Fumarate hydratase (FH) deficiency (OMIM 606812) is a rare autosomal recessive defect of the Krebs Tricarboxylic Acid (TCA) cycle caused by mutations in fumarate hydratase (FH) gene (OMIM 136850). The FH gene on chromosome 1q42 encodes two isoenzymes, one in the cytosol and the other in the mitochondrial matrix (Bourgeron et al., Reference Bourgeron, Chretien, Poggi-Bach, Doonan, Rabier, Letouze and Rustin1994).
Patients with fumarase deficiency can present with poor feeding, microcephaly, fumaric aciduria, and limb dystonia. Neurological symptoms can include severe developmental delay, infantile spasms, seizures, and regression (Allegri et al., Reference Allegri, Fernandes, Scalco, Correia, Simoni, Llerena and de Oliveira2010; Kerrigan et al., Reference Kerrigan, Aleck, Tarby, Bird and Heidenreich2000). All patients described so far appear to have had a defect of both cytosolic and mitochondrial fumarase. Infantile lethality is commonplace in this rare inborn error of metabolism. Carriers of a single FH gene mutation are at risk of developing the hereditary leiomyomatosis and renal cell cancer (Hereditary leiomyomatosis and renal cell cancer [HLRCC], OMIM 136850) phenotype.
We identified FH deficiency in a dichorionic diamniotic (DCDA) twin pregnancy. Our patients demonstrated hepatic disease as part of their clinical phenotype, and a novel mutation was identified in the FH gene.
Case Report
Our patients were the products of a non-consanguineous Caucasian family with no family history of twinning. The antenatal period was unremarkable, with ultrasound scans performed at 11, 19, and 30 weeks gestation indicating a DCDA twin pregnancy of morphologically normal males. They were delivered via emergency lower segment cesarean section at 32 weeks gestation for premature onset of labor.
Both neonates developed hyaline membrane disease requiring assisted ventilation. At 6 months of age they presented for pediatric assessments in view of global developmental delay, poor feeding, and extreme irritability.
Abnormal neurological examination findings included postnatal microcephaly, hypotonia, dystonic limb posturing, cortical visual impairment, convergent strabismus, no developmental milestone attainment, tongue thrusting and an unsafe/uncoordinated oral motor swallow mechanism, and features of the brain stem dysfunction. An initial diagnosis of cerebral palsy was postulated, but surmised to be unlikely as there was no obstetric or postnatal setting to explain hypoxic ischemic encephalopathy.
Brain magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS) identified global cerebral atrophy, corpus callosum thinning, hypoplastic brainstem, and hyperintense lesions in the basal ganglia, especially in the caudate and thalamic nuclei, with an elevated lactate peak on MRS.
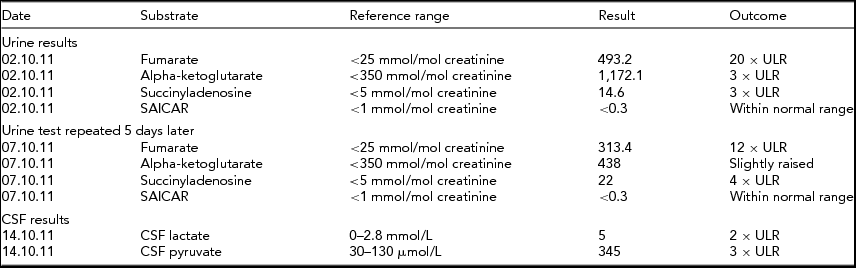
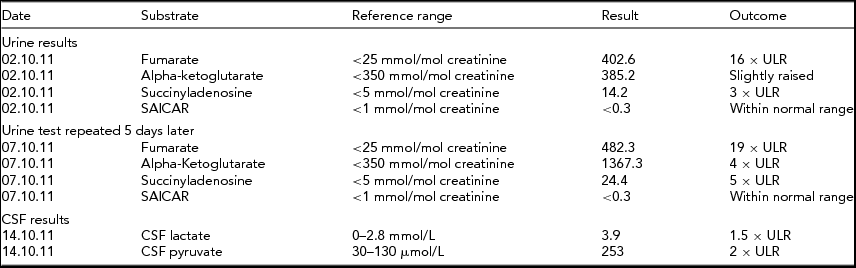
Cerebrospinal fluid had elevated lactate and pyruvate, further suggesting a mitochondrial disease process. Urine organic acids were suggestive of FH deficiency as repeated samples indicated high fumarate, alpha-ketoglutarate, and succinyladenosine (see Tables 1 and 2).
TABLE 1 Biochemical Results for Twin 1

The biochemical results found for twin 1 indicate persistent high levels of fumarate, indicative of fumarase deficiency. Raised alpha-ketoglutarate can be increased in this condition and raised levels of succinyladenosine can be secondary to increased fumarate levels.
ULR = Upper limits of reference range.
TABLE 2 Biochemical Results for Twin 2

The biochemical results found for twin 2 are similar to those for twin 1 and there are persistent high levels of fumarate, indicative of fumarase deficiency. Raised alpha-ketoglutarate can be increased in this condition and raised levels of succinyladenosine can be secondary to increased fumarate levels.
ULR = Upper limits of reference range.
Fumarase enzyme activity was assayed on cultured fibroblasts for both twins and was undetectable in both patients’ cell lines (see Table 3 and Figure 1).
TABLE 3 Fumarase Enzymology Results

The fumarase enzymology resulted in undetectable fumarase levels in both patients, with deficient control values as expected for the assay.

FIGURE 1 Example of kinetic spectrophotometric trace. Note: Fumarase enzyme levels are tested spectrophotometrically on whole cell homogenates in an assay that measures the rate of dehydration of L-malate to fumarate at a maximum absorption of 250 nm over 5 min, therefore obtaining an estimate of the level of fumarase enzyme activity in cell extracts.
Sequence analysis of the FH gene showed the siblings were compound heterozygous for c.1037G>A (p.Gly346Asp) in exon 7, and c.1431_1433dup (p.Lys477dup) in exon 10. The p.Lys477dup mutation has been described previously (Loeffen et al., Reference Loeffen, Smeets, Voit, Hoffmann and Smeitink2005) and was inherited from their mother. The p.Gly346Asp mutation, inherited from their father, is a novel mutation hitherto unreported in the medical literature; however, silico analysis (http://genetics.bwh.harvard.edu/pph2/, http://blocks.fhcrc.org/sift/SIFT.html) predicted this to be a pathogenic mutation. A prenatal diagnostic test was performed via chorionic villous sampling at 12 weeks gestation for a subsequent unaffected pregnancy.
Over the subsequent months both boys developed hepatomegaly with a hard cirrhotic liver edge on clinical palpation, and pitting peripheral edema suggestive of liver cirrhosis and hepatic synthetic dysfunction. However, this clinical component of their phenotype was not actively investigated further, as a non-invasive palliative care approach to their management had been adopted to maximize quality of life. Both boys succumbed to their disease three months apart in their second year of life.
Discussion
Fumarate hydratase deficiency is a rare inborn error of metabolism, with less than 50 cases reported to date (Allegri et al., Reference Allegri, Fernandes, Scalco, Correia, Simoni, Llerena and de Oliveira2010). Most affected infants have demonstrated severe global developmental delay, encephalopathy, microcephaly, seizures, and hypotonia (Allegri et al., Reference Allegri, Fernandes, Scalco, Correia, Simoni, Llerena and de Oliveira2010; Kerrigan et al., Reference Kerrigan, Aleck, Tarby, Bird and Heidenreich2000; Ottolenghi et al., Reference Ottolenghi, Hubert, Allanore, Brassier, Altuzarra, Mellot Draznieks and de Lonlay2011). The biochemical clues suggestive of FH deficiency include elevated urine excretion of fumarate, alpha-ketoglutarate, and succinyladenosine. Our cases highlight the importance of considering a neuro-metabolic etiology in infants with an encephalopathy, especially where there is no clear history to indicate hypoxic ischemic encephalopathy. An accurate diagnosis of neuro-metabolic diseases in infants is usually of most importance for predicting prognosis and for determining reproductive options. However, carriers of the FH gene are at risk of HLRCC, and thus accurate diagnosis opens the issue of cascade testing and tumor surveillance.
Mitochondrial disorders present with a range of hepatic clinical phenotypes, including acute fulminant hepatic failure, hepatic synthetic failure, cholestatic jaundice, steatosis, hepatomegaly, and cirrhosis (Lee & Sokol, Reference Lee and Sokol2007). Hepatic involvement has only been reported in four infants with FH deficiency to date, manifesting with cholestasis and hepatomegaly (Allegri et al., Reference Allegri, Fernandes, Scalco, Correia, Simoni, Llerena and de Oliveira2010; Walker et al., Reference Walker, Mills, Hall, Millward-Sadler, English and Chalmers1989; Zeman et al., Reference Zeman, Krijt, Stratilova, Hansikova, Wenchich, Kmoch and Houstek2000). Our siblings demonstrated hepatomegaly and hard cirrhotic liver edge on palpation, while tissue samples were not obtained in view of the palliative nature of their clinical management at the time.
Of the two compound heterozygous mutations identified, the c.1431_1433 dup (p.Lys477dup) mutation in exon 10 has been previously described to be deleterious (Loeffen et al., Reference Loeffen, Smeets, Voit, Hoffmann and Smeitink2005) and has been reportedly found nine times on the the FH gene mutation database (Bayley et al., Reference Bayley, Launonen and Tomlinson2008). The second mutation, c.1037G>A (p.Gly346Asp) in exon 7, has not been previously reported but is predicted (via in silico analysis) to be a pathogenic change. Pathogenic mutations in the FH gene have included 57% missense, 27% frameshift and nonsense mutations, and diverse deletions, insertions, and duplications (Bayley et al., Reference Bayley, Launonen and Tomlinson2008). To date, genotype–phenotype correlations in FH deficiency are lacking (Bayley et al., Reference Bayley, Launonen and Tomlinson2008); however, our siblings demonstrated a very severe clinical–biochemical phenotype.
Approximately one-third of monozygotic twins will be DCDA (Weber & Sebire, Reference Weber and Sebire2010), which is the most likely explanation as to why such a rare inborn error of metabolism has manifested in this twin pregnancy. Rare autosomal recessive inborn errors of metabolism have been infrequently described in the literature secondary to monozygous twinning, including mitochondrial neurogastrointestinal encephalomyopathy (MNGIE; Schupbach et al., Reference Schupbach, Vadday, Schaller, Brekenfeld, Kappeler, Benoist and Mattle2007), sialic acid storage (Martin et al., Reference Martin, Slaugh, Natowicz, Pearlman, Orvisky, Krasnewich and Gahl2003), and aspartylglucosaminuria (Opladen et al., Reference Opladen, Ebinger, Zschocke, Sengupta, Ben-Omran, Shahbeck and Hoffmann2012). Our patients represent the first reported case of FH deficiency in twins.
The FH gene has also been shown to act as a tumor suppressor gene, predisposing carriers to autosomal dominant benign fibroid tumors of skin, uterus, hereditary leiomyomatosis, and renal cell carcinoma (HLRCC), as well as multiple cutaneous and uterine leiomyosarcoma (MCUL) later in life (Deschauer et al., Reference Deschauer, Gizatullina, Schulze, Pritsch, Knoppel, Knape and Gellerich2006; Tomlinson et al., Reference Tomlinson, Alam, Rowan, Barclay, Jaeger, Kelsell and Aaltonen2002). Tumor surveillance programs are well described for select genetic cancer predisposition syndromes, for example, juvenile polyposis coli. The tumor surveillance required for FH mutation carriers is not clear at this stage. We elected to perform 6-monthly abdominal ultrasounds in the parents.
Our cases highlight the importance of considering a neuro-metabolic diagnosis in patients with a ‘cerebral palsy phenotype’, especially when there has been no clear indication of a hypoxic event. The diagnosis of FH deficiency can not only provide some certainty for the parents in terms of natural history progression, but is also a diagnosis with relevance of their own health status, given the cancer predisposition associated with being an FH carrier.