Obsessive compulsive symptoms (OCS) are characterized by recurrent, persistent, and intrusive anxiety-provoking thoughts or images (obsessions) and subsequent repetitive behaviors (compulsions) performed to reduce anxiety and/or distress caused by the obsessions. When a person has these obsessions and/or performs compulsions for more than one hour a day and these thoughts and rituals significantly interfere with his/her daily life routines, the person fulfills the criteria for obsessive compulsive disorder (OCD). The lifetime prevalence of OCD is 0.5–2% (American Psychiatric Association, 1994; Grabe et al., Reference Grabe, Meyer, Hapke, Rumpf, Freyberger, Dilling and John2000), but obsessions are much more prevalent in the general population — as high as 72% (Rachman & de Silva, Reference Rachman and de Silva1978; Salkovskis & Harrison, Reference Salkovskis and Harrison1984) and the prevalence of OC symptoms reaches up to 20% (Fullana et al., Reference Fullana, Mataix-Cols, Caspi, Harrington, Grisham, Moffitt and Poulton2009).
Numerous twin (Jonnal et al., Reference Jonnal, Gardner, Prescott and Kendler2000; van Grootheest et al., Reference van Grootheest, Cath, Beekman and Boomsma2005) and family studies (Hettema et al., Reference Hettema, Neale and Kendler2001; Nestadt et al., Reference Nestadt, Samuels, Riddle, Bienvenu, Liang, LaBuda, Walkup, Grados and Hoehn-Saric2000) have indicated the importance of genetic as well as environmental risk factors with regard to the etiology of OCD. Heritabilities for OCD have been estimated to be between 27–47% in adults and 45–65% in children (Jonnal et al., Reference Jonnal, Gardner, Prescott and Kendler2000; van Grootheest et al., Reference van Grootheest, Cath, Beekman and Boomsma2005), and linkage and association studies have mainly pointed towards functional deficits of genes involved in serotonergic, glutamatergic, and dopaminergic neural signaling (for review see Nicolini et al., Reference Nicolini, Arnold, Nestadt, Lanzagorta and Kennedy2009). Potential environmental risk factors for OCD include traumatic early life experiences, perinatal problems, streptococcal infection, psychosocial stress, aspects of parenting (e.g., parental overprotection), pregnancy, divorce, and emotional neglect (Albert et al., Reference Albert, Maina, Bogetto and Ravizza2000; Alonso et al., Reference Alonso, Menchon, Mataix-Cols, Pifarre, Urretavizcaya, Crespo, Jiménez, Vallejo and Vallejo2004; Cath et al., Reference Cath, van Grootheest, Willemsen, van Oppen and Boomsma2008; Geller et al., Reference Geller, Wieland, Carey, Vivas, Petty, Johnson, Reichert, Pauls and Biederman2008; Grisham et al., Reference Grisham, Anderson and Sachdev2008; Lin et al., Reference Lin, Katsovich, Ghebremichael, Findley, Grantz, Lombroso, King, Zhang and Leckman2007; Wilcox et al., Reference Wilcox, Grados, Samuels, Riddle, Bienvenu, Pinto, Cullen, Wang, Shugart, Liang and Nestadt2008).
Over the last two decades, neuroimaging studies have indicated several neurobiological changes underlying the psychological and behavioral dysfunction of OCD. Results from structural and functional magnetic resonance imaging (sMRI/fMRI) studies mainly point to volume differences and altered regional brain activation in the ventral prefrontal cortex (PFC), dorsolateral prefrontal cortex (DLPFC), basal ganglia, anterior cingulate cortex (ACC), and thalamus (Menzies et al., Reference Menzies, Chamberlain, Laird, Thelen, Sahakian and Bullmore2008; Radua & Mataix-Cols, Reference Radua and Mataix-Cols2009; Radua et al., Reference Radua, van den Heuvel, Surguladze and Mataix-Cols2010; Rotge et al., Reference Rotge, Guehl, Dilharreguy, Tignol, Bioulac, Allard, Burbaud and Aouizerate2009). These findings have contributed to the widely accepted neuroanatomical model of OCD involving the direct and indirect cortico-striato-thalamo-cortical (CSTC) loops (Mataix-Cols & van den Heuvel, Reference Mataix-Cols and van den Heuvel2006; Saxena & Rauch, Reference Saxena and Rauch2000). The direct loop functions as a self-reinforcing feedback loop that contributes to the initiation and continuation of behaviors. The indirect loop functions as a negative feedback loop important for inhibiting and switching between behaviors. It has been hypothesized that an imbalance between these loops, resulting in a hyperactive ventral and hypoactive dorsal frontal-striatal system, might mediate OC symptomatology (Mataix-Cols & van den Heuvel, Reference Mataix-Cols and van den Heuvel2006; Saxena & Rauch, Reference Saxena and Rauch2000). Although disturbance in these CSTC loops may represent the main neurological basis for OCD, several imaging studies also suggest the involvement of other brain regions, such as amygdala, hippocampus, and parietal areas in OCD (Menzies et al., Reference Menzies, Chamberlain, Laird, Thelen, Sahakian and Bullmore2008; Pujol et al., Reference Pujol, Soriano-Mas, Alonso, Cardoner, Menchon, Deus and Vallejo2004; Valente et al., Reference Valente, Miguel, Castro, Amaro, Duran, Buchpiguel, Chitnis, McGuire and Busatto2005). Therefore, Menzies and colleagues proposed an extended model that includes these brain areas that are functionally connected to the ventral and dorsal frontal-striatal circuits (Menzies et al., Reference Menzies, Chamberlain, Laird, Thelen, Sahakian and Bullmore2008).
One of the core behavioral features associated with OC symptomatology is the inability to inhibit thoughts and/or behaviors. The process of inhibitory control has been linked to frontal-striatal networks, but the ACC and its interactions with the DLPFC may also play a crucial role because this circuitry has been repeatedly linked to conflict monitoring and adjustments in control (Kerns et al., Reference Kerns, Cohen, MacDonald, Cho, Stenger and Carter2004; Melcher et al., Reference Melcher, Falkai and Gruber2008). Numerous imaging studies in OCD specifically focused on the neurobiological processes underlying inhibitory control by exposing both OCD patients and controls to cognitive tasks that are developed to measure inhibitory control, such as response interference in the Stroop color-word and Eriksen Flanker task (Melcher et al., Reference Melcher, Falkai and Gruber2008). Regarding task performance, in which prolonged reaction times and error rates are generally considered a direct indicator for cognitive conflict or interference, OCD patients have been repeatedly described to be in the normal range (Fitzgerald et al., Reference Fitzgerald, Welsh, Gehring, Abelson, Himle, Liberzon and Taylor2005; Maltby et al., Reference Maltby, Tolin, Worhunsky, O'Keefe and Kiehl2005; Nakao et al., Reference Nakao, Nakagawa, Yoshiura, Nakatani, Nabeyama, Yoshizato, Kudoh, Tada, Yoshioka and Kawamoto2005; Page et al., Reference Page, Rubia, Deeley, Daly, Toal, Mataix-Cols, Giampietro, Schmitz and Murphy2009; Viard et al., Reference Viard, Flament, Artiges, Dehaene, Naccache, Cohen, Mazet, Mouren and Martinot2005; Yucel et al., Reference Yucel, Harrison, Wood, Fornito, Wellard, Pujol, Clarke, Phillips, Kyrios, Velakoulis and Pantelis2007). However, some studies showed prolonged reaction times during high-conflict trials, suggestive for impaired inhibitory control in OCD patients (Menzies et al., Reference Menzies, Achard, Chamberlain, Fineberg, Chen, Del Campo, Sahakian, Robbins and Bullmore2007; van den Heuvel et al., Reference van den Heuvel, Veltman, Groenewegen, Witter, Merkelbach, Cath, van Balkom, van Oppen and van Dyck2005). Furthermore, of interest for the present study, Menzies et al. found delayed response inhibition on the stop-signal task in OCD patients as well as in unaffected first-degree relatives of OCD patients, suggesting familial vulnerability of this aspect of OCD (Menzies et al., Reference Menzies, Achard, Chamberlain, Fineberg, Chen, Del Campo, Sahakian, Robbins and Bullmore2007). Even if performance was intact, there is evidence that OCD patients show a different pattern of brain activation during the execution of tasks measuring inhibitory control. Most neuroimaging studies that investigated inhibitory control showed a higher response conflict-related increase in ACC activity in OCD patients than in controls (Fitzgerald et al., Reference Fitzgerald, Welsh, Gehring, Abelson, Himle, Liberzon and Taylor2005; Maltby et al., Reference Maltby, Tolin, Worhunsky, O'Keefe and Kiehl2005; Page et al., Reference Page, Rubia, Deeley, Daly, Toal, Mataix-Cols, Giampietro, Schmitz and Murphy2009; Ursu et al., Reference Ursu, Stenger, Shear, Jones and Carter2003). Increases in regional activity during high-conflict trials has also been reported for frontal-striatal regions (orbitofrontal cortex, caudate and thalamus), as well as the DLPFC and cerebellum, and temporal and parietal regions (Maltby et al., Reference Maltby, Tolin, Worhunsky, O'Keefe and Kiehl2005; Nakao et al., Reference Nakao, Nakagawa, Yoshiura, Nakatani, Nabeyama, Yoshizato, Kudoh, Tada, Yoshioka and Kawamoto2005; Nakao et al., Reference Nakao, Nakagawa, Yoshiura, Nakatani, Nabeyama, Sanematsu, Togao, Yoshioka, Tomita, Kuroki and Kanba2009; Page et al., Reference Page, Rubia, Deeley, Daly, Toal, Mataix-Cols, Giampietro, Schmitz and Murphy2009; van den Heuvel et al., Reference van den Heuvel, Veltman, Groenewegen, Witter, Merkelbach, Cath, van Balkom, van Oppen and van Dyck2005). However, the findings regarding the direction of activation changes are not consistent, because hypoactivation of the ACC and the caudate, and temporal, and parietal regions during response interference has also been reported (Nakao et al., Reference Nakao, Nakagawa, Yoshiura, Nakatani, Nabeyama, Yoshizato, Kudoh, Tada, Yoshioka and Kawamoto2005; Nakao et al., Reference Nakao, Nakagawa, Yoshiura, Nakatani, Nabeyama, Sanematsu, Togao, Yoshioka, Tomita, Kuroki and Kanba2009; Page et al., Reference Page, Rubia, Deeley, Daly, Toal, Mataix-Cols, Giampietro, Schmitz and Murphy2009; Yucel et al., Reference Yucel, Harrison, Wood, Fornito, Wellard, Pujol, Clarke, Phillips, Kyrios, Velakoulis and Pantelis2007). These inconsistencies may be explained by methodological differences between studies, such as the paradigm used to measure response interference, heterogeneity of patient groups and differences in sample size, scanning modalities/parameters, and analysis methods. However, there may also be ‘true’ variability in the underlying neurobiology of response interference in OCD. That is, it may be that dysfunction of different brain regions may lead to highly comparable changes at the behavioral level, because these regions are part of the same brain network involved in controlling behaviors. Such heterogeneity in affected brain regions could reflect a differential influence of environmental and genetic risk factors for OCD impacting on different parts of the brain.
Most brain imaging studies compare groups of affected individuals with healthy controls. These standard case-control designs cannot disentangle differences in brain function that are due to environmental risk factors from those that are due to genetic risk factors. A distinction between genetically and environmentally mediated neurobiological changes that underlie the development of OCD can be accomplished by using a discordant/concordant monozygotic (MZ) twin design (de Geus et al., Reference de Geus, van ‘t Ent, Wolfensberger, Heutink, Hoogendijk, Boomsma and Veltman2007; den Braber et al., Reference den Braber, van ‘t Ent, Cath, Wagner, Boomsma and de Geus2010; den Braber et al., Reference den Braber, van ‘t Ent, Boomsma, Cath, Veltman, Thompson and de Geus2011; van ‘t Ent et al., Reference van ’t Ent, van Beijsterveldt, Derks, Hudziak, Veltman, Todd, Boomsma and de Geus2009; Wolfensberger et al., Reference Wolfensberger, Veltman, Hoogendijk, Boomsma and de Geus2008). Excluding post-twinning de novo mutations, all MZ twins begin life with identical genomes, so behavioral discordances are likely to arise from differential exposure to environmental influences. Consequently, differences in brain function between the high-risk twin and the low-risk co-twin from MZ discordant pairs reflect environmental effects on the brain, rather than effects of genetic variation. In contrast, to maximize detection of the effects of genetic risk factors for OCD, neuroimaging results can be compared between MZ twins who both score high on OC symptoms and MZ twins who both score very low on OC symptoms. These MZ concordant high-scoring and low-scoring twins likely represent either high or low familial vulnerability for OCD. This familial vulnerability could be due to shared environmental or genetic vulnerability. However, shared family environment has not been found to contribute to OC behavior in adults (Clifford et al., Reference Clifford, Murray and Fulker1984; Jonnal et al., Reference Jonnal, Gardner, Prescott and Kendler2000; van Grootheest et al., Reference van Grootheest, Cath, Beekman and Boomsma2007). Therefore, a comparison between MZ concordant high and MZ concordant low pairs on OC symptoms is likely to reveal functional activation differences due to influences of genetic risk factors.
In previous studies by our group, we applied the discordant/concordant twin design to investigate both white matter volume differences and planning-related activation changes in the brains of subjects with an environmental etiology or genetic predisposition for OC symptoms (den Braber et al., Reference den Braber, van ‘t Ent, Cath, Wagner, Boomsma and de Geus2010; den Braber et al., Reference den Braber, van ‘t Ent, Boomsma, Cath, Veltman, Thompson and de Geus2011). The results from these studies suggest that brain regions affected in environmentally mediated OC symptoms are distinct from those affected in genetically mediated OC symptoms. Interestingly, observed white matter changes and planning-related changes in brain activity converge on the CSTC loops. Neurobiological changes in OC symptoms induced by environmental risk factors involve the dorsal frontal CSTC loop (dorsolateral prefrontal region), whereas neurobiological changes in OC symptoms induced by genetic risk factors seem to involve regions implicated in the ventral frontal CSTC loop (inferior frontal region).
The present study aimed to examine the differential impact of non-shared environmental versus genetic influences for OC symptomatology on inhibitory control related functional brain activation. To this end we compared performance and fMRI data during the Stroop color-word and the Eriksen Flanker task, between twins scoring low and twins scoring high on OC symptoms from discordant MZ pairs, and between concordant pairs where both twins scored either low or high on OC symptoms.
Methods
Participants
The twin pairs included in this study were recruited from the Netherlands Twin Register (NTR) (Boomsma et al., Reference Boomsma, de Geus, Vink, Stubbe, Distel, Hottenga, Posthuma, van Beijsterveldt, Hudziak, Bartels and Willemsen2006). Surveys were sent to twin families, including the Padua Inventory Abbreviated (PI-R-ABBR) (Cath et al., Reference Cath, van Grootheest, Willemsen, van Oppen and Boomsma2008; van Oppen et al., Reference van Oppen, Hoekstra and Emmelkamp1995). Completed PI-R-ABBR questionnaires were returned by 815 MZ twin pairs (222 male, 593 female). From this sample we selected twin pairs aged between 18 and 60 years who scored discordant, concordant high or concordant low for OCS. A twin pair was classified as discordant for OC symptoms if one twin scored OCS high (> 16) and the co-twin scored OCS low (≤ 7). A twin pair was classified as concordant high for OC symptoms if both twins scored ≥ 15, with at least one twin scoring ≥ 16. A twin pair was classified as concordant low for OC symptoms if both twins scored ≤ 7. These PI-R-ABBR cut-off scores were derived from sensitivity and specificity measurements in an independent sample of OCD patients when compared to clinical controls (n = 120, M = 20.7, SD = 8.1; sensitivity = .74, specificity = .72 at the best cut-off point of 16); (Cath et al., Reference Cath, van Grootheest, Willemsen, van Oppen and Boomsma2008). For more details on sample selection refer to den Braber et al. (2010). A final sample of 71 MZ twin pairs participated in this MRI study, including 20 discordant, 23 concordant high and 28 concordant low twin pairs (Table 1). The MRI protocol could not be completed by two subjects (metal artifact, panic attack). In the final sample (n = 140), two twins with high OCS scores from the discordant group and five twins with high OCS scores from the concordant high group met clinical diagnosis for OCD. Furthermore, three twins with high OCS scores, one twin with a low OCS score from the discordant group, and six twins from the concordant high group used selective serotonin reuptake inhibitors.
TABLE 1 Twin sample demographics
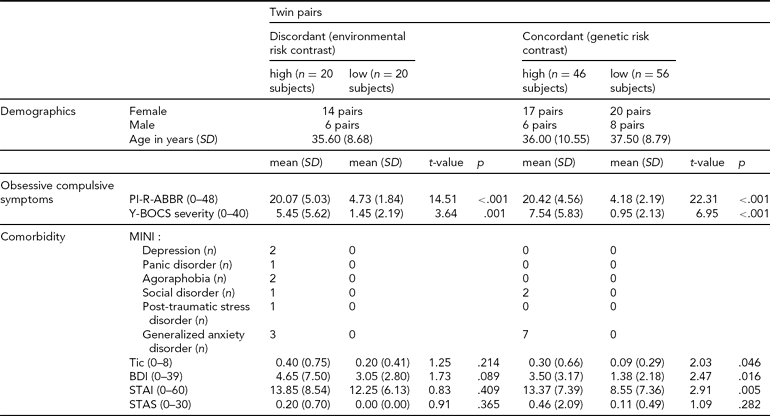
Twin pairs = number of female and male twin pairs, Age = age at time of magnetic resonance imaging (MRI) examination, PI-R-ABBR = mean Padua Inventory Abbreviated scores, SD = standard deviation, Y-BOCS severity = mean Yale–Brown Obsessive–Compulsive Scale severity scores, MINI (depression, panic disorder, agoraphobia, social disorder, post-traumatic stress disorder, generalized anxiety disorder) = number of subjects with current comorbid disorder (measured using the Mini International Neuropsychiatric Interview), Tic = mean tic scores at time of MRI, BDI = mean Beck Depression Inventory scores at time of MRI, STAI = mean State Trait Anxiety Inventory scores at time of MRI, STAS = mean State Trait Anger Scale scores at time of MRI.
Protocol
Participants were administered diagnostic interviews and questionnaires, including questions on demography, life events, comorbidity, type and severity of OC symptoms, tics, state anger, and state anxiety (for a detailed description of the administered diagnostic interviews and questionnaires, please refer to den Braber et al., Reference den Braber, van ‘t Ent, Cath, Wagner, Boomsma and de Geus2010). All twins were asked to collect mucosal cell samples for DNA extraction to test zygosity. The ethical review board of the VU University Medical Center approved the study. All participants provided written informed consent.
Task Paradigms
Stroop. During the Stroop color-word task, subjects had to report the ink color of written color words. Dutch translations of the words ‘red’, ‘yellow’, ‘blue’, and ‘green’ were used that were written in any of these four colors. Word meaning and ink color could be either congruent (e.g., the word ‘green’ written in green) or incongruent (e.g., the word ‘red’ written in blue). The correct answer had to be indicated by pressing buttons: left middle finger for ink color yellow, left forefinger for green, right forefinger for red and right middle finger for blue. The task was administered in 18 blocks of similar stimulus types. Of these, 3 blocks contained congruent and 3 blocks contained incongruent color-word stimuli. In each individual block, 16 words were presented for 2 seconds, separated by small intervals of 200 ms. The other 12 blocks consisted of words with an emotional content, which were not used here (for a full description of the task we refer to van den Heuvel et al., Reference van den Heuvel, Veltman, Groenewegen, Witter, Merkelbach, Cath, van Balkom, van Oppen and van Dyck2005). The subjects were asked to respond to the stimuli as fast and as accurately as possible. The onset of each individual stimulus, together with the subject's response, was recorded such that the data could be analyzed in an event-related manner. Total task duration was ± 10 minutes.
Flanker. In the Flanker task, subjects had to indicate, as quickly as possible, the direction of a central target arrow (i.e. ‘<‘ left hand press; ‘>‘ right hand press) which was surrounded by four task-irrelevant flankers of the same size and shape. The direction of the Flanker arrows could be either congruent (‘< < < < <‘ or ‘> > > > >‘) or incongruent (‘< < > < <‘ or ‘> > < > >‘) with the direction of the central target arrow. Flankers and targets were displayed simultaneously. The task was administered in an event-related design. During the task 120 congruent and 120 incongruent trials were presented in random order. Stimuli were shown for 200 ms; the interstimulus interval consisted of a period of gray screen after each stimulus (randomized between 600 and 1600 ms) and a subsequent fixation cross for 1000 ms before the next stimulus. Total task duration was ± 10 minutes.
For both the Stroop and Flanker tasks, stimuli were projected on a screen at the end of the MRI scanner table, viewed by the participants through a mirror. Two magnet-compatible response boxes were used to record the subject's performance. Before the experiment, the subjects practiced a number of trials on a computer outside the scanner, and again inside the scanner, before the actual start of the experimental session.
Image Acquisition
The MRI session consisted of a structural part of about 6 minutes and a functional part of about 20 minutes (Stroop ± 10 minutes; Flanker ± 10 minutes). The participant remained inside the scanner and was asked to minimize head movement during and between consecutive runs. To reduce motion artifacts, the participant's head was immobilized using foam pads.
MRI was performed on a 3.0 T Intera MR system (Philips, Medical Systems, Best) with a standard SENSE receiver head coil. The anatomical scan consisted of 182 coronal slices with a 3D gradient-echo T1-weighted sequence (flip angle 8°; repetition time, TR = 9.69 ms; echo time, TE = 4.60 ms; matrix 256 × 256 pixels; voxel size, 1.00 × 1.00 × 1.20 mm). For fMRI, an echo planar imaging (EPI) sequence (flip angle 80°; TR = 2300 ms; TE = 30 ms; matrix 96 × 96 pixels; field of view 220 × 220 mm) was used, covering the whole brain (40 axial slices; 2.29 mm × 2.29 mm in-plane resolution; 3.0 mm slice thickness). For the Stroop task a total of 260 and for the Flanker a total of 250 EPI volumes were scanned per subject.
Data Analysis
MRI data were analyzed using SPM5 (Wellcome Department of Imaging Neuroscience, London, UK). EPI scans were slice time corrected, realigned and normalized to the standard MNI (Montreal Neurological Institute) brain of SPM. Subsequently, data were resliced to 3 mm × 3 mm × 3 mm voxels and spatially smoothed using an 8 mm isotropic Gaussian kernel. After high-pass filtering (cut-off 128 seconds), functional scans were analyzed in the context of the general linear model using delta functions convolved with a canonical hemodynamic response function. Error trials and head-movement parameters were modeled as regressors of no interest. Post hoc analysis of subject motion during the scans, based on the functional scan realignment parameters, indicated that the twins with high OC symptom scores did not exhibit significantly larger head movements compared to the twins with low OC symptom scores. For each subject and task, contrast images were computed for simple main effects of congruent and incongruent trials, as well as the effect of response interference (incongruent minus congruent trials). For all contrasts, only trials with correct reactions were included.
Statistical Tests
Survey-based and interview-based variables and task performance data were investigated using a mixed-model analysis of variance (ANOVA; Mixed Models Linear menu item in SPSS; SPSS, Chicago, Illinois) with twin-pair type (discordant versus concordant) and OC symptom score (high versus low) as two fixed factors, and family as a random factor to account for within-twin pair dependence. Statistical results with regard to questionnaire and task performance data were considered significant at p < .05, Bonferroni corrected.
First-level functional MRI contrast estimates for ‘Stroop interference’ and ‘Flanker interference’ were entered into second-level analyses available in SPM5. Differences in contrast estimates between OCS high-scoring and OCS low-scoring twins from discordant pairs were investigated by paired sample t-tests. Differences in contrast estimates between concordant OCS high and concordant OCS low twin pairs were assessed using an ANOVA group comparison. To account for within-twin pair correlations of fMRI signals, first-level results of the twin and co-twin of each concordant pair were entered as repeated measures. For main task effects of selected contrasts, we set an individual voxel threshold of p < .05, corrected for multiple comparisons (false discovery rate: FDR), with a minimal cluster extent of 100 voxels. Group differences, masked with the appropriate main task effect (mask thresholded at p < .05, uncorrected), are reported at an uncorrected individual voxel threshold of p < .005, with a minimal cluster extent of 10 voxels.
Results
Questionnaire and Interview Data
As expected, OCS high-scoring compared to low-scoring twins in both the discordant and concordant groups showed significantly higher scores on the PI-R-ABBR and Yale-Brown Obsessive Compulsive Scale (Y-BOCS) symptom severity (see Table 1). In addition, high-scoring twins were more often diagnosed with current comorbid disorders, which were absent in the low-scoring twins.
Task Performance
Across all individuals, reaction times for both the Stroop and the Flanker task were significantly delayed after incongruent compared to congruent stimuli. [Stroop: incongruent M = 988.92, SD = 167.44 ms vs. congruent M = 826.27, SD = 148.84 ms, F(1,139) = 279.82, p < .001; Flanker: incongruent M = 522.54, SD = 193.28 ms vs. congruent M = 527.11, SD = 193.10 ms, F(1,139) = 5.24, p < .05]. In addition, for the Stroop task, percentages of trials with correct reactions were significantly reduced after incongruent stimuli [incongruent M = 80.4, SD = 15.3 vs. congruent M = 95.0, SD = 6.4, F(1,139) = 171.02, p < .001]. For the Flanker task, no significant reduction was found in percentage of trials with correct reactions after incongruent stimuli [incongruent M = 97.8, SD = 4.4 vs. congruent M = 98.0, SD = 3.9, F(1,139) = 0.21, p = .649].
In Table 2, response interference effects, quantified by computing differences in response latency and response accuracy between incongruent and congruent stimulus trials, are displayed separately for the discordant and concordant twin sample. Response latencies did not differ significantly between OCS high-scoring and low-scoring twins from both the discordant and concordant sample. For response accuracy, a smaller effect of response interference was found in the discordant high-risk relative to their discordant low-risk co-twins during the Flanker task.
TABLE 2 Response Interference Effects on Task Performance
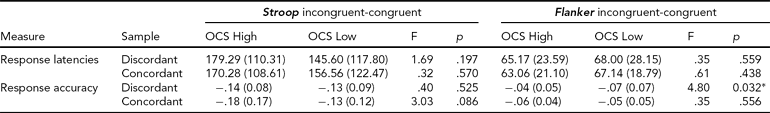
Effects of information conflict on Stroop task (left) and Flanker task (right) behavior, measured by computing differences in response latencies (top rows) and response accuracy (bottom rows) on incongruent relative to congruent stimulus trials. OCS = obsessive compulsive symptoms. Columns F and p indicate results from statistical tests on OCS-related differences between selected discordant and concordant twin samples.
* = statistically significant difference.
Functional Imaging
Main Effects. Figure 1 and Table 3 show brain areas with significant fMRI-BOLD (blood-oxygen-level-dependent) activations across all subjects, during Stroop interference (Figure 1: top; Table 3: left), and Flanker interference (Figure 1, bottom; Table 3, right). In both the Stroop and Flanker tasks, response interference was associated with enhanced activation of bilateral occipital, parietal, temporal, and caudate/putamen regions, as well as several prefrontal lobe regions including the ACC, DLPFC, premotor, and inferior frontal cortices. For the Stroop task, increased activation was also noted in left and right thalamus, whereas for the Flanker task an additional cluster was observed in left and right postcentral gyrus.

FIGURE 1 Main effects of fMRI-BOLD activation, across all participating twins. Glass brain overviews depict brain activity patterns for ‘Stroop interference’ (top) and ‘Flanker interference’ (bottom). Anatomical renderings on the right illustrate locations of functional brain activation for Stroop interference (top) and Flanker interference (bottom), across all participating twins.
TABLE 3 Brain Activity for Stroop and Flanker Interference Contrasts Across Both the Discordant and Concordant Sample

Anatomical locations of significant clusters for main effects of fMRI BOLD activation during Stroop interference incongruent-congruent trials (left) and Flanker interference incongruent-congruent trials (right). ACC = anterior cingulate cortex, MNI = Montreal Neurological Institute. MNI coordinates and t-values are listed for the voxels with the largest effect size.
Environmental Risk: OCS High-Scoring Versus Low-Scoring Twins From Discordant Pairs
Paired comparisons between the high-risk twin and the low-risk co-twin from discordant pairs revealed significant clusters of increased activation to response interference, but located in different brain regions for the Stroop and Flanker tasks. For the Stroop task, a single cluster of increased activation was found in the right DLPFC (Table 4 and Figure 2, label A). For the Flanker task, increased activation was found in the left middle temporal gyrus, left cingulate gyrus, and right cerebellum (Table 4 and Figure 2, labels B, C, and D, respectively).
TABLE 4 Environmental Risk: Brain Activation Differences Between Monzygotic Twins With High and Low Obsessive Compulsive Symptoms in the Discordant Sample

Differences in brain activation to trials with information conflict in discordant high compared to low risk twins. Test: test for significant increases (high > low) or decreases (high < low) in OCS high relative to OCS low scoring twins. MNI coordinates: location of voxel with largest effect size; Z score: z-value of voxel with largest effect size.

FIGURE 2 Most significant clusters, overlaid on MR sections, from statistical evaluations of OCS-related differences in brain activation to Stroop and Flanker trials with response interference. Left panels: hyperactivations (top row) and hypoactivations (bottom row) for discordant high compared to low-risk twins (environmental contrast). Right panels: hyperactivations (top row) and hypoactivations (bottom row) for concordant high-risk twins compared to low-risk twins (genetic contrast). OCS = obsessive-compulsive symptoms, A = Right dorsolateral prefrontal gyrus, B = Left middle temporal gyrus, C = Left cingulate gyrus, D = Right cerebellum, E = Left precentral gyrus, F = Left dorsolateral prefrontal gyrus, G = Left middle frontal gyrus, H = Left precuneus, I = Right angular gyrus, J = Left inferior parietal gyrus, K = Right inferior parietal gyrus, L = Right thalamus, M = Left inferior parietal gyrus.
High-risk discordant twins also showed an area of reduced activation during response interference, exclusively in the Stroop task, in the left precentral gyrus (Table 4 and Figure 2, label E).
Genetic Risk: Concordant High-Scoring Versus Concordant Low-Scoring Twin Pairs
Table 5 and Figure 2 (right) show clusters of OCS-related differences for brain activation to response interference between the concordant high and concordant low twin pairs. Concordant high compared to concordant low twin pairs showed several significant clusters of increased activation to response interference, again in different brain regions for the Stroop and Flanker tasks. For the Stroop task, high-risk twins from concordant pairs showed relatively increased activity in regions of the left DLPFC, left middle frontal gyrus, left precuneus, right angular gyrus, and bilateral inferior parietal gyrus (Figure 2, labels F, G, H, I, J and K, respectively). For the Flanker task, there was a single cluster of increased activation in high-risk twins in the right thalamus (Figure 2, label L).
TABLE 5 Genetic Risk: Brain Activation Differences Between Monzygotic Twins With High and Low Obsessive Compulsive Symptoms in the Concordant Sample

Differences in brain activation to trials with information conflict in concordant high compared to low risk twins.
High-risk concordant twins also showed an area of reduced activation during response interference, exclusively in the Flanker task, in the left inferior parietal gyrus (Figure 2, label M).
There were 10 subjects (3 discordant high, 7 concordant high) with current comorbid disorders. Removing these subjects from the analyses did not affect the pattern of results.
Discussion
Task performance and brain activation during Stroop color-word and Flanker interference were compared within MZ twin pairs discordant for OC symptoms, and between groups of pairs scoring very low or very high on OC symptoms. These comparisons examined the differential impact of non-shared environmental versus genetic and shared environmental risk factors for OC symptomatology on inhibitory control related functional brain activation. Shared family environment has never been reported to influence OC behavior in adult twin studies (Clifford et al., Reference Clifford, Murray and Fulker1984; Jonnal et al., Reference Jonnal, Gardner, Prescott and Kendler2000; van Grootheest et al., Reference van Grootheest, Cath, Beekman and Boomsma2007). Therefore, familial risk factors for this trait were taken to translate mainly to genetic risk.
Analysis of task-related behavior indicated classical effects of response interference on response latencies and response accuracy for both the Stroop and Flanker tasks. In line with previous studies, task performances of twins high and low on OC symptoms were comparable, with the exception of marginally reduced interference in high-scoring compared to low-scoring twins of the discordant sample during the Flanker task, which was mainly due to the fact that high-scoring twins made fewer errors during incongruent trials than their low-scoring co-twins.
The fMRI main effects of our study indicated that highly similar brain processes were active during Stroop and Flanker task performance. Brain areas involved include bilateral occipital, parietal, temporal, and caudate/putamen regions, as well as several frontal lobe regions including the ACC, the DLPFC, and the premotor and inferior frontal cortices. These results highly overlap with findings from previous studies that investigated response interference using these paradigms (Kerns et al., Reference Kerns, Cohen, MacDonald, Cho, Stenger and Carter2004; van den Heuvel et al., Reference van den Heuvel, Veltman, Groenewegen, Witter, Merkelbach, Cath, van Balkom, van Oppen and van Dyck2005; van ‘t Ent et al., Reference van ’t Ent, van Beijsterveldt, Derks, Hudziak, Veltman, Todd, Boomsma and de Geus2009). OC symptom status clearly affected this pattern of brain activation, with increased conflict-related DLPFC activity in high-risk compared to low-risk twins from both the discordant and concordant samples during the Stroop task. It has been hypothesized that evaluative and control functions are represented by a dorsolateral prefrontal – anterior cingulate cortical circuit, where the ACC is involved in detecting the occurrence of conflict and the DLPFC in performance adjustments (Melcher et al., Reference Melcher, Falkai and Gruber2008). A study that examined predictions of this conflict hypothesis indeed showed more ACC activity in high-conflict correct trials and error trials, which was associated with adjustments in behavior on the next trial that reflect improved control (Kerns et al., Reference Kerns, Cohen, MacDonald, Cho, Stenger and Carter2004). In addition, this study showed that trials exhibiting the largest adjustments in behavior following conflict were associated with increased activity in the DLPFC. Furthermore, previous studies provided evidence for hyperactivity in this dorsolateral prefrontal anterior cingulate circuit during cognitive control in OCD patients compared to healthy controls (Maltby et al., 2005; Schlosser et al., Reference Schlosser, Wagner, Schachtzabel, Peikert, Koch, Reichenbach and Sauer2010) and showed that OCD patients exhibited enhanced dorsal ACC to DLPFC connectivity, in agreement with the hypothesis that OCD is related to an overactive control system (Schlosser et al., Reference Schlosser, Wagner, Schachtzabel, Peikert, Koch, Reichenbach and Sauer2010). During Stroop interference there was no evidence for increased conflict-related activity in the ACC in our study, but increased conflict-related activity in the ACC was observed in OCS high-scoring compared to low-scoring twins from the discordant sample during Flanker interference. In addition, this increase in ACC activity during response interference was accompanied by a better performance of the OC symptom high-scoring twins compared to their low-scoring co-twins.
While the ACC was not significantly more activated in high-scoring versus low-scoring twins during Stroop interference, the DLPFC, involved in performance adjustments, showed increased conflict-related activity in the OCS high-scoring twins from discordant OCS pairs as well as in twins from concordant OCS pairs. This finding suggests that, although the degree of color-word conflict detection was the same, OCS high-scoring subjects have a higher propensity to adjust their behavior following conflict compared to low-scoring subjects. This supports the assumption of an overactive control network in OC symptomatology. As the DLPFC was affected in both the high-scoring twins from discordant and concordant pairs, it seems to act as a final common pathway for both genetic and environmental risk factors for OC symptomatology. Deviant DLPFC activity during inhibitory control may be closely correlated with the actual behavioral deficits of the disorder.
Response conflict-related brain alterations in OCS high-scoring compared to low-scoring twins were also observed in several other regions of the brain. Regions affected in the discordant group were different from those observed in the concordant group. Brain regions showing different activation patterns in twins with high OC symptoms scores compared with those with low OC symptoms scores that were present in only the discordant group and, therefore, are likely related to environmental risk factors for OC symptomatology include the precentral gyrus (Stroop), middle temporal gyrus and cerebellum (Flanker). Brain regions showing different activation patterns in twins with high OC symptoms scores compared with those with low OC symptoms scores that were present in only the concordant group and, therefore, are likely related to genetic risk factors for OC symptomatology include the middle frontal gyrus, precuneus, angular gyrus, parietal gyrus (Stroop), and thalamus (Flanker).
An environmentally mediated increase in temporal activity and a genetically mediated increase in parietal activity have also been observed in a previous study by our group that used the discordant/concordant twin design to study OC symptom-related brain alterations during a cognitive planning paradigm (den Braber et al., Reference den Braber, van ‘t Ent, Cath, Wagner, Boomsma and de Geus2010). OCD-related alterations in temporal and parietal cortices have been found by others as well, and have therefore been included in an extended model for OCD (Menzies et al., Reference Menzies, Chamberlain, Laird, Thelen, Sahakian and Bullmore2008). An altered function of these regions might, through their functional connections with the ventral and dorsal PFC, lead to an imbalance of the direct and indirect pathway of the CSTC networks, which could subsequently induce OC behavior. Abnormalities in thalamic volume and function in OCD have been extensively reported (Menzies et al., Reference Menzies, Chamberlain, Laird, Thelen, Sahakian and Bullmore2008). The thalamus is implicated in the CSTC model of OCD. It is the key region in modulating subcortical input to the frontal cortex, stimulates output of frontal brain regions, and plays a crucial role in the processing of sensory inputs, thereby mediating behaviors, emotion, and cognition (Sherman & Guillery, Reference Sherman and Guillery2002). Therefore, disturbances within this structure are likely to be coupled to the cognitive and behavioral deficits seen in OCD patients. Further research into the association between these structures and the control network is warranted.
A total of 10 subjects included in this study had comorbid diagnoses which could have confounded our result. Although excluding these subjects did not change the patterns of results, comorbid traits that do not meet threshold for clinical diagnoses in the remaining subjects with high levels of OC symptoms may have influenced the results. This is a limitation of the design used, as the selection for high levels of OC symptoms will by necessity lead to co-selection for comorbid traits.
In summary, the present study demonstrates decreases as well as increases in brain activation during the inhibition of distracting information in OCS high-scoring compared to low-scoring subjects. A robust increase in DLPFC activity during response interference was observed in both the high-scoring twins of the discordant sample as well as the high-scoring twins of the concordant sample, marking this structure as a possible key region for disturbances in inhibitory control in OCD.
Acknowledgments
We thank Gabriëlla Blokland, Myrle Kemperman, Mira Geirnaert and Judith Wagner for help with MRI data collection. Funded by NWO (MW 904-61-193; MaGW-nr: 400-07-080; MagW 480-04-004).









