To date, most genetically informative research examining the origin of human traits has relied on twin studies to derive heritability estimates and related statistics (e.g. Polderman et al., Reference Polderman, Benyamin, de Leeuw, Sullivan, van Bochoven, Visscher and Posthuma2015). Twin studies are based on comparisons of monozygotic (MZ) and dizygotic (DZ) twins to decompose the etiology of individual differences into genetic, shared environment and nonshared environment components. In this way, twin studies are focused on identifying etiological influences that make twins in the same generation more similar or different to one another. While twin heritability estimates go some way toward explaining why traits run in families, within-generation studies miss some of the picture — because it is also of interest to understand the factors underlying correlations between generations.
Parents and children are similar to one another, to a degree, in almost all measurable traits. These include physical characteristics (e.g. Jaaskelainen et al., Reference Jaaskelainen, Pussinen, Nuutinen, Schwab, Pirkola, Kolehmainen and Laitinen2011), personality traits (e.g. Boutwell & Beaver, Reference Boutwell and Beaver2010), psychopathology (e.g. Micco et al., Reference Micco, Henin, Mick, Kim, Hopkins, Biederman and Hirshfeld-Becker2009), cognitive ability (e.g. Bouchard & McGue, Reference Bouchard and McGue1981), educational attainment (e.g. Hertz et al., Reference Hertz, Jayasundera, Piraino, Selcuk, Smith and Verashchagina2007) and observed behaviors, such as cigarette smoking (Chassin et al., Reference Chassin, Presson, Seo, Sherman, Macy, Wirth and Curran2008). Cross-trait correlations are also found between generations, for example, between parent substance use and child psychopathology (Vidal et al., Reference Vidal, Vandeleur, Rothen, Gholam-Rezaee, Castelao, Halfon and Preisig2012). Such parent–child associations may arise through one or more of the following possible mechanisms: (1) Parents may have a direct effect on their children, influencing offspring development in some way through their behavior. For example, parental affection may increase feelings of self-worth in their children (McAdams et al., Reference McAdams, Rijsdijk, Narusyte, Ganiban, Reiss, Spotts and Eley2017). (2) Children may inherit genetic variants associated with the parent and child traits of interest. For example, genetic factors associated with depression in parents have been found to manifest as conduct problems in adolescent offspring, partially accounting for the phenotypic association between the parent and child traits (Silberg et al., Reference Silberg, Maes and Eaves2010). This is an example of passive gene–environment correlation, whereby the child’s genotype is correlated with the environment in which they are reared (with their environment characterized by the parent’s genetically influenced trait of interest; Plomin et al., Reference Plomin, DeFries and Loehlin1977). Accounting for passive gene–environment correlation is of crucial importance in distinguishing possible causal effects of parent–child interactions from the effects of genetic relatedness. (3) Parents and children share their environments at various levels. They inhabit the same or overlapping cultures, neighborhoods, extended families and nuclear families. Environmental influences operating at one or more of these levels can increase or induce correlations between parent and child traits. (4) Children may have a direct effect on their parents. For example, child anxiety symptoms can prospectively predict future anxiety symptoms in mothers (Ahmadzadeh et al., Reference Ahmadzadeh, Eley, Leve, Shaw, Natsuaki, Reiss and McAdams2019); and children may influence the parenting that they receive (Avinun & Knafo, Reference Avinun and Knafo2014; Oliver et al., Reference Oliver, Trzaskowski and Plomin2014).
While studies of twins and their parents can be useful in understanding the nature of associations between parent and child traits, they can only ever tell us about the role of offspring genes in these associations because we only have information on the relatedness between people within the offspring generation (the twins’ generation). In the children-of-twins (CoT) design, it is possible to explore the effects of parents on children, and vice versa, while accounting for the potential confounding effects of parent and child genes (and thus passive gene–environment correlation) and shared family environments.
The CoT design involves studying samples of twins and their children (D’Onofrio et al., Reference D’Onofrio, Turkheimer, Eaves, Corey, Berg, Solaas and Emery2003; Fischer, Reference Fischer2018; Heath et al., Reference Heath, Kendler, Eaves and Markell1985; McAdams et al., 2014, Reference McAdams, Hannigan, Eilertsen, Gjerde, Ystrom and Rijsdijk2018; Nance & Corey, Reference Nance and Corey1976; Silberg et al., Reference Silberg, Maes and Eaves2010). Children inherit 50% of their DNA from each of their parents. As depicted in Figure 1, because MZ twins share all of their segregating genes, when both twins in an MZ twin-pair have children, their offspring are just as genetically related to their own parent (genetic correlation = .50) as they are to their parent’s genetically identical twin (avuncular genetic correlation = .50). In contrast, because DZ twins share 50% of their segregating genes on average, when both twins in a DZ twin-pair have children, their offspring are more genetically related to their own parent (genetic correlation = .50) than they are to their parent’s non-identical twin (avuncular genetic correlation = .25). Subsequently, cousins who are offspring of MZ twins are more genetically related to each other (genetic correlation = .25) compared to the offspring of DZ twins (genetic correlation = .125). Comparisons between avuncular correlations in these extended families linked by MZ versus DZ twin parents thus provide researchers with a natural, quasi-experiment for the study of associations between parent and child phenotypes.
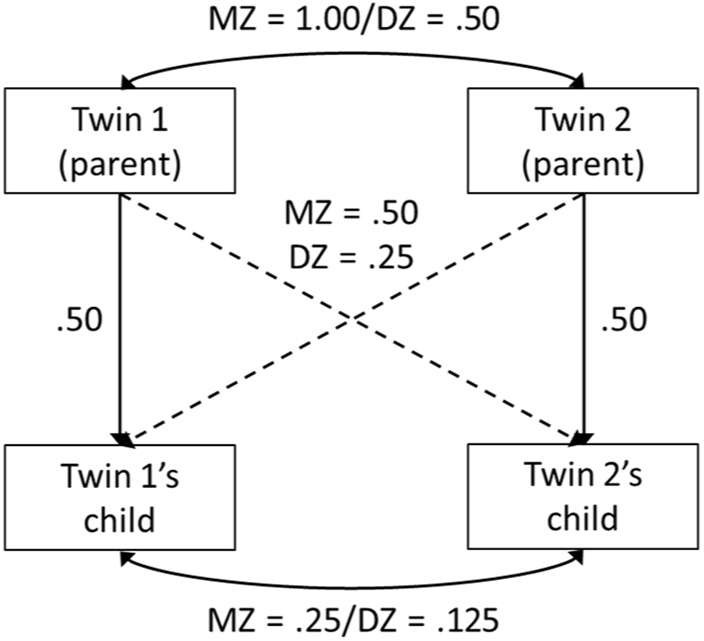
Fig. 1. Genetic correlations for monozygotic (MZ) and dizygotic (DZ) twin-pairs and their children.a
Here, we describe the procedural and measurement aspects of the first British CoT sample, the Children of the Twins Early Development Study (CoTEDS). CoTEDS is a spin-off from the Twins Early Development Study (TEDS; Rimfeld et al., Reference Rimfeld, Malanchini, Spargo, Spickernell, Selzam, McMillan and Plomin2019). The TEDS sample includes approximately 10,000 contactable twin-pairs who have been followed longitudinally from infancy through adulthood. At the time of writing, TEDS twins were aged 22–25 years old. The zygosity of TEDS twins was assigned using a parent-reported questionnaire of physical similarity, which is found to be over 95% accurate (Price et al., Reference Price, Freeman, Craig, Petrill, Ebersole and Plomin2000), and DNA testing was undertaken where zygosity remained unclear (the current sample includes 64% DZ, 33% MZ and 3% unknown). As TEDS twins begin to have children of their own, they are invited to join CoTEDS with their offspring. In the initial stages, CoTEDS has been designed to partially mirror the early years of TEDS data collection, to create a two-generation dataset that includes many of the same phenotypic measures on parents and offspring at corresponding stages of early development. New phenotypes are also being assessed in CoTEDS, relating specifically to parent and offspring mental health, as well as parenting behaviors and feelings.
Research Aims
CoTEDS has been designed to address several types of research questions. Our primary aim is to use the two-generation, longitudinal dataset to understand genetic and environmental factors that underpin intergenerational transmission of common traits within families, with a specific focus on the transmission of cognitive and mental health-related phenotypes. Our secondary aim is to understand the degree to which parenting affects child development and vice versa. Third, in TEDS adults, we aim to examine the genetic and environmental factors that influence individuals’ experiences and behaviors during parenthood.
From the outset, CoTEDS has been designed with the goal of being able to replicate our analyses across other samples and triangulate our findings with those arising through use of alternative genetically sensitive research designs. For this reason, we have built in overlap between CoTEDS and TEDS as well as other genetically informative cohort studies to ensure that we assess many of the same phenotypes at corresponding stages of development. To date, these cohorts primarily include the prospective adoption study, the Early Growth and Development Study (EGDS; Leve et al., Reference Leve, Neiderhiser, Shaw, Ganiban, Natsuaki and Reiss2013), and two transgenerational prospective observational studies, the Norwegian Mother and Child Birth Cohort Study (MoBa; Magnus et al., Reference Magnus, Birke, Vejrup, Haugan, Alsaker, Daltveit and Stoltenberg2016) and the Avon Longitudinal Study of Parents and Children (ALSPAC; Boyd et al., Reference Boyd, Golding, Macleod, Lawlor, Fraser, Henderson and Smith2013). Ongoing data collection from EGDS, MoBa and ALSPAC encapsulates longitudinal phenotypic and genomic information on parents and offspring from prenatal stages through childhood. As CoTEDS progresses, we will explore opportunities for further overlap with additional databases. By ensuring that we employ a combination of research methodologies in our work, we will be more likely to reach valid and robust research conclusions (Rutter et al., Reference Rutter, Pickles, Murray and Eaves2001).
Recruitment
Parents are recruited to CoTEDS from the sample of approximately 20,000 contactable adult twins who remain enrolled in the TEDS. The initial TEDS recruitment strategy, retention information and sample characteristics have been described in detail elsewhere (Haworth et al., Reference Haworth, Davis and Plomin2013; Oliver & Plomin, Reference Oliver and Plomin2007; Rimfeld et al., Reference Rimfeld, Malanchini, Spargo, Spickernell, Selzam, McMillan and Plomin2019; Trouton et al., Reference Trouton, Spinath and Plomin2002). Recruitment to CoTEDS commenced in March 2016 (data collection was launched the following year), with all TEDS twins invited to register their existing offspring online. CoTEDS registration for existing offspring and/or pregnancies is advertised to all TEDS families (both twins and their parents) during TEDS data collection, on the TEDS website, in the annual TEDS newsletter, on social media, annual email circulars and by word of mouth when researchers have contact with TEDS families. Recruitment efforts are continually maintained.
CoTEDS registration includes a short screening procedure to confirm that the inclusion criteria are met for data collection: (1) the child must be a biological offspring of the TEDS twin and (2) the child must have regular contact with the TEDS twin (for twins not living with their offspring, we require that they have at least 1–3 h contact time per week; contact time is recorded for use as a covariate in analyses). We aim for twins to register their offspring at birth; however, there is no maximum child age for registration. Furthermore, we aim to register as many biological children per TEDS twin as possible, including twins in the offspring generation (as of May 2019, 2.4% of all registered births in CoTEDS are multiples [13 twin–pairs], as compared to national statistics showing that 1.6% of all British births in 2017 were twins; Ghosh, Reference Ghosh2019). Child ages at registration are detailed in Table 1 for all registrations between March 2016 and May 2019. These children are registered to 435 twins (79.8% female), which includes 45 twin-pairs (where both twins in a pair have at least one registered child; 51% MZ) and 345 individual twins (of which 46% are from an MZ pair).
Table 1. Child ages at CoTEDS registration and the total registered sample in May 2019a
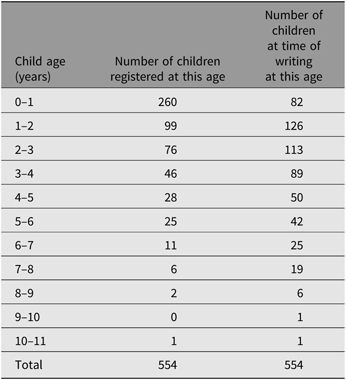
a Children registered between March 2016 and May 2019. New children registered on a continual basis.
At each wave of data collection, twins provide informed consent and are given the option to share contact details for their child’s co-parent (this may be the child’s other biological parent and/or the twin’s partner). Co-parents are recruited to take part in CoTEDS for the equivalent single wave of data collection. Co-parents are not recontacted for CoTEDS unless the twin nominates them again at a subsequent wave of data collection. The nature of the relationship between CoTEDS children and the co-parent providing data at each wave is carefully tracked.
Data Collection Protocol
Data collection is continually maintained alongside recruitment. The first wave of data collection (Wave 1) was launched in May 2017, involving a parent-reported questionnaire for the parents of 1-year-old CoTEDS children. The target child age for questionnaire completion is 12 months, but data are collected for all children between 12 and 23 months, with child age included as a covariate for analyses. The questionnaire is sent to participants to complete in their own time, taking approximately 60 min to complete online or on paper. Baseline information is collected for general demographic data and the composition and living situation of the immediate family. A battery of measures, described below, is then completed to assess several child, parenting, parent and socioecological phenotypes. Items relate to the perinatal period, first 12 months of the child’s life and the weeks prior to questionnaire completion. Quality control items are used to monitor participant attention and validity of responses in sections that use large matrices of items measured along the same Likert scale. These quality control items require participants to select a specific response to the Likert scale. During data analysis, researchers will have the option to exclude participant responses on any given measure if quality control items are answered incorrectly. Prior to the launch of Wave 1, the full questionnaire battery was piloted in a sample of 195 community volunteers with infant children, who also provided quantitative and qualitative feedback on the questionnaire. Psychometric properties and participant feedback were assessed for all scales, and questionnaire edits made where appropriate.
Data collection commenced for the second wave of data collection (Wave 2) in October 2018, for the parents of 2-year-old children (target age 24 months). An adapted, age-appropriate version of the Wave 1 questionnaire is used at Wave 2, with the addition of three parent-assessed tests of child cognitive ability that are completed by parents after the questionnaire (see Table 2; Saudino et al., Reference Saudino, Dale, Oliver, Petrill, Richardson, Rutter and Plomin1998). The Wave 2 battery was piloted in a sample of 210 community volunteers with infant children, who again provided quantitative and qualitative feedback. As with Wave 1, the Wave 2 pilot data were used to inform the composition of the final Wave 2 questionnaire. At the time of writing, we are developing the third wave of data collection (Wave 3) for the parents of 3-year-old children. As depicted in Figure 2, all parents complete data collection waves as applicable to their child’s age at CoTEDS registration. Children who are registered before their first birthday follow the standard protocol, with parents invited to complete Waves 1–3 on, or shortly after, the child’s corresponding birthday (see Figure 2, black arrows). Any parents registering a child older than 23 months will be invited to retrospectively complete a subset of baseline questions from Wave 1, after they have completed any other applicable waves of data collection (see Figure 2, grey arrows).
Table 2. Summary of measures included in CoTEDS Waves 1 and 2
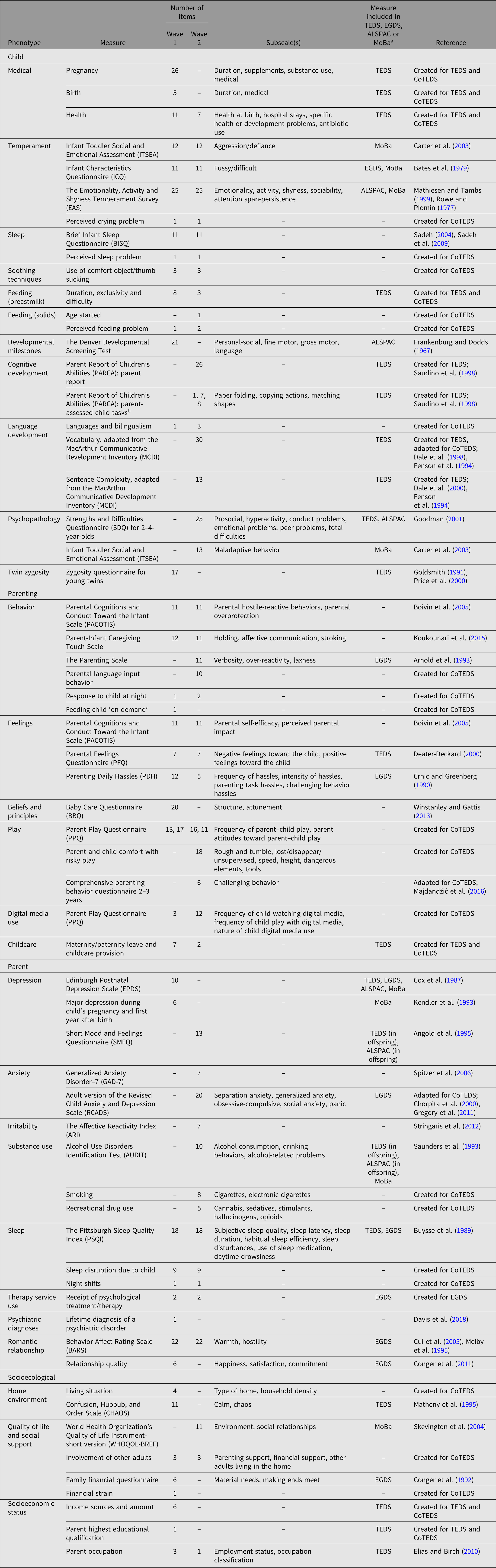
a TEDS, Twins Early Development Study; EGDS, Early Growth and Development Study; ALSPAC, Avon Longitudinal Study of Parents and Children; MoBa, Norwegian Mother and Child Birth Cohort Study.
b Parent-assessed tasks of child cognitive ability — completed by parents with the child, after the questionnaire battery.
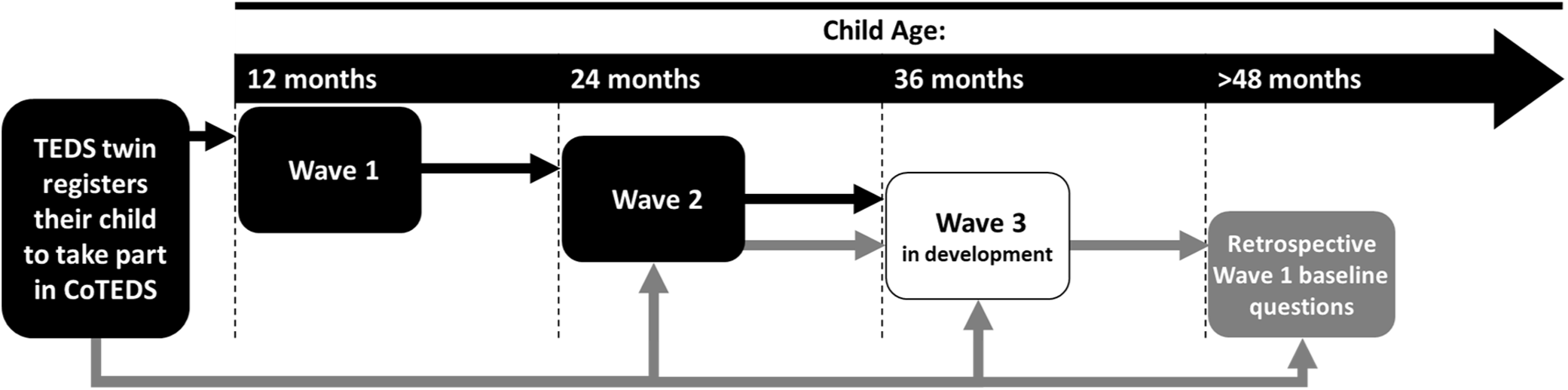
Fig. 2. Data collection protocol in May 2019.a
Measures, Waves 1 and 2
Within the space constraints of the Wave 1 and 2 questionnaires, we included key phenotypes that have previously been theoretically or empirically related to the development of cognitive and mental health-related traits in children. Where possible, we have used well-established, documented and validated measures. Where no sample-appropriate, questionnaire-based measure with adequate psychometric properties could be found, we designed our own. A summary of the measures, including number of items and overlap between CoTEDS Waves 1 and 2, is outlined in Table 2. For some phenotypes, the number of items differs between waves if edits were made to ensure that measures were age-appropriate and/or to accommodate space constraints in each questionnaire. Table 2 also details intentional measurement overlap with other genetically informative cohort studies.
Future Directions
Data Collection
Following the development and launch of Wave 3, we plan to continue with further waves of data collection to be completed as the children of TEDS twins grow older, including questionnaire-based measurements of new age-appropriate phenotypes post infancy (e.g. comprehensive measures of child psychopathology and cognitive development, as well as parenting measures relating to early and middle childhood). Alongside questionnaire-based measures, we plan to collect other forms of data, for example, using observational methods to examine parent–child interactions (e.g. Ginsburg et al., Reference Ginsburg, Grover, Cord and Ialongo2006; Oliver & Pike, Reference Oliver and Pike2019). We will focus on developing our study design to enable longitudinal data collection from a range of sources within our ever-growing sample. New possibilities include harnessing in-home technologies to reach families, for example, using video calls or gamified mobile applications to remotely examine traits and parent–child relationships. Future directions include plans to collect genotype data from the children and partners of TEDS twins, to maximize learning from our two-generation pedigree analyses with state-of-the-art methods in statistical genetics.
Data Analyses
Opportunities to examine genetic and environmental intergenerational effects will be rich in CoTEDS, using longitudinal data relating to the phenotypes described in Table 2, alongside data from the main TEDS study. The number of twin-pairs with children required to reach 80% power to detect intergenerational genetic transmission of varying magnitudes has been estimated elsewhere by McAdams et al. (Reference McAdams, Hannigan, Eilertsen, Gjerde, Ystrom and Rijsdijk2018). Crucially, the authors show that the required number of twin-pairs is reduced if data on two or more children are included per twin (i.e. using the multiple-children-of-twins [MCoT] design). Furthermore, by examining twin phenotypes measured in TEDS and offspring phenotypes measured in CoTEDS, we can also include twin-pairs where only one twin in the pair has children in CoTEDS. Including these ‘incomplete’ extended families will allow us to maximize the number of avuncular associations in extended MCoT models. We therefore expect that our first genetically informed intergenerational analyses in CoTEDS will use data from all combinations of twin-pairs with children (i.e. one child per twin, two children per twin and incomplete extended families). Recruiting nonbiological offspring of TEDS twins to CoTEDS (e.g. children conceived via egg or sperm donors or adopted children) will be another possible avenue to increase power for extended MCoT analyses in the future. Further analyses will have the potential to span three generations across time, by combining longitudinal data on TEDS twins, their parents and their offspring. Hence, we will also be able to ask questions relating to societal and cultural changes across generations. Triangulating this work with analyses in other datasets will enable us to make a robust contribution to the literature regarding associations between parents and children during early child development.
Summary
CoTEDS will include genetically sensitive, prospective information on both parents and their offspring from infancy. As the number of children born to TEDS twins continues to increase over the coming years, CoTEDS is in place to develop an invaluable resource for the examination of genetic and environmental factors that shape child development, helping us to better understand the role that parents play in this process.
Acknowledgments
We thank the families involved in the Twins Early Development Study (TEDS) and Children of TEDS (CoTEDS) for their ongoing participation and support for our research. We thank the research teams who have managed and coordinated TEDS and CoTEDS data collection. With special thanks to Rachel Ogden, Meredith Han, Laura Okonajiofor and Rebecca Watkins-Muleba.
Financial support
CoTEDS data collection and the positions of T.M. and Y.A. are funded by a Sir Henry Dale Fellowship awarded to T.M., jointly funded by the Wellcome Trust and the Royal Society (107706/Z/15/Z). T.E. is part funded by a program grant from the UK Medical Research Council (MR/M021475/1), the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. TEDS, and the position of A.M., is supported by a program grant to R.P. from the UK Medical Research Council (MR/M021475/1 and previously G0901245), with additional support from the US National Institutes of Health (HD044454; HD059215). R.P. is supported by a Medical Research Council Research Professorship award (G19/2) and an European Research Council Advanced Investigator award (295366). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. The views expressed are those of the authors.
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Ethical approval was granted by the Psychiatry, Nursing and Midwifery Research Ethics Subcommittee, King’s College London.






