Conventional studies were conducted on mutational damages both by SCE (sister chromatid exchanges) as well as by scoring chromosomal aberrations on more than 600 individuals exposed to methyl isocyanate gas in Bhopal (central India) in order to assess the genotoxicity imposed by the exposure. This short note re-emphasizes the importance of certain chromatin dots that were seen emanating from chromosomes which are decidedly early indicators of neoplastic transformations. MDs have always been found in all breast, colon, and bone cancer patients we have studied.
Results and Discussion
Lymphocyte cultures were routinely carried out and chromosomes were stained with Giemsa banding as per standard techniques, and approaches have been published (Goswami, Reference Goswami1986; Goswami & Chang, Reference Goswami and Chang2001). Small chromatin dots measuring 1.5–3 microns were observed emanating from different chromosome in several metaphases, as well as seen freely near the specific chromosomes (Figures 1–4). As far back as 1973, a few chromatin dots of variable sizes were already encountered in squashes of brain tumor tissues, that is, medulloblastoma, ependymoma, and tuberculoma, along with hyper and hypoploid chromosome counts (Dharker et al., Reference Dharker, Chaurasia and Goswami1973). Studies were then extended to various malignancies and the presence of MDs was recorded in all of them. Intriguingly, only selective chromosomes are involved (chromosomes 1, 3, 4, 5, 9, 11, 16; Goswami, Reference Goswami2001; Goswami, & Chang, Reference Goswami and Chang2001) in emanating MDs.
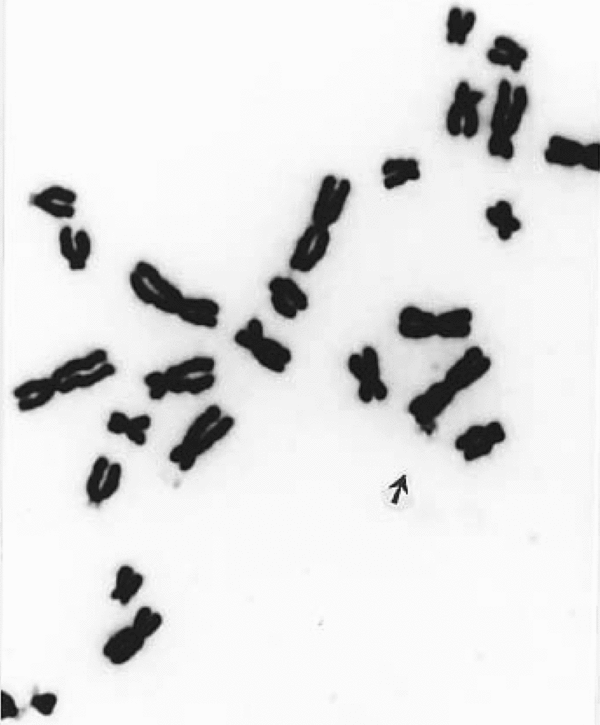
FIGURE 1 A part of the metaphase plate stained with Feulgen's method showing two darkly stained dots emanating from the terminal part of human chromosome 1; this patient died of breast cancer nearly 2 years after the study.
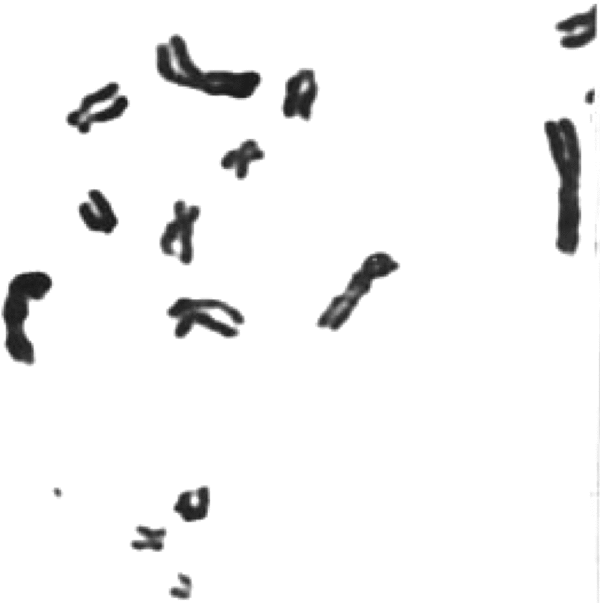
FIGURE 2 A part of a metaphase spread from the same slide to show a free marker dot (ca 1,500).

FIGURE 3 Giemsa banded metaphase showing a detached marker dot; it appears faintly stained due to change in the focusing chromosomes. Note that most of the marker dots photographed showing fine fibrillar connections with some part of any chromosome (see Figure 4) can be seen only by changing focus, thereby making the chromosome surface a little unclear.
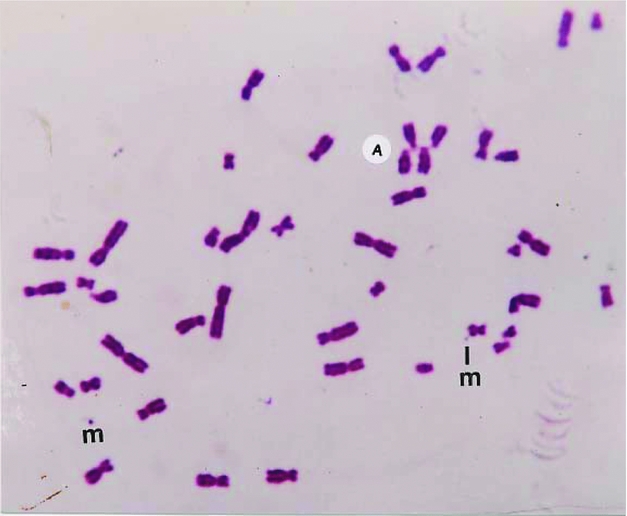
FIGURE 4 A major part of Giemsa stained metaphase exhibiting a detaching marker dot still attached by a fine fibril. Marker dots (m), acrocentric (A) associations, and translocations are also present in this methylisocyanate exposed person.
With a view to establish that the MDs frequently found in patients with malignancy are actually chromatin structures observed as freely found dots (Figures 2 and 3) or seen emanating from chromosomes (Figure 4), they were also stained with conventional Feulgen's reaction. It has been now well established that these MDs are DNA containing chromatin structures, which most likely are the outcome of some additional molecular mechanism.
It appears that the molecular attenuation of chromatin structures movable from chromosomes is related with triggering neoplastic transformations (Goswami, Reference Goswami1993). These dots appear only in those metaphases that exhibit translocations and acrocentric associations, which are again precursors to firm installation of chromosomal mutagenesis in cells as established since the time of Boveri (German, Reference German1974).
Acknowledgments
I am grateful to the Late Professor T. C. Hsu for his initial encouragement during 1973 and to surgeons in hospitals at Gwalior and Bhopal for facilitating this long-term study (1970–2002).






