Individuals behave in ways conducive to good health because, among other reasons, they believe doing so brings a better future than they would otherwise experience (Clarke et al., Reference Clarke, Goodwin, Messias and Eaton2008; Dozois & Beck, Reference Dozois, Beck, Dobson and Dozois2008; Goldston et al., Reference Goldston, Kelley, Reboussin, Daniel, Smith, Schwartz and Hill1997; Grenard et al., Reference Grenard, Munjas, Adams, Suttorp, Maglione, McGlynn and Gellad2011; Nsamenang & Hirsch, Reference Nsamenang and Hirsch2015). Not everyone, however, shares such optimism. This fact has led to scholarly (Case & Deaton, Reference Case and Deaton2015; Centers for Disease Control and Prevention, 2003) and popular (Stobbe, Reference Stobbe2018) speculation that health declines in populations in which optimism wanes. Such speculation motivates the present research. We describe ‘collective optimism’ as a characteristic of healthy populations and illustrate how it may manifest in the frequency of twins among male births — a fundamental biological indicator of a population’s willingness to invest in the future.
We, like others, argue that optimism implies a willingness to invest in the future (Herzberg et al., Reference Herzberg, Glaesmer and Hoyer2006; Hooker et al., Reference Hooker, Monahan, Shifren and Hutchinson1992) and assume, also like others (Rose & Day, Reference Rose and Day1990), that individuals in any population form a Gaussian distribution on optimism. A person’s position in this distribution can presumably change over time for reasons peculiar to him or her. Some persons will, therefore, become more optimistic while others become less. Although these persons change places in the distribution, the mean and range of the distribution remain essentially unchanged.
We also embrace the argument that optimism arises or declines not only within individuals but also among them (Bennett, Reference Bennett2011; Biggs, Reference Biggs2003; Chambers & Windschitl, Reference Chambers and Windschitl2004; Olson, Reference Olson2006; Segerstrom & Nes, Reference Segerstrom and Nes2006; Segerstrom & Sephton, Reference Segerstrom and Sephton2010). Parties to the myriad relationships among members of a population share expectations with family, friends and others, known and unknown, regarding the circumstances they can realize together in the future. The optimism of one person will likely affect the expectations of others. Much literature reports, for example, that the willingness to invest money and effort in new and old enterprises appears ‘contagious’, implying a ‘collective’ optimism (Anglin et al., Reference Anglin, McKenny and Short2018). We build on this construct and argue that optimism in the population functions as an informal insurance mechanism. Those encouraging optimism among others have, in effect, a surplus above their current need. They contribute their surplus to a pool of collective optimism available to all to draw from when needed.
Collective optimism will actuarially suffice to serve the population if the circumstances that cause participants to contribute to and withdraw from the pool remain peculiar to them. But, as with any insurance scheme, the pool can become smaller than needed when an ambient stressor simultaneously taxes the optimism of many participants, thereby reducing contributions while increasing withdrawals. Under this circumstance, the range and mean of optimism in the population shifts left, collective optimism shrinks and investment in the future declines.
A decline in collective optimism has implications for population health. Less optimism in the population implies that fewer persons have expectations that investments to improve their circumstances will ‘pay off’ (Hamermesh & Soss, Reference Hamermesh and Soss1974). Suicide provides an extreme but intuitive example of how collective optimism might manifest in population health. Assume, for argument, that epidemiologists had computed the average value of optimism, η, among all persons who committed suicide over many years. Going forward, persons falling below η would presumably contribute disproportionately to the incidence of suicide (Ribeiro et al., Reference Ribeiro, Huang, Fox and Franklin2018). The incidence would, moreover, increase when collective optimism declined because the left shift of the population would put more persons below η (Jones Reference Jones1986, Sia et al., Reference Sia, Williams, Pasco, Jacka, Brennan-Olsen and Veerman2018; Wray et al., Reference Wray, Colen and Pescosolido2011; Yip et al., Reference Yip, So, Kawachi and Zhang2014).
Equating increases in the incidence of suicide to decreases in collective optimism may appear tautological because suicide implies the end of investment in the future. But collective optimism, if real, should also manifest in indicators of population health other than suicide. Consistent with this expectation, depression correlates inversely with the expected benefit of living a healthy lifestyle (Goldman-Mellor et al., Reference Goldman-Mellor, Caspi, Harrington, Hogan, Nada-Raja, Poulton and Moffitt2014) and of complying with treatment (Clarke et al., Reference Clarke, Goodwin, Messias and Eaton2008; Dozois & Beck, Reference Dozois, Beck, Dobson and Dozois2008; Goldston et al., Reference Goldston, Kelley, Reboussin, Daniel, Smith, Schwartz and Hill1997; Grenard et al., Reference Grenard, Munjas, Adams, Suttorp, Maglione, McGlynn and Gellad2011; Nsamenang & Hirsch, Reference Nsamenang and Hirsch2015). Lost optimism thereby becomes, as intuition might suggest, a risk factor for somatic illnesses (Bagner et al., Reference Bagner, Pettit, Lewinsohn and Seeley2010; Bennett & Elliot, Reference Bennett and Elliot2005; Kang et al., Reference Kang, Kim, Bae, Kim, Shin, Yoon and Kim2015).
We move beyond intuitive, if not tautological, connections and suggest that collective optimism, if real, should affect the most fundamental investments humans make. We argue that shifts in collective optimism affect the outcomes of pregnancy because gestation requires investment not only from prospective parents but also from their social networks and communities (Abernethy, Reference Abernethy1994; Balbo et al., Reference Balbo, Billari and Mills2013; Easterlin, Reference Easterlin1976; Lester & Yang, Reference Lester and Yang1992).
More than half of gestations end spontaneously without a live birth (Bruckner & Catalano, Reference Bruckner and Catalano2018). Abortuses, moreover, do not appear randomly sampled from their conception cohorts. Had they survived to birth, they would have more likely exhibited low reproductive fitness in that they would have required more maternal resources than would more robust infants, and yet would have produced fewer, if any, grandchildren to carry maternal genes into the future (Hardy et al., Reference Hardy, Hardy, Jacobs, Lewallen and Hassold2016; Quenby et al., Reference Quenby, Vince, Farquharson and Aplin2002; Salker et al., Reference Salker, Teklenburg, Molokhia, Lavery, Trew, Aojanepong and Roelen2010; Vanneste et al., Reference Vanneste, Voet, Le Caignec, Ampe, Konings, Melotte and Fryns2009). Natural selection, therefore, has apparently conserved mutations that enable females to spontaneously abort fetuses unlikely, if born, to survive to reproductive age (Brosens & Gellersen, Reference Brosens and Gellersen2010). The literature refers to the distillation of conception cohorts into more reproductively fit birth cohorts as ‘selection in utero’ (Bruckner & Catalano, Reference Bruckner and Catalano2018).
The mechanisms that regulate selection in utero appear to ‘sense’ not only the capacity of a fetus, if born, to thrive in the prevailing environment, but also the resources the mother may have to biologically and behaviorally invest in that infant. Some nonconscious ‘decisional biology’ then chooses whether or not the prospective mother invests further in the gestation (Catalano et al., Reference Catalano, Bruckner, Avalos, Stewart, Karasek, Kariv and Casey2017). Gestations of similar fetuses in the same environment do not, however, all spontaneously abort, suggesting that women must differ in willingness to invest in risky gestations.
The willingness to invest in risky gestations suggests an optimism that might occur only during pregnancy and not correlate with optimism in investments that women can report making. Philosophical and neuroscience literature implies, however, that biases and preferences exhibited in investments we can report making also affect those made without conscious awareness (Damasio, Reference Damasio1994; Jeannerod, Reference Jeannerod1997; Koestler, Reference Koestler1967; Ryle, Reference Ryle2009; Soon et al., Reference Soon, He, Bode and Haynes2013; Tobler et al., Reference Tobler, O’Doherty, Dolan and Schultz2007). Consistent with this argument, research reports that the ratio of twins to singletons among Swedish male newborns varies positively with population consumer confidence, a marker of collective economic optimism (Karasek et al., Reference Karasek, Goodman, Gemmill, Falconi, Hartig, Magganas and Catalano2015). That research focused on male twins because they have historically proved poor investments, from an evolutionary perspective, in that their gestations have produced fewer grandchildren than those of singletons or of female twins (Dufour & Sauther, Reference Dufour and Sauther2002; Lummaa, Reference Lummaa2001; Lummaa et al., Reference Lummaa, Pettay and Russell2007). This low reproductive fitness appears attributable to the high death rates among small male infants in general and male twins — the smallest of male infants — in particular (Bolund et al., Reference Bolund, Lummaa, Smith, Hanson and Maklakov2016). Indeed, male twins appear particularly sensitive to threats, such as political terrorism, to communities or broader collectives (Catalano et al., Reference Catalano, Saxton, Gemmill and Hartig2016).
Based on the above arguments, we theorize that temporal variation in suicide among women of reproductive age gauges that population’s collective optimism and willingness to make investments in the future, including the biological investment in gestation. We hypothesize, therefore, that the incidence of suicide among women of reproductive age will predict the male twin ratio. We use data from contemporary Sweden to test the hypothesis that the monthly number of suicides among women of reproductive age correlates inversely with the frequency of twins among live male births. We conduct our test in Sweden to take advantage of high-quality data describing birth outcomes (Källén & Källén, Reference Källén and Källén2003) as well as the incidence of suicide (Tøllefsen et al., Reference Tøllefsen, Hem and Ekeberg2012).
Selection in utero implies that the male twin ratio does not fall in the same month that suicides occur but 3 or 4 months later. Research suggests that selection against small for gestational age males occurs disproportionately between the 18th and 23rd weeks of gestation (Goldhaber & Fireman, Reference Goldhaber and Fireman1991; Li et al., Reference Li, Odouli, Wi, Janevic, Golditch, Bracken and Iriye2002; Owen & Matthews, Reference Owen and Matthews2003; Taylor, Reference Taylor, Fraser and McKusick1969). Given that live-born Swedish male twins gestate, on average, for 37 weeks (Cheung et al., Reference Cheung, Yip and Karlberg2000), we predict that the expected inverse correlation will appear 14–19 weeks (i.e., 37 weeks less 23 or 18 weeks) after suicides.
Methods
Data
We obtained sex-specific monthly counts of suicide among Swedes aged 20 through 49 years from the registry of causes of death maintained by the Swedish National Board of Health and Welfare. We also procured the monthly numbers of live-born male and female singletons and twins from the Medical Birth Registry and also maintained by the National Board of Health and Welfare. Both sets of time series covered the 528 months starting January 1973 and ending December 2016 (most recent month available at the time of the analyses). The data on the birth outcomes are of good quality, with little undercounting (Källén & Källén, Reference Källén and Källén2003). As for suicide data, one review of reliability assessments shows the Swedish data to be relatively accurate, though with some misclassification (e.g., as accidents) and underreporting (yet less than 10%; Tøllefsen et al., Reference Tøllefsen, Hem and Ekeberg2012). In line with the recommendation of Björkenstam et al. (Reference Björkenstam, Johansson, Nordström, Thiblin, Fugelstad, Hallqvist and Ljung2014), we excluded deaths of undetermined intent from our suicide variable.
We computed the monthly odds of a twin among live-born twin and singleton males and females (i.e., all male or female twins born live in month t divided by male or female singletons born live in month t). We used the monthly incidence (i.e., count) of suicides among Swedish women aged 20 through 49 as our independent variable.
Analyses
Our test proceeded through four steps. First, we created our dependent variable by computing the monthly ratios of the odds of a twin among male births to the odds among female births. Our theory implies that this ratio should correlate inversely over time with the incidence of suicide among women aged 20 through 49. Using the odds ratio allows us to minimize the effect of phenomena — for example, the use of assisted reproductive technology or changes in total fertility — that could affect temporal variation in the likelihood of twin births regardless of sex (Tandberg et al., Reference Tandberg, Bjørge, Børdahl and Skjaerven2007). We transformed the odds ratios to natural logarithms (i.e., logit) to make their distributions nearer normal and to allow us to express results as percent changes in the odds ratio.
Our second step followed from the fact that patterns often found in time-series data violate an important assumption of correlational tests. Such tests essentially ask whether two variables differ similarly from their statistically expected values in the same cases. Such tests typically assume that the values of such variables exhibit normal distributions and independence from each other. These assumptions allow specifying the mean of a variable as its expected value. Time series, however, often violate the assumption of independence because they exhibit ‘autocorrelation’ or trends, cycles (e.g., seasonality), or the tendency to remain elevated or depressed, or to oscillate, after high or low values. The expected value of such a series is not its mean but rather the values predicted from autocorrelation. In our second step, we used Box–Jenkins methods (Box et al., Reference Box, Jenkins, Reinsel and Ljung2015) to detect and model autocorrelation in the monthly twin odds ratios. Box–Jenkins methods use differencing (i.e., subtracting values at t from those at t + 1), as well as moving average and autoregressive parameters to express autocorrelation in time series. These well-described methods appear frequently in social and natural sciences research, as well as, although less commonly, in epidemiologic and clinical research (De Gooijer & Hyndman, Reference De Gooijer and Hyndman2006). The fitted values of a Box–Jenkins model become the expected values for a correlational test and the residuals of the model measure the difference between expected and observed values.
Third, we used Box–Jenkins methods to estimate the expected values of suicides among Swedish aged 20 through 49.
Fourth, we estimated the following test equation formed by adding the residuals of the Box–Jenkins model for suicide among women of reproductive age to the Box–Jenkins model for the monthly male to female twin odds ratios.
 $${\Delta ^{\it d}}{[({T_{{\rm{mt}}}}/{S_{{\rm{mt}}}})/({T_{{\rm{ft}}}}/{S_{{\rm{ft}}}})]^e} = c + {{\rm{\omega }}_0}{\Delta ^d}{X_t} + \ldots + {{\rm{\omega }}_7}{\Delta ^d}{X_t}_{ - 8} + (1 - \theta {B^{q}})/(1 - {\rm{\phi }}{B^{p}}){a_t}$$
$${\Delta ^{\it d}}{[({T_{{\rm{mt}}}}/{S_{{\rm{mt}}}})/({T_{{\rm{ft}}}}/{S_{{\rm{ft}}}})]^e} = c + {{\rm{\omega }}_0}{\Delta ^d}{X_t} + \ldots + {{\rm{\omega }}_7}{\Delta ^d}{X_t}_{ - 8} + (1 - \theta {B^{q}})/(1 - {\rm{\phi }}{B^{p}}){a_t}$$T mt is the number of male twins born in month t. S mt is the number of male singletons born in month t. T ft is the number of female twins born in month t. S ft is the number of female singletons born in month t. Δd is the difference operator indicating that [(T mt/S mt)/(T ft/S ft)]e has been differenced at lag d (i.e., values at time t subtracted from those at t + d). c is a constant. ω1 through ω7 gauge percent change in the logit of the twin odds ratio when the observed incidence of suicide among women of reproductive age differs from expected by 1. X t through X t−8 are the residuals of the Box–Jenkins model of suicide among females of reproductive age in months t through t−8. θ is a moving average parameter that measures the fraction of a at t remembered at time t + q. Moving average parameters best express ‘short memory’ or quick (i.e., over relatively few months) regression to expected values. B is the backshift operator or value of [(T mt/S mt)/(T ft/S ft)]e at t−p. ϕ is an autoregressive parameter that measures the fraction of [(T mt/S mt)/(T ft/S ft)]e at t remembered at time t + p. Autoregressive parameters best express ‘long memory’ or slow (i.e., over several or more months) regression to expected values. a t is the residual of the model at month t.
Our theory predicts that either or both, ω3 and ω4 (i.e., coefficients for suicides 3 and 4 months before births) will fall significantly (i.e., p < .05, two-tailed test) below 0. We included suicides in the same month as births as well as in months 1, 2, 5, 6, 7 and 8 before births as falsification tests. Coefficients for these months should not differ from zero.
Results
Swedish authorities attributed the deaths of 7,725 Swedish women aged 20 through 49 years to suicide during our 44-year test period spanning 1973 through 2016. The mean of monthly suicides in this group was 14.63 (SD = 5.18) with a range from 3 to 34 over the 528 test months.
The 2,337,313 males born in the test period included 55,664 twins (i.e., 2.38%). The 2,209,736 females included 54,447 twins (i.e., 2.46%). The monthly male-to-female twin odds ratio had a mean of 0.977 (SD = 0.164) with a range from 0.534 to 1.702.
Step 2 in our test, identifying and modeling autocorrelation in the logit of the twin odds ratio, yielded the Box–Jenkins parameters shown in Eq. (2) below:
 $${[({T_{{\rm{mt}}}}/{S_{{\rm{mt}}}})/({T_{{\rm{ft}}}}/{S_{{\rm{ft}}}})]^e} = - 0.038 + 1/\left( {1 + 0.110{B^{11}}} \right){a_t}$$
$${[({T_{{\rm{mt}}}}/{S_{{\rm{mt}}}})/({T_{{\rm{ft}}}}/{S_{{\rm{ft}}}})]^e} = - 0.038 + 1/\left( {1 + 0.110{B^{11}}} \right){a_t}$$The residuals of the equation exhibited no autocorrelation, and the estimated coefficients exceeded at least twice their standard errors. The autoregressive parameter at 11 implies that high or low values ‘echoed’ nearly a year later. This echo suggests a seasonality in the likelihood of a twin among male births over and above any seasonality exhibited by female twins.
Step 3 in our test, identifying and modeling autocorrelation in the incidence of suicide among women aged 20 through 49, yielded the Box–Jenkins parameters shown in Eq. (3) below:
 $${Z_t} = - 0.151 + (1 - 0.808{B^{12}})/(1 - 0.123{B^3}){a_t}$$
$${Z_t} = - 0.151 + (1 - 0.808{B^{12}})/(1 - 0.123{B^3}){a_t}$$The residuals of the equation exhibited no autocorrelation, and the estimated coefficients exceeded at least twice their standard errors. The moving average parameter at 12 implies seasonality in which high or low values ‘echoed’ a year later. The autoregressive parameter at 3 suggests a quarterly echo.
The coefficients in our test equation, estimated in step 4, appear in Table 1. The first column shows results for the full model with all lags of the suicide variable. The second column shows results for the pared model (i.e., model without parameters found non-significant in the full model). Both estimations find, as hypothesized, coefficients significantly (p < .05; two-tailed test) less than zero for suicides 4 months before the month of births.
Table 1. Estimated coefficients (standard errors in parentheses) for full and pared equations predicting the Swedish sex-specific twin odds ratio from suicides among women aged 20 through 49 and from autocorrelation (N = 528 months beginning 1/1973)
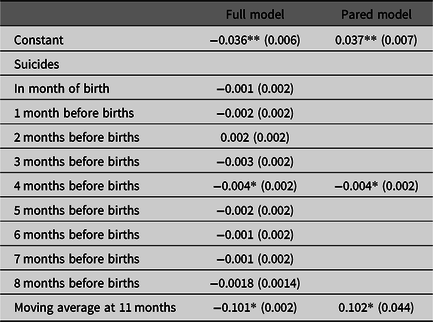
Note: *p < .05, two-tailed test, **p < .01, two-tailed test.
We used methods offered by Chang et al. (Reference Chang, Tiao and Chen1988) to determine whether outliers in the dependent variable could have inflated the confidence intervals for error terms and led us to falsely accept the null for associations other than those when suicide preceded births by 4 months. Adjusting for outliers did not produce additional significant (p < .05; two-tailed test) parameters.
Intuition suggests that optimism among women of reproductive age simply samples among that of the population at large. If this were so, we would expect that repeating our test with suicides among, for example, males of reproductive age would produce the same results. We pursued this test by substituting suicides among males 20 through 49 for those among females and proceeding through the steps described above. We found no association.
Figure 1 shows residuals from Eq. (2) above, which measure the degree to the odds of a twin among male births in month t differ from expected (i.e., from the odds among females and from autocorrelation), plotted, over residuals from Eq. (3) that measure the degree to which suicides among women of reproductive age in month t−4 differed from expected (i.e., from autocorrelation). The association we found appears as the downward slope in the line that best fits the data.
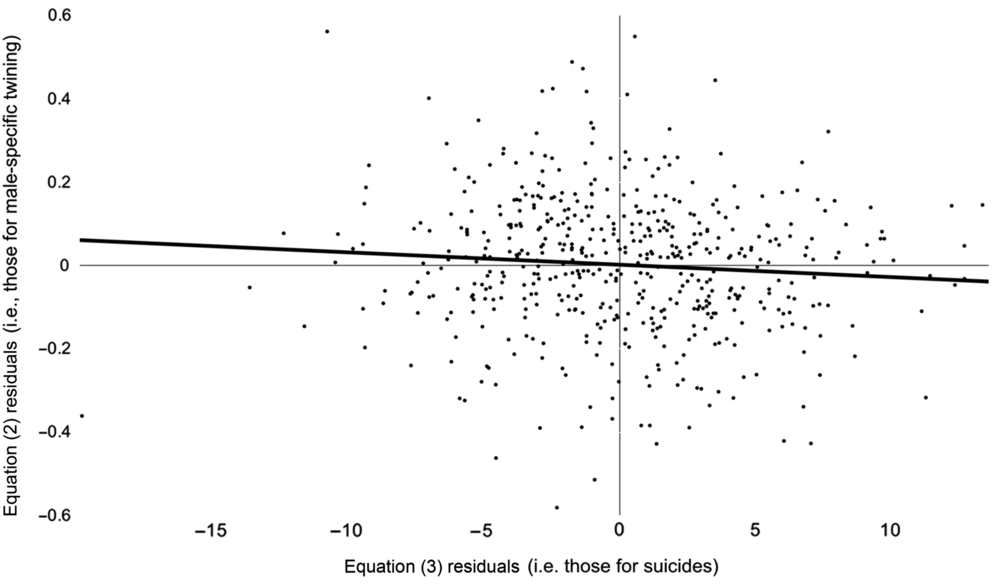
Fig. 1. Monthly (January 1973 through December 2016) differences between observed and expected (from autocorrelation) logits of Swedish male-specific twinning plotted, with trend line, over differences between observed and expected (from autocorrelation) suicides among women of reproductive age.
Our finding implies that the ratio of the odds of a twin among males to the odds of a twin among females changed by .4% four months after suicides among Swedish women of reproductive age differed by 1 from the value expected from autocorrelation. Differences from expected suicides ranged from −20 to 14 suicides during our test period, suggesting that the response in odds of a twin among males compared to a twin among females ranged from an increase of 8% to a decrease of 5.6%.
We attempted to provide a perhaps more interpretable result by creating a ‘months of collective optimism’ variable. We did so by rescoring our independent variable to one for months in which suicides fell below expected and zero otherwise. Estimating our test equation with this predictor yielded a coefficient of −0.035 (p < .05; two-tailed test) for the suicide variable four months before births. This coefficient implies that over the most recent 44 years for which we have data, a decrease in suicides among Swedish women aged 20 through 49 below expected levels preceded by 4 months an increase of 3.7% in the odds of a twin among male births compared to the odds among female births.
Discussion
Consistent with our theory, we find that the likelihood of a twin among male births in Sweden varies inversely with the incidence of suicide among women of reproductive age. Also consistent with theory, the association appears 4 months after the month in which suicides exceed those expected from seasonality and other forms of autocorrelation. We attribute this correlation to sensitivity of selection in utero to the willingness of women of reproductive age to invest in the future and to the availability for those women of collective optimism.
We observed no association between male suicides and the likelihood of a twin among male live births. This finding may reflect that reproductive-aged females, the only population in which selection in utero operates, may respond differently to stimuli/provocations than do men of reproductive age. Suicides among men and women of reproductive age correlate, for example, at only .5 over our 528 test months and controlling for autocorrelation reduces the correlation to .07. Research reports, moreover, that suicide among men and women responds differently to optimism-affecting conditions such as economic activity (Chang et al., Reference Chang, Stuckler, Yip and Gunnell2013; Corcoran et al., Reference Corcoran, Griffin, Arensman, Fitzgerald and Perry2015).
We argue that mechanisms by which humans signal the need for, and share, optimism should be considered elements of public health infrastructure that deserve investment and enhancement. Identifying and testing the efficacy of these mechanisms seem, moreover, worthy agenda for population health researchers. Obvious questions this work should address include whether these mechanisms should vary by age, gender or race/ethnicity (Manski, Reference Manski2004; Syme, Reference Syme2007). Less intuitive questions might include whether making the shared resource pool more concrete (e.g., social media explicitly dedicated to optimism exchange) would increase the use of collective optimism (Kim, Reference Kim2018).
Strengths of our study include that we use high-quality data on both births and suicide. These data allowed us to apply rigorous time-series methods that preclude type I errors arising from shared autocorrelation. Removing autocorrelation from the dependent and independent variables implies, by axiom, that the association we found cannot arise from, for example, shared seasonality or trends. Nor can it arise from any ‘third variables’ that affect the gestation of twins in general because our use of the male/female ratio of the odds of a twin birth as the dependent variable controls for general confounders.
We acknowledge that greater optimism may lead less fertile couples to pursue assisted reproduction technologies that disproportionately produce multiple births. This circumstance, however, cannot explain our results. We found more or fewer than expected twins among male newborns 4 months after changes in the incidence of suicides among females of reproductive age. If collective optimism affected the male twin ratio by changing who attempted pregnancy and the methods they used, the association would appear not 4 but rather 8 or 9 months after changes in the incidence of suicide.
We do not offer the association we found as strong evidence that collective optimism explains selection in utero against male twins more parsimoniously than do other theories. Rather, we offer the above analysis as an early intuitive test in what we hope will become a string of increasingly confirmatory, and likely more complex, tests. Our test, moreover, contributes to the literature describing the sensitivity of male twin births to change in the social environment (Catalano et al., Reference Catalano, Saxton, Gemmill and Hartig2016; Karasek et al., Reference Karasek, Goodman, Gemmill, Falconi, Hartig, Magganas and Catalano2015).
Future research should test at least three hypotheses implied by our argument but not testable with data available to us. First, our argument suggests that male–male twin gestations should respond more to collective optimism, however measured, than do other twin sets.
A second hypothesis arises from the assumption that maternal and environmental factors influence the likelihood of birthing dizygotic more than monozygotic twins. Maternal weight gain during pregnancy, for example, reportedly influences the likelihood of dizygotic twins surviving to birth (Reddy et al., Reference Reddy, Branum and Klebanoff2005). This circumstance suggests that indicators of collective optimism might predict gestational weight gain among women contributing to monthly birth cohorts.
The increasing use of imaging early in gestation may allow a test of a third hypothesis suggested by our arguments. Results from such imaging should lead researchers to determine whether the incidence of ‘vanishing twin syndrome’ (i.e., conversion of more than a third of twin gestations to singletons via absorption of the smaller fetus) varies with indicators of collective optimism (Landy & Keith, Reference Landy and Keith1998).
Assessing the validity and usefulness of collective optimism as a construct, and the use of the suicide rate as its proxy, will require more critical consideration than we provide here. We believe, however, that such consideration appears justified given the potential of the construct to connect the collective experiences of populations with indicators of their health. Efforts to identify the observable circumstances that not only undermine but also build and restore collective optimism seem important because they could lead to empirical tests of the widely held suspicion that such circumstances affect the health of populations (Stobbe, Reference Stobbe2018).
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.




