Pook-Than, Jiang, Lam, Miriami and Snyder2012; Chen, Xia et al., Reference Chen, Xia, Tu, Duan, Kukurba, Li-Pook-Than, Xie and Snyder2018; Schussler-Fiorenza Rose et al., Reference Schussler-Fiorenza, Contrepois, Moneghetti, Zhou, Mishra, Mataraso and Snyder2019). Precision medicine depends on data science, especially machine learning (Karczewski & Snyder, Reference Karczewski and Snyder2018; Trachana et al., Reference Trachana, Bargaje, Glusman, Price, Huang and Hood2018). There is enthusiasm for the potential of big data and machine learning for precision medicine and health, but few examples are currently available in the literature. MZ twins provide a unique opportunity for practicing precision medicine because they are perfectly matched in terms of age, gender, genome and early-life environmental factors.
We plan to collect personal, multidimensional, dense, dynamic data clouds of MZ twins using a longitudinal big data approach. Participants will regularly receive multiomic tests, physiological examinations, total body and ocular imaging, and will be continuously monitored using wearable devices. Biological samples such as blood, saliva, urine and stool will be collected for obtaining multi-omic data, including DNA methylation, transcriptome, metabolome, proteome, lipidome, antibodome and microbiome. Continuous monitoring using wearable sensors is ongoing, collecting the lifestyle and physiological information for near work, physical activity, sleep, body composition and heart rate, as well as environmental information such as temperature, rainfall, humidity, wind speed, particulate matter and radiation exposure. In addition, various phone applications are applied to allow participants to easily report changes in health and lifestyle, such as diet, exercise, stress, mood and disease. The integrated cloud data enable deep profiling to create a huge ‘personal biology map’ for each MZ pair. We are currently seeking multidisciplinary collaborators for analysis of the deep personal dataset using big data and machine learning technologies with the aim to (1) identify the key differences within MZ twins resulting from nongenetic influences by exploring deep longitudinal profiling; (2) discover novel molecular biomarkers, behavior or environmental factors that may impact human health; and (3) develop prediction models for medical conditions using integrated measurements. GTES data collection is still ongoing and will last a lifetime. We are open to new collaborations. Any requests for cooperation or biobank data sharing can be made by contacting Professor He Mingguang ([email protected]).
Financial support
The research funded by National Natural Science Foundation of China (81600763) and Fundamental Research Funds of the State Key Laboratory. Prof. Mingguang receives support from the University of Melbourne Research Accelerator Program and the CERA Foundation. The Centre for Eye Research Australia receives Operational Infrastructure Support from the Victorian State Government. The sponsor or funding organization had no role in the design or conduct of this research.
Conflict of interest
The authors have no financial or other conflicts of interest concerning this study.





